Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Characterization
| Ri a | Ri b | Yield % (v/w) | Identification c | 1.17 |
|---|---|---|---|---|
| Compound ID | ||||
| 938 | 1076 | α-Pinene | Ri, MS d, CoGC e | 86.8 f |
| 980 | 1118 | β-Pinene | Ri, MS, CoGC | 2.5 |
| 993 | 1174 | Myrcene | Ri, MS, CoGC | 3.2 |
| 1013 | 1159 | δ-3-Carene | Ri, MS | 2.4 |
| 1030 | 1203 | Limonene | Ri, MS, CoGC | 2.2 |
| 1057 | 1255 | γ-Terpinene | Ri, MS, CoGC | 0.3 |
| 1143 | 1532 | Camphor | Ri, MS, CoGC | T |
| 1152 | 1683 | trans-Verbenol | Ri, MS | T |
| 1165 | 1587 | Pinocarvone | Ri, MS | T |
| 1182 | 1864 | p-Cymen-8-ol | Ri, MS | T |
| 1189 | 1706 | α-Terpineol | Ri, MS | 0.4 |
| 1217 | 1725 | Verbenone | Ri, MS | 0.1 |
| 1284 | 1597 | Bornyl acetate | Ri, MS, CoGC | T |
| 1477 | 1726 | d-Germacrene | Ri, MS | T |
| 1515 | 1776 | δ-Cadinene | Ri, MS | T |
| 1604 | 2160 | Cedrol | Ri, MS, CoGC | T |
| Monoterpene hydrocarbons | 97.3 | |||
| Oxygenated monoterpenes | 0.8 |
2.2. Antibacterial Activity
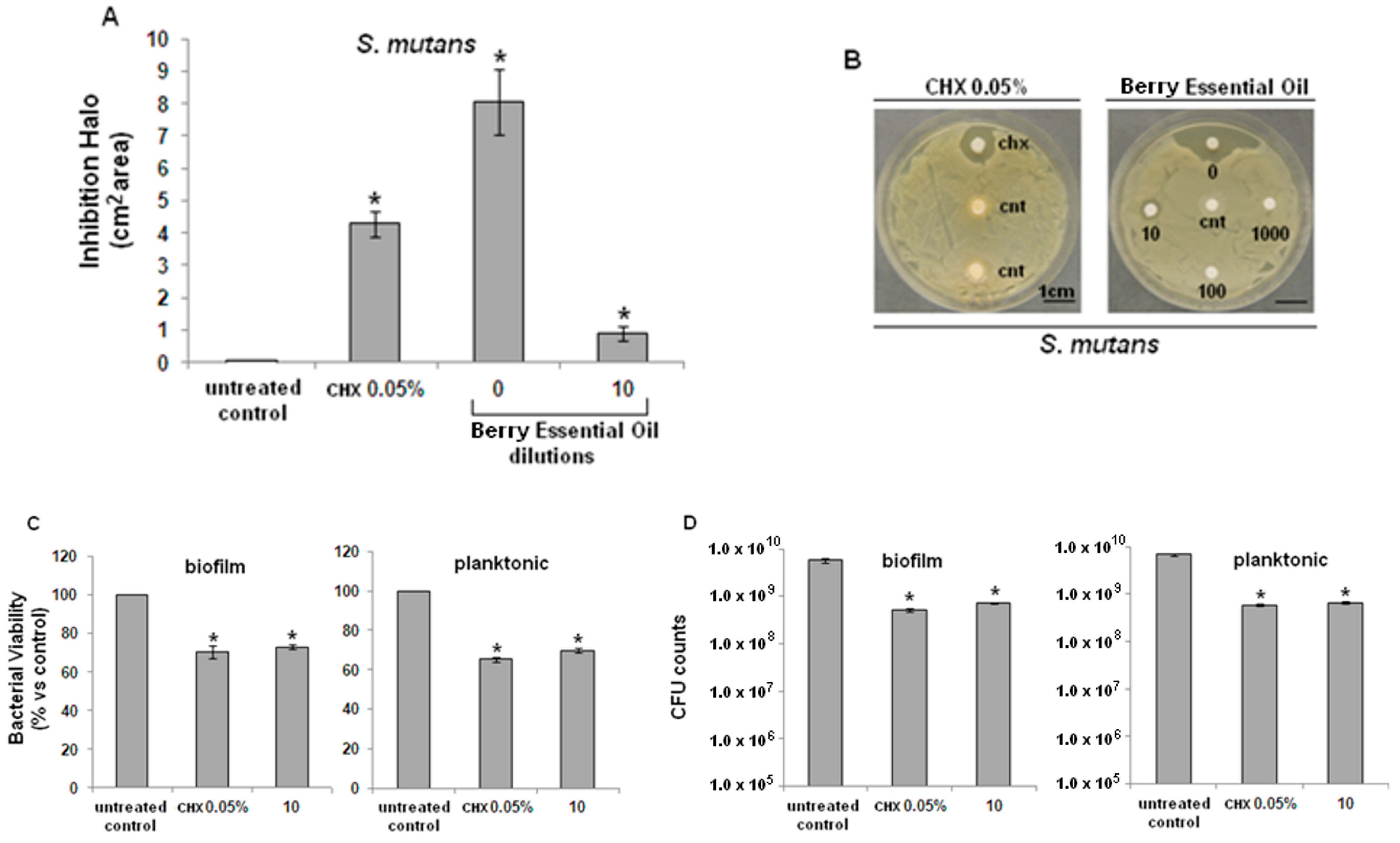
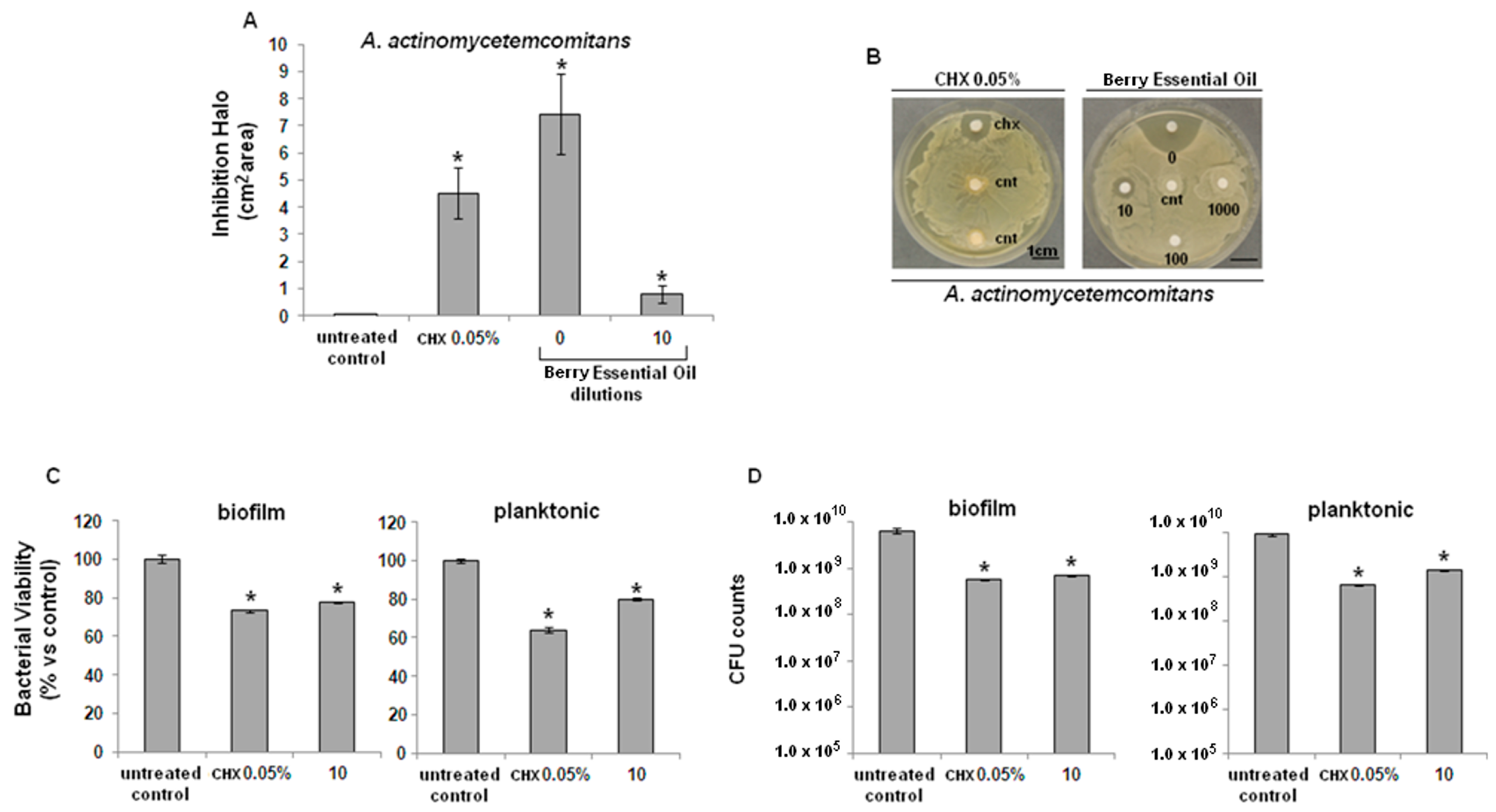
2.3. Cytocompatibility
2.4. Discussion

3. Experimental Section
3.1. Essential Oils
3.1.1. Plant Material
3.1.2. Essentials Oil Extraction
3.1.3. Essential Oil Analysis
3.2. Antibacterial Activity of Essential Oils
3.2.1. Bacterial Strains and Growth Conditions
3.2.2. Biofilm and Planktonic Bacterial Cells
3.2.3. Essential Oil Treatment
3.2.4. Inhibition Halo
3.2.5. Bacterial Cell Viability
3.2.6. Colonies Forming Units (CFU) Counts
- number of colonies = countable single round colonies;
- dilution factor = dilution made from the initial 1 mL suspension;
- serial dilution = 1–6 ten-fold dilution areas where colonies were counted.
3.3. Cytocompatibility Evaluation of Essential Oils
3.3.1. Cells
3.3.2. Cell Viability Determination
3.4. Statistical Analysis of Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, T.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, M.A. Everything is everywhere: But the environment selects: Ubiquitous distribution and ecological determinism in microbial biogeography. Stud. Hist. Philos. Biol. Biomed. Sci. 2008, 39, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Dörfer, C.E.; Schlattmann, P.; Page, L.F.; Thomson, W.M.; Paris, S. Socioeconomic Inequality and Caries: A Systematic Review and Meta-Analysis. J. Dent. Res. 2015, 94, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Southerland, J.H.; Taylor, G.W.; Moss, K.; Beck, J.D.; Offenbacher, S. Commonality in chronic infammatory diseases: Periodontitis, diabetes and coronary artery disease. Periodontol. 2000 2006, 40, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, G.D.; Hu, F.Z.; Sotereanos, N.; Sewicke, J.; Parvizi, J.; Nara, P.L.; Arciola, C.R. What role do periodontal pathogens play in osteoarthritis and periprosthetic joint infections of the knee? J. Appl. Biomater. Funct. Mater. 2014, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; Chiesa, R.; Arciola, C.R.; Rimondini, L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. J. Biomed. Mater. Res. A 2015, 103, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Saino, E.; Rimondini, L.; Pedeferri, M.P.; Visai, L.; Cigada, A.; Chiesa, R. Electrochemically induced anatase inhibits bacterial colonization on Titanium Grade 2 and Ti6Al4V alloy for dental and orthopedic devices. Colloids Surf. B Biointerfaces 2011, 88, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J. Acquiring and maintaining a normal oral microbiome: Current perspective. Front. Cell Infect. Microbiol. 2014, 4, 85. [Google Scholar] [CrossRef]
- Varoni, E.; Tarce, M.; Lodi, G.; Carrassi, A. Chlorhexidine (CHX) in dentistry: State of the art. Minerva Stomatol. 2012, 61, 399–419. [Google Scholar]
- Brambilla, E.; Ionescu, A.; Gagliani, M.; Cochis, A.; Arciola, C.R.; Rimondini, L. Biofilm formation on composite resins for dental restorations: An in situ study on the effect of chlorhexidine mouthrinses. Int. J. Artif. Organs 2012, 35, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Goltz, S.; Nietzsche, S.; Jentsch, H.; Pfister, W. Efficacy of chlorhexidine digluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int. 2011, 42, 687–700. [Google Scholar]
- Hidalgo, E.; Dominguez, C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol. In Vitro 2001, 15, 271–276. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bruhn, G.; Richter, S.; Netuschil, L.; Brecx, M. Clinical controlled study on plaque and gingivitis reduction under long-term use of low-dose chlorhexidine solutions in a population exhibiting good oral hygiene. Clin. Oral Investig. 2001, 5, 89–95. [Google Scholar]
- Cochis, A.; Fracchia, L.; Martinotti, M.G.; Rimondini, L. Biosurfactants prevent in vitro Candida albicans biofilm formation on resins and silicon materials for prosthetic devices. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, A.D.; Dicks, L.M. Calcium orthophosphate-based bone cements (CPCs): Applications, antibiotic release and alternatives to antibiotics. J. Appl. Biomater. Funct. Mater. 2012, 10, 2–11. [Google Scholar] [PubMed]
- Hammer, K.A.; Heel, K.A. Use of multiparameter flow cytometry to determine the effects of monoterpenoids and phenylpropanoids on membrane polarity and permeability in staphylococci and enterococci. Int. J. Antimicrob. Agents 2012, 40, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; El Beyrouthy, M.; Ouaini, N.; Iriti, M.; Eparvier, V.; Stien, D. Chemical composition and antimicrobial activity of the essential oil of Juniperus excelsa M. Bieb. growing wild in Lebanon. Chem. Biodivers. 2014, 11, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Fadli, M.; Chevalier, J.; Saad, A.; Mezrioui, N.E.; Hassani, L.; Pages, J.M. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 38, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, M.; Vardar-Unlü, G.; Vural, N.; Donmez, E.; Ozbas, Z.Y. Chemical composition, antibacterial and antifungal activity of the essential oil of Thymbra spicata L. from Turkey. Chem. Nat. Compd. 2009, 23, 572–579. [Google Scholar]
- Weli, A.M.; Al-Hinai, S.R.K.; Hossain, M.M.; Al-Sabahi, J.N. Composition of essential oil of Omani Juniperus excelsa fruit and antimicrobial activity against foodborne pathogenic bacteria. J. Taibah Univ. Sci. 2014, 8, 225–230. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Zheljazkov, V.D.; Carvalho, C.R.; Astatkie, T.; Jeliazkova, E.A.; Rosa, L.H. Dual Extraction of Essential Oil and Podophyllotoxin from Creeping Juniper (Juniperus horizontalis). PLoS ONE 2014, 9, e106057. [Google Scholar] [CrossRef] [PubMed]
- Nizar, Y.S.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar]
- Takarada, K.; Kimizuka, R.; Takahashi, N.; Honma, K.; Okuda, K.; Kato, T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol. Immunol. 2004, 19, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.C.; Harper, S.; Ricci-Nittel, D.; Lux, R.; Shi, W. In-vitro evidence for efficacy of antimicrobial mouthrinses. J. Dent. 2010, 38, 16–20. [Google Scholar] [CrossRef]
- Quintas, V.; Prada-López, I.; Prados-Frutos, J.C.; Tomás, I. In situ antimicrobial activity on oral biofilm: Essential oils vs. 0.2% chlorhexidine. Clin. Oral Investig. 2015, 19, 97–91. [Google Scholar] [CrossRef] [PubMed]
- Tsourounakis, I.; Palaiologou-Gallis, A.A.; Stoute, D.; Maney, P.; Lallier, T.E. Effect of essential oil and chlorhexidine mouthwashes on gingival fibroblast survival and migration. J. Periodontol. 2013, 84, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lower-Nedza, A.D.; Hong, M.; Jie, S.; Wang, Z.; Yingmao, D.; Tschiggerl, C.; Bucar, F.; Brantner, A.H. Chemical composition and antimicrobial activity of three essential oils from Curcuma wenyujin. Nat. Prod. Commun. 2013, 8, 523–526. [Google Scholar] [PubMed]
- Helmi, Z.; Al Azzam, K.M.; Tsymbalista, Y.; Ghazleh, R.A.; Shaibah, H.; Aboul-Enein, H. Analysis of Essential Oil in Jerusalem Artichoke (Helianthus tuberosus L.) Leaves and Tubers by Gas Chromatography-Mass Spectrometry. Adv. Pharm. Bull. 2014, 4, 521–526. [Google Scholar] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter susceptibility testing of microbes growing on peg lids: A miniaturized biofilm model for high-throughput screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the essential oil is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzimonti, B.; Cochis, A.; Beyrouthy, M.E.; Iriti, M.; Uberti, F.; Sorrentino, R.; Landini, M.M.; Rimondini, L.; Varoni, E.M. Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells. Molecules 2015, 20, 9344-9357. https://doi.org/10.3390/molecules20059344
Azzimonti B, Cochis A, Beyrouthy ME, Iriti M, Uberti F, Sorrentino R, Landini MM, Rimondini L, Varoni EM. Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells. Molecules. 2015; 20(5):9344-9357. https://doi.org/10.3390/molecules20059344
Chicago/Turabian StyleAzzimonti, Barbara, Andrea Cochis, Marc El Beyrouthy, Marcello Iriti, Francesca Uberti, Rita Sorrentino, Manuela Miriam Landini, Lia Rimondini, and Elena Maria Varoni. 2015. "Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells" Molecules 20, no. 5: 9344-9357. https://doi.org/10.3390/molecules20059344
APA StyleAzzimonti, B., Cochis, A., Beyrouthy, M. E., Iriti, M., Uberti, F., Sorrentino, R., Landini, M. M., Rimondini, L., & Varoni, E. M. (2015). Essential Oil from Berries of Lebanese Juniperus excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells. Molecules, 20(5), 9344-9357. https://doi.org/10.3390/molecules20059344











