Abstract
The anthocyanin extract from a domestic Perilla cultivar (Perilla frutescens var. acuta) were isolated and characterized with high mass accuracy and multi-dimensional fragmentation by means of ultra-performance liquid chromatography (UPLC) and electrospray ionization-ion trap-time of flight mass spectrometry analysis (ESI-IT-TOF-MSn). The new developed and applied LC-MS method focused on in-depth screening of anthocyanin compounds with similar structures which also provided a new approach of anthocyanin characterization without the use of external standards. Selective detection of interested anthocyanins was achieved utilizing extracted ion chromatogram (EIC) analysis, while MSn spectra were recorded to allow identification of the anthocyanin based on characteristic fragmentation patterns. Seven anthocyanins including one feruloyl (Cyanidin 3-O-feruloylglucoside-5-O-glucoside), two caffeoyl (Cyanidin 3-O-caffeoylglucoside-5-O-glucoside, Cyanidin 3-O-caffeoylglucoside-5-O-malonylglucoside) and four coumaroyl substituted anthocyanins (Cis-shisonin, Malonyl-cis-shisonin, Shisonin, and Malonyl-shisonin) were identified. Annexin-V FITC/PI flow cytometric assay was performed to analyze the influence of anthocyanin extract of P. frutescens var. acuta on cell apoptosis. The results suggested that Perilla anthocyanins can induce Hela cell apoptosis by a dose dependent manner.
1. Introduction
Perilla (Perilla frutescens (L.) Britt.) is an annual herbaceous plants native to Eastern Asia. It is well-known in Chinese medicine and has a long history of cultivation in China as a source of medicine and spices. Among the common varieties of P. frutescens, three are widely used by local people which are generally known as var. frutescens, var. crispa and var. acuta (Figure 1). Seeds of P. frutescens var. frutescens are considered as a rich oil source and its leaves are edible and also used as a preservative in the food industry. Similarly, P. frutescens var. crispa are also used as fresh vegetables by people from the Far East, but more research is focused on its medicinal properties [1,2]. P. frutescens var. acuta are particularly known for prevention and treatment of seafood poisoning by fishes or crabs contaminated with bacteria that produce various toxins, and are also shown to have other bio-activities like anti-oxidative, anti-inflammatory and anti-allergic abilities [3,4,5].

Figure 1.
Three natural varieties of Perilla frutescens (a) P. frutescens var. frutescens; (b) P. frutescens var acuta; (c) P. frutescens var crispa.
Figure 1.
Three natural varieties of Perilla frutescens (a) P. frutescens var. frutescens; (b) P. frutescens var acuta; (c) P. frutescens var crispa.

Different chemotypes have been found within varieties of P. frutescens, which lead to the diversity of leaf and stem colors, indicating the existence of a great range of anthocyanins in these plants. Related researches on anthocyanin contents in var. crispa have shown that the red color was given by the presence of a cyanidin-type anthocyanin, cyanidin 3-(6-p-coumaroylglucoside)-5-malonylglucoside, malonyl-shisonin [6], and other less abundant anthocyanin compounds that accumulate in the epidermal cells of leaves and stems of P. frutescens [7]. On the other hand, biological activities like antioxidant effects have been widely studied for P. frutescens varieties which indicated their medical potentials [4,8]. However, only limited studies were available for anthocyanin constituents and anticancer activity of P. frutescens var. acuta, though anthocyanins from other plant materials have exhibited a wide range of anticancer properties including antioxidant effects, anti-cell proliferation, induction of apoptosis, anti-inflammatory effects, anti-angiogenesis and et al. [9,10]. It is possible that Perilla anthocyanins have anticancer potential.
In the present study, therefore, an electrospray ionization-ion trap-time of flight mass spectrometer equipped with an ultra-performance liquid chromatograph (UPLC-ESI-IT-TOF-MSn) was used to identifying anthocyanin compounds in leaves of P. frutescens var. acuta. UPLC-ESI-IT-TOF-MS are complementary alternatives for characterizing isomers or anthocyanins with similar structures which show similar retention times and spectroscopic characteristics on HPLC-DAD analysis [11]. These soft ionization, multi stage fragmentation and high resolution techniques provide mass spectra with intact molecular ion and high accuracy of full-fragment spectra making it possible to define the distribution of anthocyanins in complex matrices. For now, to our knowledge, high mass accuracy and MSn measurements of anthocyanins appear to be limited in literature. Thus, one of the objectives of this study was to develop an advanced LC-MS system to confirm the identity of anthocyanins which have similar structures without the use of reference standards. Another objective was to investigate the induction ability on cell apoptosis of its extract according to DAPI staining and Annexin-V FITC/PI flow cytometric assay since it has been reported that anthocyanins can induce apoptosis through both intrinsic (mitochondrial) and extrinsic (FAS) pathways [9].
2. Results and Discussion
2.1. Total Anthocyanin Content (TAC)
The total monomeric anthocyanin content in the leaves of P. frutescens var. acuta was 52.5 mg/100 g dry weight, expressed as cyanidin-3-O-glucoside equivalents and calculated as the mean value of three measurements and the standard deviation. In a previous report, total anthocyanin content of two var. frutescens cultivars from different regions of China are 1.6 mg/g (dry matter) and 1.8 mg/g (dry matter), respectively, whereas two var. crispa cultivars from China and Japan are 0.7 mg/g (dry matter) and 1.6 mg/g (dry matter), respectively. Apart from the difference between P. frutescens varieties, different levels of structural anthocyanin gene expression related to cultivation environment and harvest time may explain this difference of anthocyanin content [1].
2.2. UPLC-ESI-IT-TOF-MSn Analysis
Although there are various reports of using conventional HPLC with DAD detection at 520 nm for the determination of anthocyanins in complex plant extracts [11], such methods cannot produce very accurate results, especially for those anthocyanins with similar structures. Despite the effect of matrix, the anthocyanins with similar structures may easily elute together as an unresolved broad peak, which increases the difficulty of qualitative analysis. On the other hand, LC-MS analysis permits more accurate results and provides benefits such as increased sensitivity and structural information compared to LC-DAD [12]. Moreover, application of ESI-IT-TOF effectively simplifies the procedure of characterization and structure elucidation and makes it possible for the identification of anthocyanins without the aid of standards, enabling the establishment of anthocyanin profile and structure through high accuracy molecular mass determination and multistage fragmentation of the ions of interest in merely a single assay.
As can be seen, seven anthocyanins were detected. Table 1 shows the detailed information obtained by UPLC-TOF-MS, including retention time, maximum absorption (λmax), theoretical and measured mass signals of molecular ion ([M]+), accurate mass, error associated with the accurate mass (ppm) and the molecule formula, while Table 2 summarizes the MSn data collected by the QIT procedure and subsequent fragment assignments. As mentioned above, the higher accuracy and sensitivity of the TOF system provided quite a sharp profile from which the main anthocyanins can be easily identified through extracted ion chromatograms (EIC). Moreover, when analysed by MSn, anthocyanins rapidly lose the sugar moiety, and the molecular ion [M]+ occurs together with the somewhat less-intense fragment ions [M-sugar moiety]+ or [M-sugar moiety-organic acid]+. These fragmentation features may provide extra information on the structure of the compound which can be useful to the identification of the anthocyanin. However, on some occasions the aglycone fragment was the only one present in the MSn spectra, and this made the TOF function of accurate mass measurement (less than 5 ppm error in the IT-TOF-MS system) crucial to the qualitative analysis. In addition, the abundances of the isotope peaks of the molecular ions ([M]+) were also monitored to confirm the identities of compounds. Therefore, UPLC-ESI-IT-TOF-MSn experiment which simultaneously performs TOF and MSn function has its unique advantages in the field of natural product characterization, especially anthocyanin identification.

Table 1.
Profile of anthocyanin compounds from P. frutescens var. acuta determined by LC-ESI-IT-TOF-MSn.
| Peak No. | Compound Assigned | Rt (min) a | λmax (nm) | Measured Signal (m/z) | Therotical Mass (m/z) | Error (ppm) | Molecular Weight (m/z) | Molecule Formula |
|---|---|---|---|---|---|---|---|---|
| 1 | Cis-shisonin | 6.27 | 283, 315, 528 | 757.1965 | 757.1974 | 1.19 | 757.1980 | C36H37O18+ |
| 2 | Cyanidin 3-O-caffeoylglucoside- 5-O-glucoside | 6.35 | 283, 318, 525 | 773.1932 | 773.1924 | 1.03 | 773.1929 | C36H37O19+ |
| 3 | Cyanidin 3-O-caffeoylglucoside- 5-O-malonylglucoside | 7.42 | 282, 317, 525 | 859.1926 | 859.1927 | 1.16 | 859.1933 | C39H39O22+ |
| 4 | Malonyl-cis-shisonin | 7.53 | 282, 313, 527 | 843.1965 | 843.1978 | 1.54 | 843.1984 | C39H39O21+ |
| 5 | Shisonin | 7.76 | 284, 315, 525 | 757.1973 | 757.1974 | 0.26 | 757.1980 | C36H37O18+ |
| 6 | Cyanidin 3-O-feruloylglucoside- 5-O-glucoside | 8.18 | 283, 316, 523 | 787.2097 | 787.2080 | 2.16 | 787.2086 | C37H39O19+ |
| 7 | Malonyl-shisonin | 8.91 | 283, 312, 528 | 843.1952 | 843.1978 | 3.08 | 843.1984 | C39H39O21+ |
a Retention time was recorded according to the peaks from extracted ion chromatogram (EIC).

Table 2.
The fragment ions and types of seven anthocyanins originated from the precursor ions [M]+ in positive ion (PI) mode from P. frutescens var. acuta.
| Anthocyanins | MSn Fragment Ions in Positive Ion Mode (m/z) (Fragment Type) a,b | ||
|---|---|---|---|
| MS1 | MS2 | MS3 | |
| Cis-shisonin | 757.1965 [M]+ | 595.1422 [M-Glc]+ 287.0556 [Cy]+ 449.1002 [M-Glc-Cou]+ | 287.0541 [Cy]+ |
| Cyanidin 3-O-caffeoylglucoside- 5-O-glucoside | 773.1932 [M]+ | 611.1394 [M-Glc]+ 287.0549 [Cy]+ 449.1051 [M-Glc-Caf]+ | 287.0543 [Cy]+ |
| Cyanidin 3-O-caffeoylglucoside- 5-O-malonylglucoside | 859.1926 [M]+ | 287.0560 [Cy]+ 535.1085 [M-Glc-Caf]+ 611.1397 [M-Glc-Mal]+ 491.1186 [M-Glc-Caf-CO2]+ 815.2015 [M-CO2]+ 449.1007 [M-Glc-Mal-Caf]+ | 287.0552 [Cy]+ 491.1178 [M-Glc-Caf-CO2]+ 449.1007 [M-Glc-Mal-Caf]+ |
| Malonyl-cis-shisonin | 843.1965 [M]+ | 595.1440 [M-Glc-Mal]+ 535.1077 [M-Glc-Cou]+ 287.0560 [Cy]+ 491.1178 [M-Glc-Cou-CO2]+ 799.2107 [M-CO2]+ | 287.0550 [Cy]+ 491.1175 [M-Glc-Cou-CO2]+ 449.1033 [M-Glc-Mal-Cou]+ |
| Shisonin | 757.1973 [M]+ | 595.1444 [M-Glc]+ 287.0537 [Cy]+ 449.1059 [M-Glc-Cou]+ | 287.0541 [Cy]+ |
| Cyanidin 3-O-feruloylglucoside- 5-O-glucoside | 787.2097 [M]+ | 625.1547 [M-Glc]+ 287.0523 [Cy]+ 449.1104 [M-Glc-Fer]+ | 287.0539 [Cy]+ |
| Malonyl-shisonin | 843.1952 [M]+ | 595.1459 [M-Glc-Mal]+ 535.1095 [M-Glc-Cou]+ 287.0543 [Cy]+ 491.1181 [M-Glc-Cou-CO2]+ 799.2019 [M-CO2]+ | 287.0551 [Cy]+ 491.1148 [M-Glc-Cou-CO2]+ 449.1062 [M-Glc-Mal-Cou]+ |
Abbreviations. Cy, cyanidin; Glc, glucoside; Cou, p-coumaroyl moiety; Caf, caffeoyl moiety; Fer, feruloyl moiety; Mal, malonyl moiety; a Precursor ions in MS2 were underlined; b Fragments were listed in the order of abundance.
2.2.1. Extracted Ion Chromatogram (EIC) Analysis
The LC-DAD chromatogram and extracted ion chromatograms (EIC) of the extract of P. frutescens var. acuta subjected to positive ion ESI-IT-TOF analysis are shown in Figure 2. EICs were obtained at each m/z value corresponding to the theoretical mass of the anthocyanins that were tentatively assigned in order to identify the compound clearly. Cyanidin 3-O-caffeoylglucoside-5-O-glucoside (2) ([M]+, m/z 773.1924), Cyanidin 3-O-caffeoylglucoside-5-O-malonylglucoside (3) ([M]+, m/z 859.1927), Cyanidin 3-O-feruloylglucoside-5-O-glucoside (6) ([M]+, m/z 787.2080) showed only one peak in EICs, while the EIC for m/z 757.1974 showed two peaks, one at Rt 6.27 min and the other at Rt 7.76 min, both presenting the same fragmentation patterns, suggesting the existence of isomers of shisonin. According to a previous report [6], the one at Rt 6.27 min was assigned as cis-shisonin (1) and the other was identified as shisonin (5), which were distinguished by their ion abundance and retention time. The EIC for m/z 843.1978 also showed two peaks, one with relatively weak intensity at Rt 7.53 min suggesting to be a cis- isomer of malonyl-cis-shisonin (4), and the other at Rt 8.91 min exhibiting higher intensity thus confirming its identity as a tran- isomer of malonyl-shisonin (7) [7]. These two pairs of isomers are the main anthocyanin components existed in the P. frutescens which is in good agreement with previous literature [6,7]. Although accurate mass measurements alone could not differentiate between cis- and trans- isomers (which have identical molecular formula), the EIC analysis easily separated the two isomers, which provided another option for reliable data about elution order and abundance of anthocyanins when the LC chromatogram cannot give sufficient information.
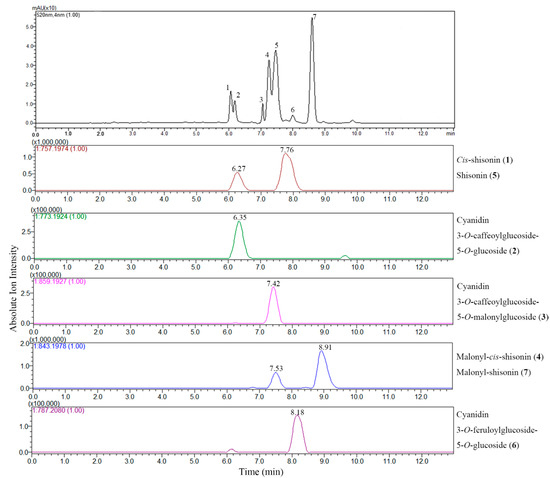
Figure 2.
LC-DAD chromatogram at 520 nm and extracted ion chromatograms (EIC) of LC-ESI-IT-TOF-MS analysis of anthocyanins in P. frutescens var. acuta extract.
Figure 2.
LC-DAD chromatogram at 520 nm and extracted ion chromatograms (EIC) of LC-ESI-IT-TOF-MS analysis of anthocyanins in P. frutescens var. acuta extract.
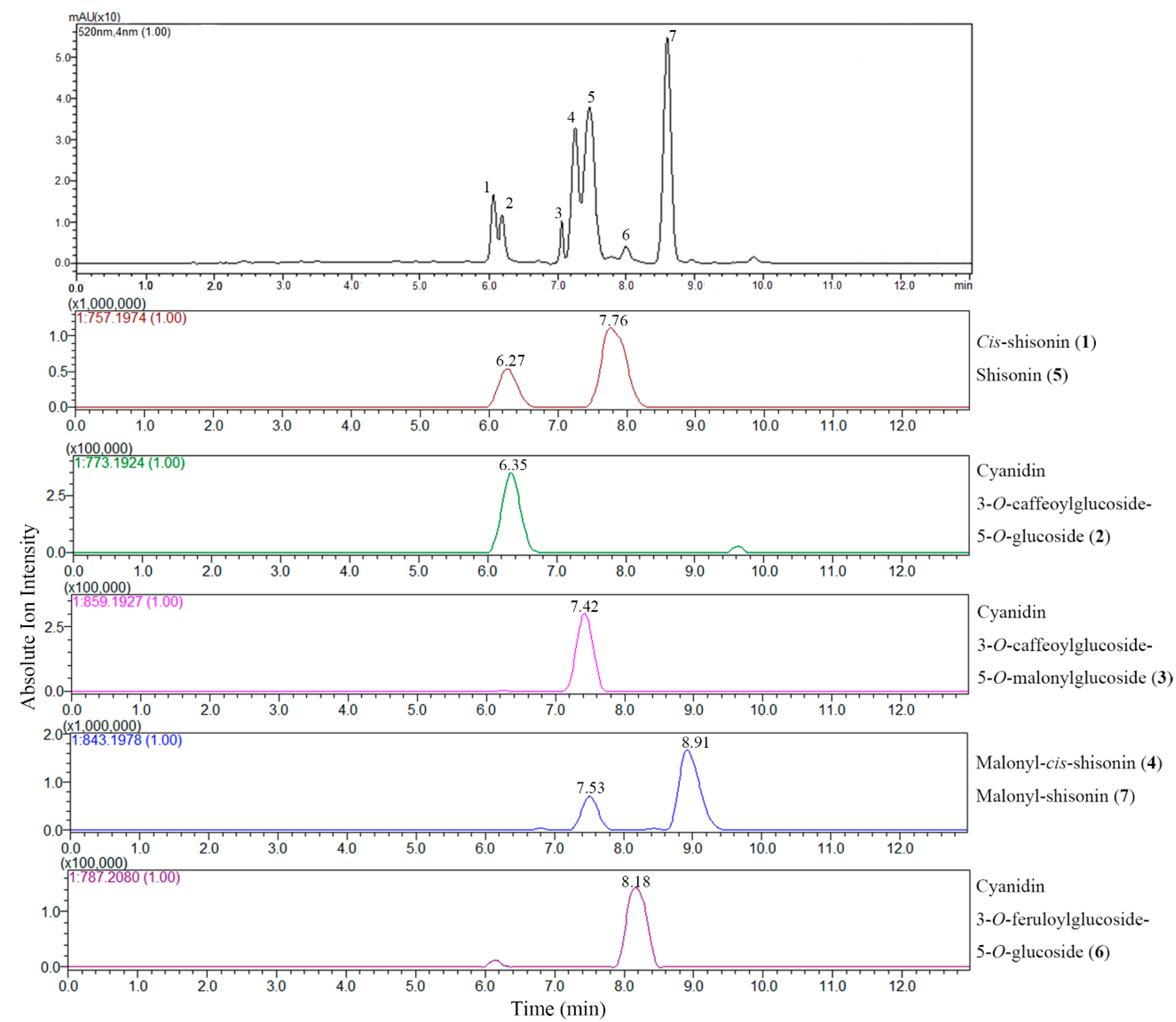
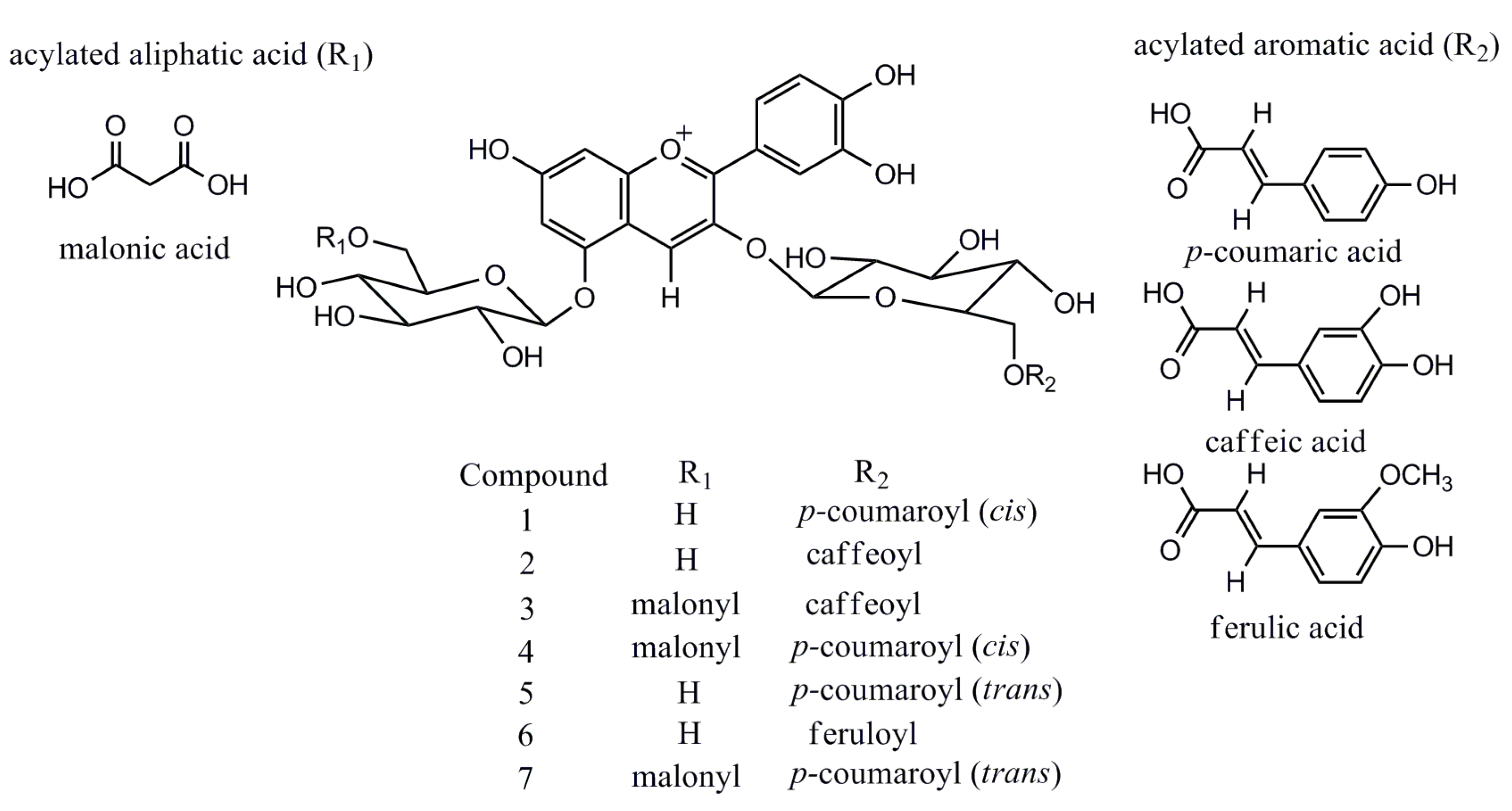
LC-MS in full scan mode has been used extensively for screening of anthocyanin profiles of P. frutescens, even though compound identification is often tentatively based on elution order, molecular ion information and fragmentation features only [6,7]. However, the EIC analysis selectively improves the sensitivity of targeted analytes, excluding the interference from other phenolic compounds in TIC, which, in this regard, is highly effective in the characterization of interested bioactive compounds in complex matrices of plant extracts. All the anthocyanins characterized in P. frutescens var. acuta extract are reported in Figure 3. Further literature searches confirm the identity of the six compounds except for a new found feruloyl substituted anthocyanin (6), which only showed a trace amount presence [6]. Although these six anthocyanins were previously identified by [6,7,8], we have documented for the first time the existence in P. frutescens var. acuta. On the other hand, sugar residues of anthocyanins can be easily acylated with organic acids, and common acylating agents include cinnamic acids such as caffeic, p-coumaric, ferulic and sinapic acid [13]. Among Perilla anthocyanins, to the best of our knowledge, coumaric and caffeic acid are common substitution moieties [6,7]. However, this is the first time that the existence of ferulic acid moiety has been identified in P. frutescens.
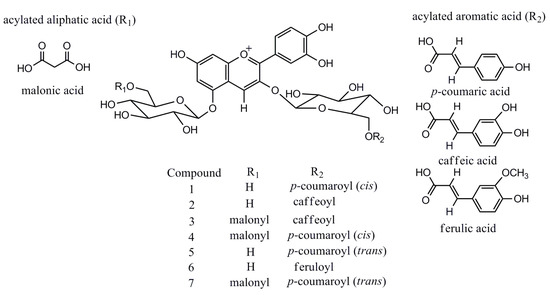
Figure 3.
Structures of major anthocyanins found in P. frutescens var. acuta.
Figure 3.
Structures of major anthocyanins found in P. frutescens var. acuta.
2.2.2. Fragmentation Patterns of Perilla Anthocyanins
MSn function was known for its capability of successive fragmentation steps which can provide more structural information on the compound. Since few reference standards for anthocyanins are available (in particular, no standards are available for derived anthocyanins in plant extracts), those anthocyanins which were tentatively assigned through accurate mass measurement can only be further identified by analyzing their fragmentation behaviors in MS2 and MS3 spectra and by referring to previous reports.
The ESI-MSn spectrum of a typical anthocyanin (malonyl-shisonin) (7) is shown in Figure 4. The TOF-MS1 scan data showed that the molecular ion was m/z 843.1952 ([M]+) which was calculated as a molecular formula of C39H39O21+. The measured mass value obtained for this molecular ion was very close to the theoretical value (m/z 843.1978) of C39H39O21+ with an error of −3.08 ppm. In comparison with the theoretical values, the obtained ratios and abundance of the isotope ions for [M]+ was also analyzed to further confirm the validity of the molecular ion, which, as a result, exactly matches with the theoretical values with an Iso score of 85.72 (100). Moreover, The MS2 fragmentation of the molecular ion ([M]+, m/z 843.1952) produced several fragment ions: the flavylium cation of cyanidin ([Cy]+, m/z 287.0547) was confirmed, based on the mass accuracy −1.05 ppm error, as compared with the theoretical mass (m/z 287.0550, C15H11O6+). Signals for neutral loss of a malonylglucoside moiety, [M-Glc-Mal]+ (m/z 595.1419) and loss of a coumaroylglucoside moiety, [M-Glc-Cou]+ (m/z 535.1077) were also observed as the main fragment ions, corresponding to cyanidin 3-O-coumaroylglucoside and cyanidin 5-O-malonylglucoside, respectively.
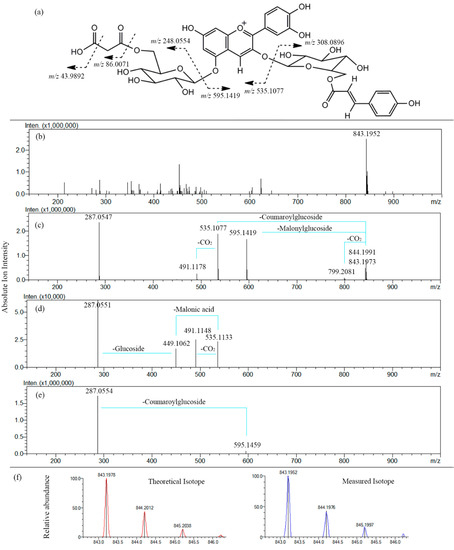
Figure 4.
ESI-MS3 analysis of malonyl-shisonin in P. frutescens var. acuta extract. Positive ion mode. Collision Energy 25%. (a) Fragmentation features; (b) MS1 scan spectrum; (c) MS2 spectrum with fragments assigned; (d) MS3 spectrum (precursor ion m/z 595.1419); (e) MS3 spectrum (precursor ion m/z 535.1077); (f) Patterns of isotopes of the molecular ion m/z 843.1952.
Figure 4.
ESI-MS3 analysis of malonyl-shisonin in P. frutescens var. acuta extract. Positive ion mode. Collision Energy 25%. (a) Fragmentation features; (b) MS1 scan spectrum; (c) MS2 spectrum with fragments assigned; (d) MS3 spectrum (precursor ion m/z 595.1419); (e) MS3 spectrum (precursor ion m/z 535.1077); (f) Patterns of isotopes of the molecular ion m/z 843.1952.
In addition to molecular ion and fragment ion assignment, high mass accuracy was also used in analyzing the neutral losses of fragment ions. For example, In MS2 spectra, the loss of CO2 (43.9892 Da) can be differentiated from the loss of C2H4O (44.0262 Da) by determining the accuracy between the observed signal (43.9892 Da) and the theoretical monoisotopic mass.
The two most abundant fragment ions (m/z 595.1419, m/z 535.1077) in the MS2 spectra were then selected as precursor ions to trigger CID for further exploration of the correlation between fragmentation patterns and structural features in MS3 spectra. However, not many new fragment ions were found in MS3. Signals at m/z 449.1062 are believed to be [M-Glc-Mal-Cou]+, corresponding to cyanidin 5-O-glucoside which was due to the loss of the entire malonyl moiety.
The observed fragmentation patterns confirmed the known structure of malonyl-shisonin and are consistent with that reported by [6,7,14]. The published data of MSn refers to a fragmentation mechanism that cleavage occurs at C-O bond between aglycone and glucoside in positive ion mode. In MS2 mode, signals corresponding to the product ions were compatible with the combination of the independent losses of the glucose and acylated glucose moieties, in agreement with previously reported data obtained from various P. frutescens varieties [6,7].The acylated aromatic acid (p-coumaric acid, caffeic acid and ferulic acid) was stable and no fragment signals were discovered, while the acylated aliphatic acid (malonic acid) easily yielded neutral fragment losses (CO2) or entire cleavage from the glucoside. Similar fragmentation patterns were observed for other anthocyanins detected in P. frutescens var. acuta.
2.3. Cell Morphology Changes during Apoptosis
It has been reported that the induction of apoptosis is one of the major mechanisms of anthocyanin-mediated cell death [15,16,17]. Since observation of cell morphology is the most direct way to know whether the cells are undergoing apoptosis, a traditional fluorescence staining method was used. Our results showed that with the increase of anthocyanin concentrations, apoptosis obviously increased in a dose dependent manner and the classical apoptotic cells were observed in 300 μg/mL anthocyanin-treated cells. Results of multiple concentrations of anthocyanin-treated cells are given in the Supplementary Material. Characteristic morphological changes of apoptosis from cell shrinkage and membrane blabbing to the formation of apoptotic bodies are shown in Figure 5. These results suggest that Perilla anthocyanins (300 μg/mL) can induce apoptosis in Hela cells after 24 h treatment.
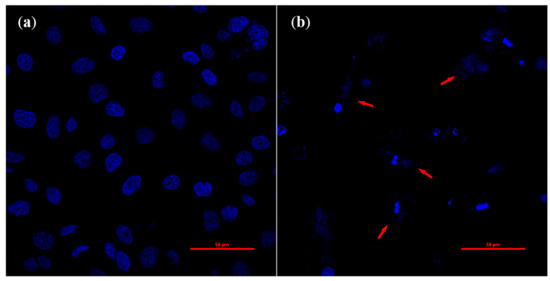
Figure 5.
Effects of Perilla anthocyanins on cell apoptosis induction (600×) after DAPI staining (a) Control (b) 300 μg/mL.
Figure 5.
Effects of Perilla anthocyanins on cell apoptosis induction (600×) after DAPI staining (a) Control (b) 300 μg/mL.
2.4. Cell Apoptosis Detected by Annexin V-FITC/PI Assay
In apoptotic cells, the membrane phospholipid phosphatidyserine (PS) is translocated from inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment. Annexin V is a phospholipid-binding protein that has a high affinity for PS; thus, it can serve as a sensitive probe for cytometric analysis of cells that are undergoing apoptosis. Propidium iodide (PI) was used with FITC Annexin V to allow the investigation of early apoptotic cells (PI negative, FITC Annexin V positive).
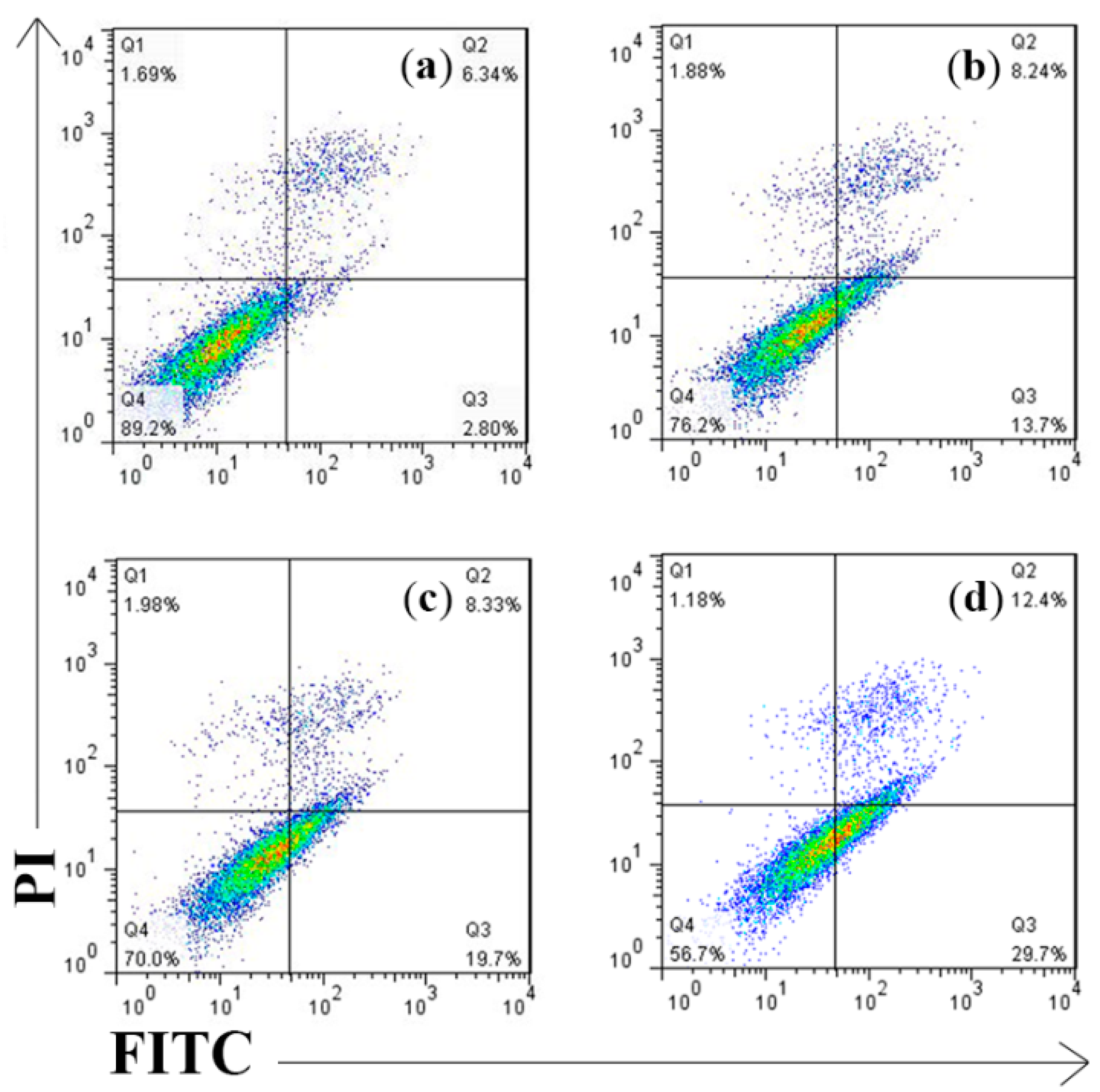
Flow cytometric analysis confirmed that the number of AV and AV/PI positive cells significantly increased in 300 μg/mL anthocyanin-treated groups at 12 h treatment. As been shown in Figure 6, the Q1(%) was 1.18% and the Q3(%) was 29.7%; thus, the respective apoptosis rates [Q2(%) + Q4(%)] were 42.1% indicating its high efficiency against Hela cells. Also, 300 μg/mL anthocyanin had a higher apoptotic ratio compared to 200 μg/mL (21.9%) and 250 μg/mL (28.0%) groups showing certain dose dependency.
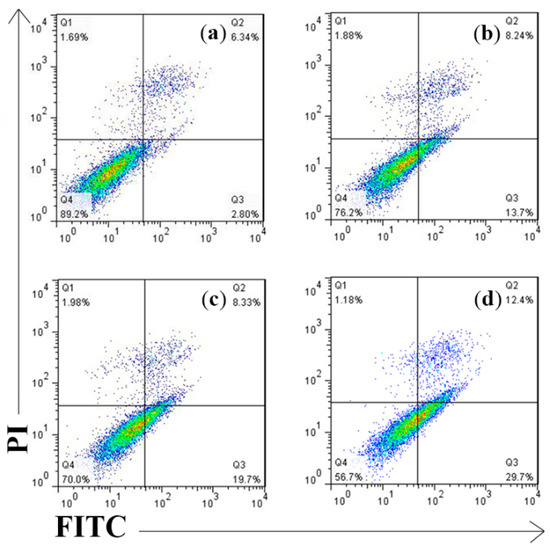
Figure 6.
Measurement of Perilla anthocyanins on apoptotic rates by Annexin V FITC/PI analysis (a) Control (b) 200 μg/mL (c) 250 μg/mL (d) 300 μg/mL.
Figure 6.
Measurement of Perilla anthocyanins on apoptotic rates by Annexin V FITC/PI analysis (a) Control (b) 200 μg/mL (c) 250 μg/mL (d) 300 μg/mL.
3. Experimental Section
3.1. Plant Material
Perilla (Perilla frutescens var. acuta) leaves with red-green fresh leaf color were collected during September from wild in Longquan area (Zhejiang Province, China) (119°6′ east, 28°01′ north). Longquan area enjoys a comfortable subtropical monsoon climate featuring precipitation, full sunshine and four distinct seasons. Approximately 20 bunches were collected. Roots and rhizomes were removed subsequently. The fresh leaves once harvested were immediately packed with plastic sample containers in an ice box and delivered to our laboratory by overnight courier to prevent deterioration.
3.2. Reagents
All HPLC and LC-MS grade chemicals, including methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). Ethanol, hydrochloric acid (HCl), acetic acid, formic acid and poly-sery HC-C18 SPE cartridges were purchased from ANPEL (shanghai, China). Hyclone fetal bovine serums (FBS), Dulbecco’s Modified Eagle Medium (DMEM), penicillin/streptomycin solution, dimethylsulfoxide (DMSO) were purchased from Thermo Fisher (Waltham, MA, USA). Trypsin-EDTA was obtained from Gibco (Invitrogen, Carlsbad, CA, USA). DAPI (4′,6-diamidine-2′-phenylindole) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Amberlite XAD-7 was obtained from ANLAND (Shanghai, China). Annexin V-FITC/PI Apoptosis Detection Kit was purchased from BD (San Jose, CA, USA).
3.3. Cell Lines
The human cervix Adenocarcinoma Hela cell line was provided by the Department of applied biology, College of bioengineering, East China University of Science and Technology, Shanghai, China. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin antibiotics solution at 37 °C in a humidified atmosphere of 5% CO2.
3.4. Extraction and Isolation
Anthocyanins were extracted according to the method described by Zou et al. [18], with some modifications. Frozen dried Perilla leaves were ground to powder by a mortar and pestle. Then, powdery material (20 g) was extracted twice with 1000 mL 45% ethanol (v/v) in water (6% acetic acid). The extraction temperature was fixed at 45 °C and the extraction time was 60 min. Magnetic stirring was used during the extraction to improve solid-liquid exchange. The supernatant was concentrated in a rotary evaporator at 40 °C and then subjected to Amberlite XAD-7 column chromatography (2.5 × 50 cm). The column was washed with 400 mL of water, and elution of the anthocyanins was carried out with 400 mL of 95% ethanol. The eluate was concentrated under reduced pressure at 40 °C.
The concentrated anthocyanin extract was further refined by solid phase extraction (SPE) in C18 cartridges. The aqueous extract of anthocyanin was passed through a sorbent C18 poly-sery cartridge previously activated with methanol and equilibrated with water. The extract adsorbed onto the cartridge was rinsed with ultra-pure water to remove water-soluble impurities, and then eluted with acidified methanol (1% formic acid, v/v) [19]. Methanol was removed under vacuum at 35 °C and the residue was freeze-dried. Approximately 12 mg purified extract was obtained from 20 g of powdery Perilla leaves.
3.5. Total Anthocyanin Content (TAC)
As an important index of anthocyanins, monomeric anthocyanin pigment content was determined by the pH differential method described by [20]. The pigment concentrations were calculated by the following formula:
Absorbance was calculated as A = (A520 − A700)pH 1.0 − (A520 − A700)pH 4.5 with a molar absorptivity (ε) of 26,900 (L·cm−1·mol−1) and molecular weight of 449.2 (g/mol). Anthocyanin content was mg/L which was then converted to mg/100 g dried sample. The data were expressed as the mean values ± SD (n = 3).
3.6. UPLC-ESI-IT-TOF-MSn Analysis
Freeze dried samples were redissolved in a mixture of water/methanol/formic acid (80:20:1, v/v/v) and were analyzed by UPLC-ESI-IT-TOF-MSn. The final concentration of the sample was 0.1 mg/mL.
UPLC-ESI-IT-TOF-MSn was recorded on an UFLCXR system incorporating two LC-20ADXR pump, a SIL-20ACXR autosampler, a CTO-20AC column oven, a DGU-20A5 vacuum degasser, a SPD-M20A Uv-Vis detector and an LCMS-IT-TOF Mass Spectrometer (Shimadzu, Kyoto, Japan). Reversed phase separation was performed using a Shim-pack XR-ODS II column (2.0 × 100 mm, 2 μm) (Shimadzu, Kyoto, Japan) with a flow rate of 0.4 mL/min thermostated at 35 °C. Mobile phase A was 1% formic acid in water and mobile phase B was methanol. The gradient profile was as follows: solvent B increased from 20%–30% in the first 3 min, and then continually increased to 50% at 20 min, and finally reconditioned with 20% B for 5 min (total run time 25 min). The injection volume was 6 μL.
The MS parameters were as follows: positive ion mode was utilized by the ESI source. The full scan mass spectra covered the range from m/z 150–1000 Da (MS1). As for MS2 and MS3 the scan range was modified according to the precursor ions. Heat block and curved desolvation line temperatures were both 200 °C. Nebulizing nitrogen gas flow was 1.5 L/min, whilst the desolvation nitrogen gas flow was 10 L/min. The interface voltage was 4.5 kV for PI. The detector voltage was 1.60 kV. The relative collision induced dissociation energy was adjusted between 20%–30%. All data were recorded and processed by Shimadzu software LCMS solution version 3.70, Formula Predictor version 1.2 and Accurate Mass Calculator (Shimadzu, Kyoto, Japan).
For quantification, the peak areas of the isolated compounds were integrated from the HPLC chromatogram at 520 nm using LCMSsolution software. Malonyl-shisonin (7) was the most predominant anthocyanin (19.0 mg/100 g) followed by shisonin (5) (13.9 mg/g), malonyl-cis-shisonin (4) (9.3 mg/g), cis-shisonin (1) (4.4 mg/g), Cyanidin 3-O-caffeoylglucoside-5-O-glucoside (2) (3.0 mg/g), Cyanidin 3-O-caffeoylglucoside-5-O-malonylglucoside (3) (1.6 mg/g), Cyanidin 3-O-feruloylglucoside-5-O-glucoside (6) (1.3 mg/g). Each anthocyanin was quantified in cyanidin-3-O-glucoside equivalents (mg/100 g dry weight).
3.7. DAPI Fluorescence Staining
Purified anthocyanins were redissolved in sterilized water with a stock concentration of 1 mg/mL. Cells treated with different concentrations (0, 100, 150, 200, 250, 300 μg/mL) of anthocyanins were placed in a 5% CO2 incubator for 24 h at 37 °C. After the incubation procedure, the cells were fixed with 4% formaldehyde for 30 min and then washed three times. The stock solution of DAPI was diluted to a concentration of 10 μM and added to the cells. After 10 min staining at room temperature, unincorporated DAPI solution was removed. Cells were mounted with anti-fluorescence quenching liquid seal pieces, and then observed under a fluorescence microscope.
3.8. Annexin-V FITC/PI Flow Cytometric Analysis
The detection was performed utilizing the Annexin-V FITC/PI apoptosis detection kit. Briefly, cells (2 × 106 cells/mL) treated with various concentrations (0, 200, 250, 300 μg/mL) of anthocyanin and cultured for 12 h were harvested, washed twice with cold PBS and resuspended with 100 μL binding buffer. Afterwards, 5 μL PI and Annexin V-FITC were added. The cells was incubated at 4 °C for 15 min in darkness and then analyzed for apoptosis on a BD FACSAria flow cytometer. Data were further evaluated by Cell Quest and FlowJo.
4. Conclusions
In our study, the characterization of anthocyanins in a rare Perilla variety cultivated in China was carried out by UPLC combined with DAD detector, TOF-MS and MSn analysis. The inherent advantages of high mass accuracy measurement and structural identification power utilizing the LCMS-IT-TOF instrument have showed us a new approach for the detailed investigation of anthocyanins in plant extracts. The developed approach involves a two-step analysis: in the first step, the EIC analysis combined with RP-LC chromatographic elution order allows tentative compound identification. Then, the structural elucidation by the second step MSn analysis ensures unambiguous identification of detected compounds. This methodology therefore offers a powerful and simple approach for the selective determination of anthocyanin derivatives. Seven anthocyanins were differentiated from P. frutescens var. acuta, most of which are consistent with the other varieties of P. frutescens. To our knowledge, the feruloylglycosylated anthocyanin was first reported in P. frutescens. Furthermore, this paper presents the first anticancer activity research on the anthocyanins in P. frutescens. The results showed that Perilla anthocyanins could induce Hela cell apoptosis. However, further studies are required to clarify the mechanism of action and the bioavailability of anthocyanins in Hela cells.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/05/9155/s1.
Acknowledgments
The authors gratefully acknowledge the SHIMADZU Center of Analytical and Measurement (Shanghai) for providing the LCMS-IT-TOF instrument.
Author Contributions
Ya-Ning Chang and Yan-Kang He conceived and designed the experiments. Yan-Kang He and You-Yuan Yao performed the research and analyzed the data. Yan-Kang He and Ya-Ning Chang wrote the paper. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol extraction from eight Perilla frutescens cultivars. C. R. Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Nitta, M.; Lee, J.; Ohnishi, O. Asianperilla crops and their weedy forms: Their cultivation, utilization and genetic relationships. Econ. Bot. 2003, 57, 245–253. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.C.; Choi, U.K. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against staphylococcus aureus using evolutionary operation factorial design technique. Int. J. Mol. Sci. 2011, 12, 2395–2407. [Google Scholar]
- Jun, H.I.; Kim, B.T.; Song, G.S.; Kim, Y.S. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014, 148, 367–372. [Google Scholar] [CrossRef]
- Oh, H.A.; Park, C.S.; Ahn, H.J.; Park, Y.S.; Kim, H.M. Effect of Perilla frutescens var. acuta kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011, 236, 99–106. [Google Scholar]
- Yoshida, K.; Kondo, T.; Kameda, K.; Goto, T. Structure of anthocyanins isolated from purple leaves of Perilla ocimoides L. var. crispa benth and their isomerization by irradiation of light. Agric. Biol. Chem. 1990, 54, 1745–1751. [Google Scholar]
- Yamazaki, M.; Nakajima, J.; Yamanashi, M.; Sugiyama, M.; Makita, Y.; Springob, K.; Awazuhara, M.; Saito, K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 2003, 62, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2009, 14, 133–140. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Huang, H.P.; Hsu, J.D.; Yang, S.F.; Wang, C.J. Hibiscus anthocyanins rich extract-induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol. Appl. Pharmacol. 2005, 205, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Campos, D.; Betalleluz, I.; Giusti, M.M.; Schwartz, S.J.; Tian, Q.; Pedreschi, R.; Larondelle, Y. High-performance liquid chromatography with photodiode array detection (HPLC-DAD)/HPLC-Mass spectrometry (MS) profiling of anthocyanins from andean mashua tubers (tropaeolum tuberosum ruiz and pavon) and their contribution to the overall antioxidant activity. J. Agric. Food Chem. 2006, 54, 7089–7097. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, J.S.; Choi, D.S.; Jung, M.Y. Characterization and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS. J. Agric. Food Chem. 2013, 61, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Bridle, P.; Timberlake, C. Anthocyanins as natural food colours—Selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- Yamazaki, M.; Makita, Y.; Springob, K.; Saito, K. Regulatory mechanisms for anthocyanin biosynthesis in chemotypes of Perilla frutescens var. crispa. Biochem. Eng. J. 2003, 14, 191–197. [Google Scholar] [CrossRef]
- Bunea, A.; Rugina, D.; Sconta, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tabaran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in b16-f10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Li, S.; Jia, Y.; Wang, L. Blueberry anthocyanins induce G2/M cell cycle arrest and apoptosis of oral cancer kb cells through down-regulation methylation of p53. Zhongguo yi Chuan Xue Hui Bian Ji 2014, 36, 566–573. [Google Scholar]
- Sehitoglu, M.H.; Farooqi, A.A.; Qureshi, M.Z.; Butt, G.; Aras, A. Anthocyanins: Targeting of signaling networks in cancer cells. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 2379–2381. [Google Scholar] [CrossRef]
- Zou, T.; Wang, D.; Guo, H.; Zhu, Y.; Luo, X.; Liu, F.; Ling, W. Optimization of microwave-assisted extraction of anthocyanins from mulberry and identification of anthocyanins in extract using HPLC-ESI-MS. J. Food Sci. 2012, 77, C46–C50. [Google Scholar] [CrossRef] [PubMed]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Characterization of anthocyanins in kenyan teas: Extraction and identification. Food Chem. 2012, 131, 31–38. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the anthocyanin extract of P. frutescens var. acuta and the Hela cell line are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
