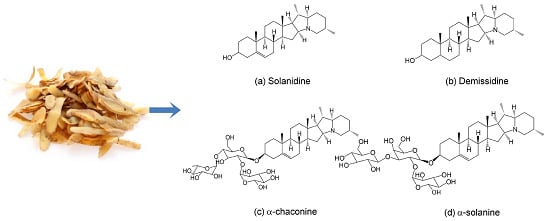
Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction
Abstract
:1. Introduction

2. Results and Discussion
2.1. Extraction of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid
| Extracts Obtained by 1 g Dried Potato Peel (DPP) in 25 mL for 24 h | α-Solanine | α-Chaconine | Solanidine | Demissidine | Total | Extraction Yield (mg/g DPP) | Total Glycoalkaloid (µg/g DPP) |
|---|---|---|---|---|---|---|---|
| (µg/mg Dried Extract) | |||||||
| Tetrahydrofuran | 0.26 ± 0.01 | 2.55 ± 0.18 | 0.97 ± 0.10 | 0.14 ± 0.01 | 3.91 ± 0.23 | 47.20 ± 0.75 | 184.55 ± 3.41 |
| Methanol | 4.29 ± 0.21 | 3.28 ± 0.26 | 2.40 ± 0.26 | 0.15 ± 0.02 | 10.12 ± 0.45 | 75.20 ± 2.10 | 761.02 ± 5.87 |
| Ethylacetate | 0.14 ± 0.01 | 0.03 ± 0.001 | 1.40 ± 0.21 | 0.14 ± 0.03 | 1.70 ± 0.14 | 38.40 ± 1.95 | 65.28 ± 1.62 |
| Chloroform | 0.14 ± 0.01 | 0.03 ± 0.002 | 1.86 ± 0.16 | 0.15 ± 0.02 | 2.18 ± 0.26 | 23.20 ± 1.65 | 50.58 ± 2.41 |
| Acetonitrile | 0.15 ± 0.02 | 0.04 ± 0.001 | 1.17 ± 0.09 | 0.14 ± 0.01 | 1.47 ± 0.19 | 34.40 ± 2.71 | 50.57 ± 2.46 |
| Acetone | 0.17 ± 0.02 | 2.30 ± 0.21 | 1.72 ± 0.07 | 0.15 ± 0.03 | 4.34 ± 0.35 | 31.20 ± 3.10 | 135.41 ± 3.55 |
| Iso-propanol | 1.01 ± 0.15 | 1.86 ± 0.19 | 0.94 ± 0.10 | 0.13 ± 0.01 | 3.94 ± 0.30 | 49.60 ± 2.95 | 195.42 ± 4.10 |
| Ethanol | 4.06 ± 0.31 | 3.90 ± 0.11 | 1.49 ± 0.22 | 0.14 ± 0.02 | 9.59 ± 0.42 | 41.60 ± 3.87 | 398.94 ± 3.86 |
| Dichloromethane | 0.86 ± 0.1 | 0.31 ± 0.02 | 0.68 ± 0.01 | 0.13 ± 0.01 | 1.97 ± 0.11 | 40.80 ± 2.52 | 80.38 ± 2.71 |
| Water | 0.69 ± 0.05 | 0.03 ± 0.001 | 0.54 ± 0.02 | 0.12 ± 0.01 | 1.38 ± 0.20 | 101.60 ± 5.20 | 140.21 ± 1.96 |
| 5% Acetic acid | 2.98 ± 0.22 | 1.54 ± 0.15 | 0.69 ± 0.01 | 0.12 ± 0.01 | 5.33 ± 0.31 | 138.40 ± 5.92 | 737.67 ± 6.84 |
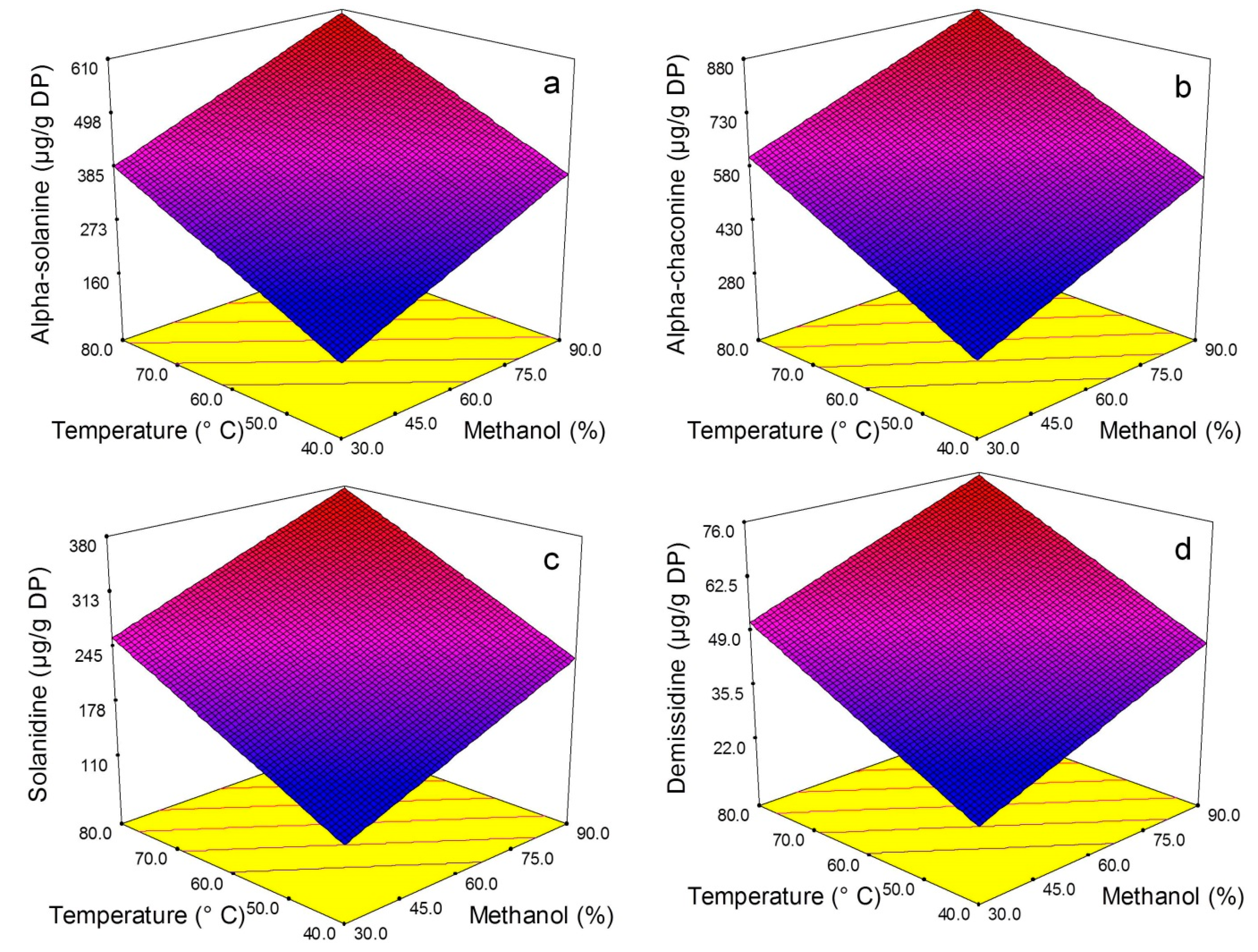
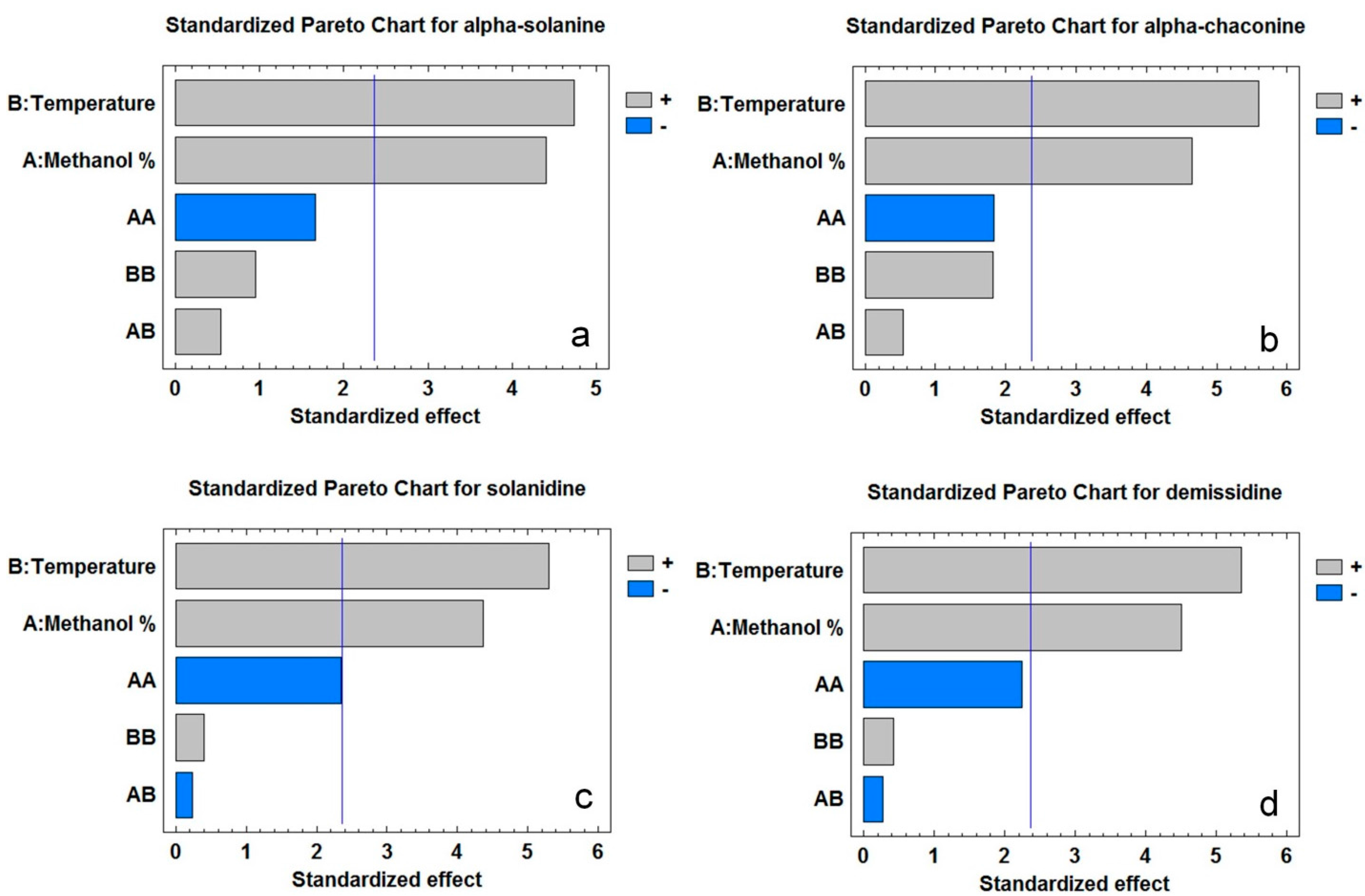
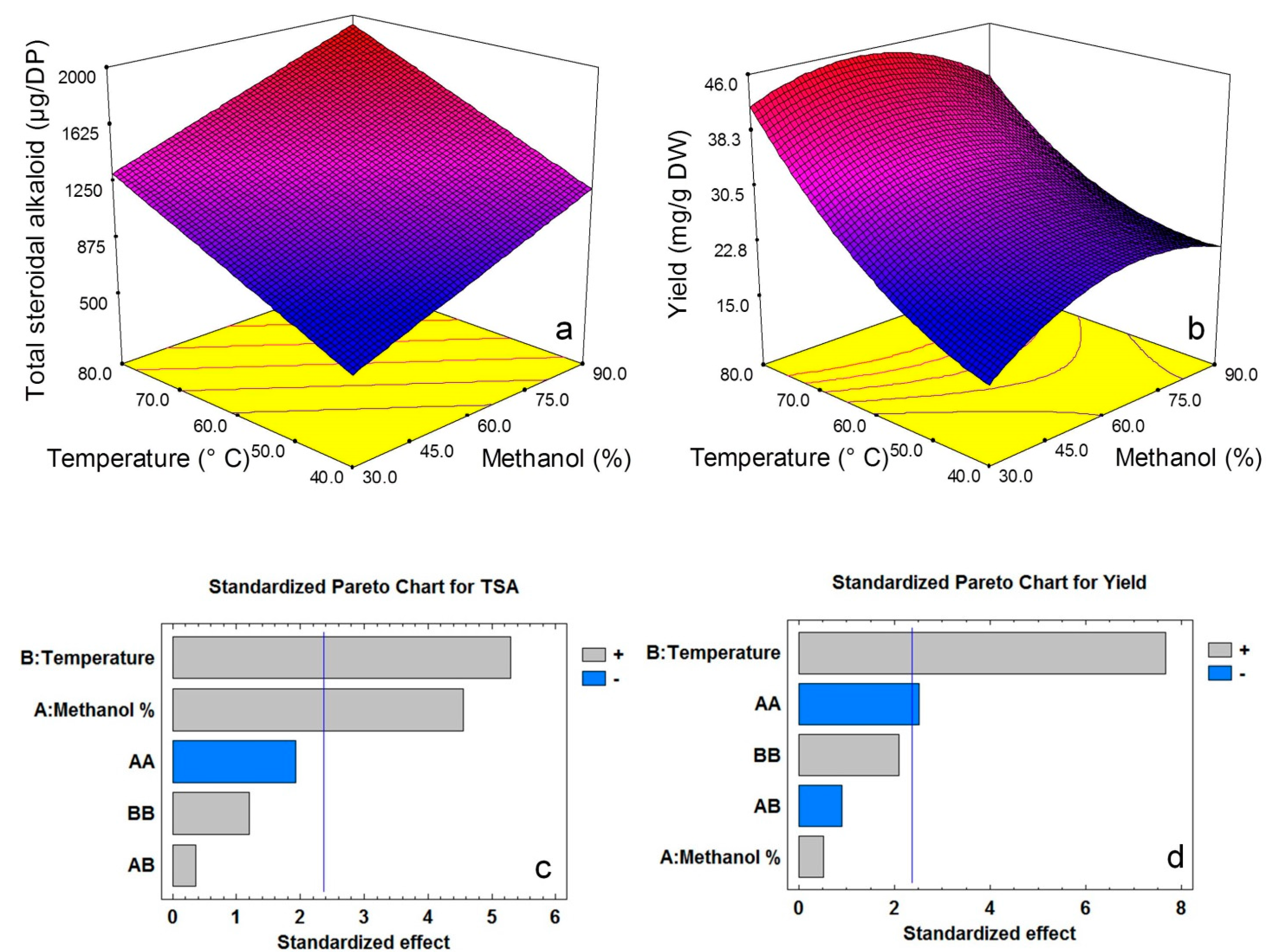
2.2. Model Fitting
| Parameters | R2 | CV | p | Lack of Fit | F-value | Second Order Polynomial Equation |
|---|---|---|---|---|---|---|
| α-solanine | 0.784 | 13.55 | 0.0005 | 0.613 | 18.55 | Y = −172.904 + 3.53787 × X1 + 5.70597 × X2 |
| α-chaconine | 0.780 | 12.05 | 0.0005 | 0.322 | 17.70 | Y = −166.793 + 4.45751 × X1 + 8.06025 × X2 |
| Solanidine | 0.783 | 12.74 | 0.0005 | 0.536 | 18.02 | Y = −91.2571 + 1.98821 × X1 + 3.61989 × X2 |
| Demissidine | 0.795 | 12.34 | 0.0004 | 0.503 | 19.42 | Y = −18.501 + 0.403972 × X1 + 0.720972 × X2 |
| Total alkaloids | 0.790 | 12.36 | 0.0004 | 0.478 | 18.79 | Y = −449.032 + 10.3792 × X1 + 18.1071 × X2 |
| Yield | 0.846 | 10.66 | 0.0005 | 0.177 | 16.46 | Y = −24.3625 + 0.794455 × X1 + 0.525292 × X2 − 0.0064205 × X12 |
2.3. Optimisation and Model Validation
| Parameter | Predicted Values (79.6 °C, 89.3% MeOH) | Experimental Values (80 °C, 89% MeOH) | (E%) | Solid/Liquid Extraction Values |
|---|---|---|---|---|
| α-Solanine (µg/g DW) | 579.9 | 597.4 ± 6.48 | 3.01 | 247.3 ± 7.54 |
| α-Chaconine (µg/g DW) | 851.0 | 873.0 ± 15.26 | 2.58 | 474.0 ± 11.23 |
| Solanidine (µg/g DW) | 365.0 | 374.0 ± 9.87 | 2.46 | 224.2 ± 8.57 |
| Demissidine (µg/g DW) | 73.0 | 75.0 ± 2.56 | 2.73 | 36.0 ± 5.46 |
| Total steroidal alkaloid (mg/g DW) | 1.87 | 1.95 ± 0.03 | 4.27 | 0.98 ± 0.02 |
| Yield (mg/g DW) | 39.2 | 37.8 ± 0.56 | 3.57 | 30.12 ± 0.78 |
3. Experimental Section
3.1. Samples and Reagents
3.2. Drying of the Peel Slurry
3.3. Pressurized Liquid Extraction (PLE)
3.4. Conventional Solid/Liquid Extraction
3.5. Identification and Quantification of Steroidal Alkaloids in Potato Peel by Ultra-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry
3.6. Statistical Analysis
3.7. Model Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schieber, A.; Saldana, M.A. Potato peels: A source of nutritionally and pharmacologically interesting compounds—A review. Food 2009, 3, 23–29. [Google Scholar]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food. Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Lojkowska, E.; Ehlenfeldt, K.; Kelman, A.; Helgeson, J.P. Fertile interspecific somatic hybrids of Solanum: A novel source of resistance to Erwinia soft rot. Phytopathology 1988, 78, 1216–1220. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G.; Weissenberg, M. Interactions between the glycoalkaloids solasonine and solamargine in relation to inhibition of fungal growth. Phytochemistry 1994, 37, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Sanford, L.; Kobayashi, R.; Deahl, K.; Sinden, S. Diploid and tetraploid Solanum chacoense genotypes that synthesize leptine glycoalkaloids and deter feeding by colorado potato beetle. Am. Potato J. 1997, 74, 15–21. [Google Scholar] [CrossRef]
- Morris, S.H.; Lee, T.H. The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): A review. Food Technol. Aust. 1984, 36, 118–124. [Google Scholar]
- Slanina, P. Solanine (glycoalkaloids) in potatoes: Toxicological evaluation. Food Chem. Toxicol. 1990, 28, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Kobayashi, H.; Dong, A.; Jing, Y.; Iwasaki, S.; Yao, X. Antineoplastic agents iii: Steroidal glycosides from Solanum nigrum. Planta Med. 1999, 65, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated jurkat and raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C. Polyphenol antioxidants from potato peels: Extraction optimization and application to stabilizing lipid oxidation in foods. In Polyphenol Antioxidants From Potato Peels: Extraction Optimization and Application to Stabilizing Lipid Oxidation in Foods, Proceedings of the National Conference on Undergraduate Research (NCUR), Ithaca College, NY, USA; 2011; pp. 1–8. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food. Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006, 72, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Denery, J.R.; Dragull, K.; Tang, C.; Li, Q.X. Pressurized fluid extraction of carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum. Anal. Chim. Acta 2004, 501, 175–181. [Google Scholar] [CrossRef]
- Herrero, M.; Arráez-Román, D.; Segura, A.; Kenndler, E.; Gius, B.; Raggi, M.A.; Ibáñez, E.; Cifuentes, A. Pressurized liquid extraction-capillary electrophoresis-mass spectrometry for the analysis of polar antioxidants in rosemary extracts. J. Chromatogr. A 2005, 1084, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yamashita, N.; Tanaka, H.; Matsukawa, H.; Tanabe, K. Development of extraction method of pharmaceuticals and their occurrences found in Japanese waste water treatment plants. Environ. Int. 2009, 35, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, W.; Thirakhupt, K.; Sitticharoenchai, D.; Rohitrattana, J.; Thongkongowm, P.; Borjan, M.; Robson, M. DDT and derivatives in indicator species of the aquatic food web of Rangsit agricultural area, Central Thailand. Ecol. Indic. 2009, 9, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L. Optimization of extraction of phenolic acids from a vegetable waste product using a pressurized liquid extractor. J. Funct. Foods 2012, 4, 842–850. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D. Subcritical water extraction of phenolic compounds from potato peel. J. Funct. Foods 2011, 44, 2452–2458. [Google Scholar]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The optimisation of extraction of antioxidants from potato peel by pressurised liquids. Food Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Rodríguez-Meizoso, I.; Cifuentes, A.; Jaime, L.; García-Blairsy Reina, G.; Señorans, F.; Ibáñez, E. Green processes based on the extraction with pressurized fluids to obtain potent antimicrobials from Haematococcus pluvialis microalgae. LWT-Food Sci. Technol. 2009, 42, 1213–1218. [Google Scholar] [CrossRef]
- Zaibunnisa, A.; Norashikin, S.; Mamot, S.; Osman, H. An experimental design approach for the extraction of volatile compounds from turmeric leaves (Curcuma domestica) using pressurised liquid extraction (PLE). LWT-Food Sci. Technol. 2009, 42, 233–238. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food. Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Samples Availability: Samples of the compounds are not available available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.B.; Rawson, A.; Aguiló-Aguayo, I.; Brunton, N.P.; Rai, D.K. Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction. Molecules 2015, 20, 8560-8573. https://doi.org/10.3390/molecules20058560
Hossain MB, Rawson A, Aguiló-Aguayo I, Brunton NP, Rai DK. Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction. Molecules. 2015; 20(5):8560-8573. https://doi.org/10.3390/molecules20058560
Chicago/Turabian StyleHossain, Mohammad B., Ashish Rawson, Ingrid Aguiló-Aguayo, Nigel P. Brunton, and Dilip K. Rai. 2015. "Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction" Molecules 20, no. 5: 8560-8573. https://doi.org/10.3390/molecules20058560
APA StyleHossain, M. B., Rawson, A., Aguiló-Aguayo, I., Brunton, N. P., & Rai, D. K. (2015). Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction. Molecules, 20(5), 8560-8573. https://doi.org/10.3390/molecules20058560