Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources
Abstract
:1. Introduction

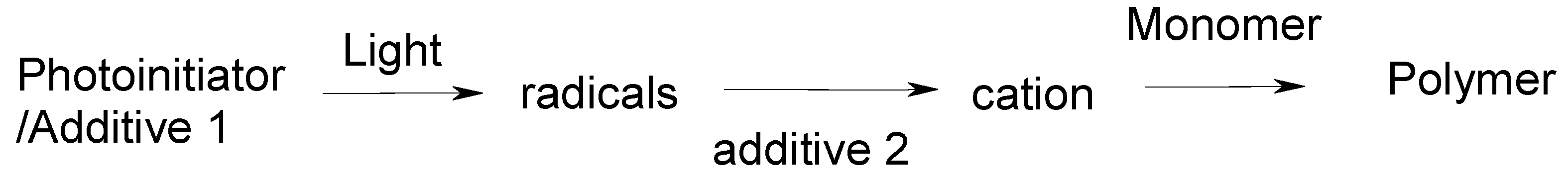
2. Backgrounds: Photoinitiation of Cationic ROP
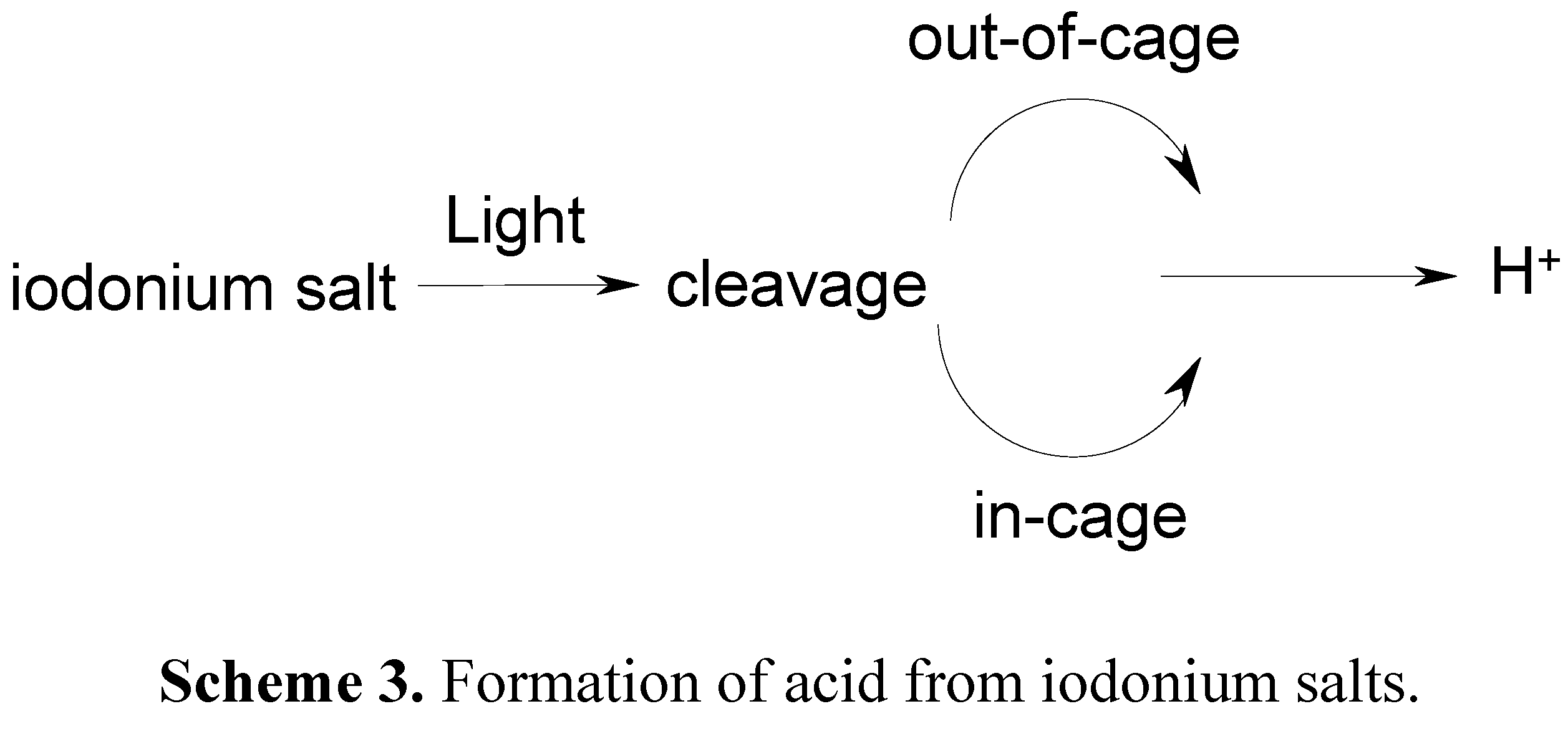
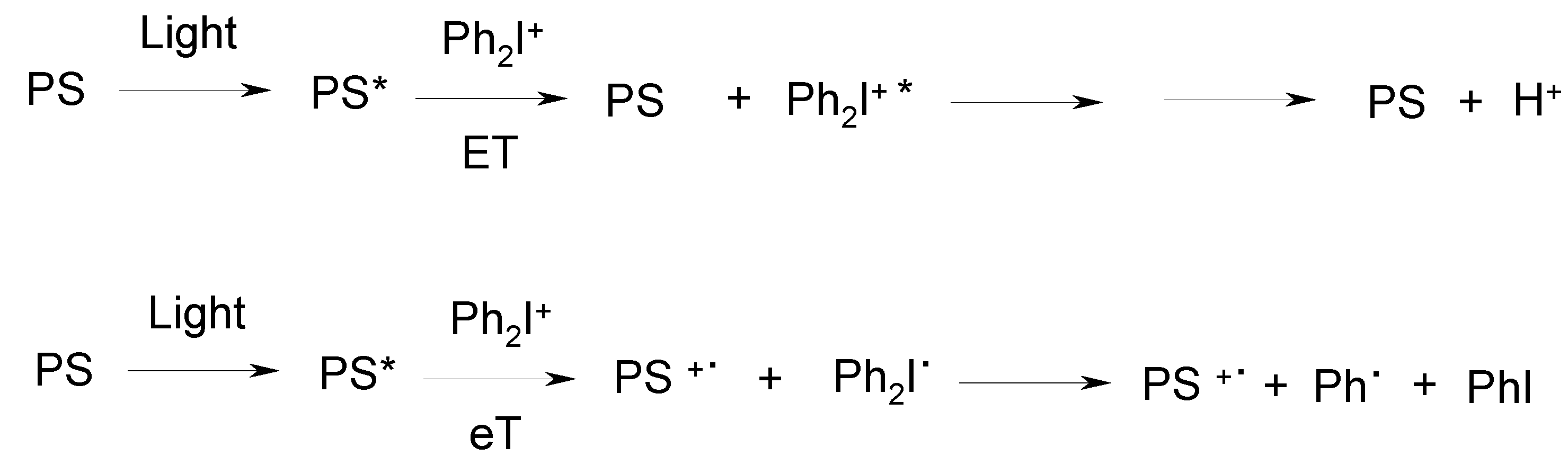
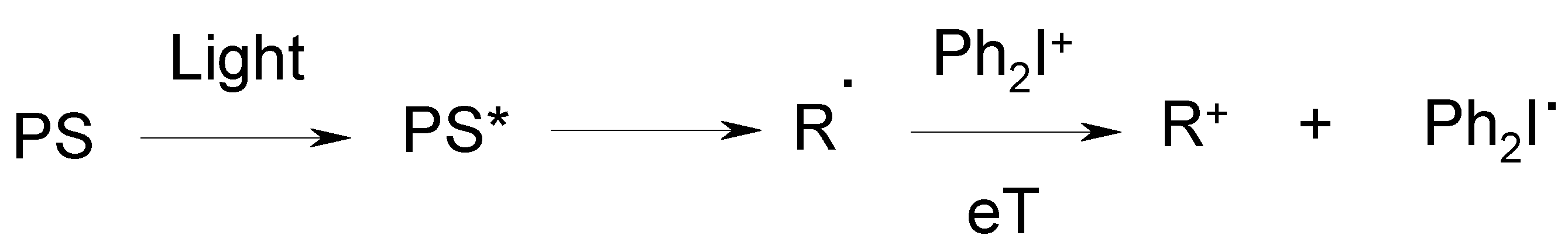
3. Development of a New Strategy and Design of Novel Cationic Photoinitiating Systems
3.1. The New Strategy
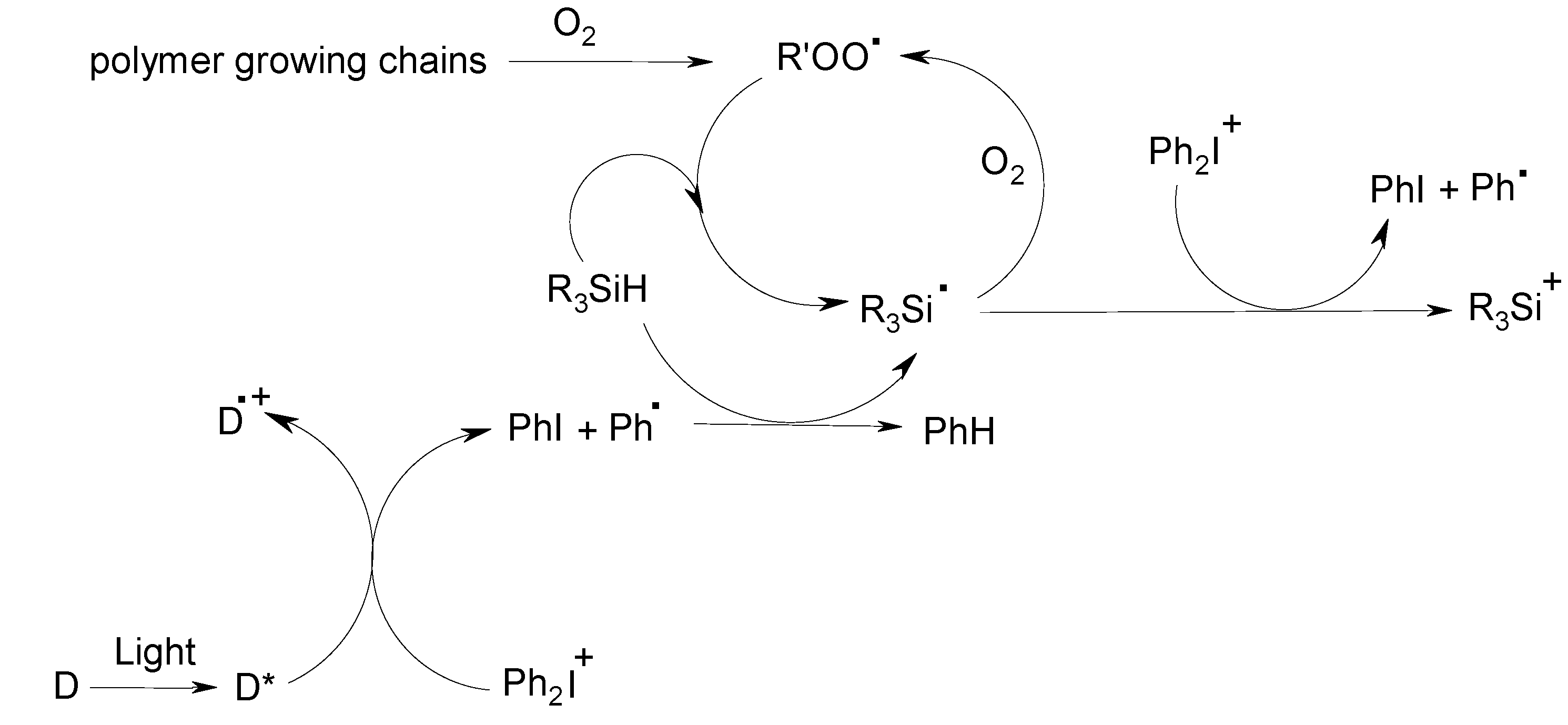
3.2. Examples of What Can Be Achieved
- -
- -
- -
- -
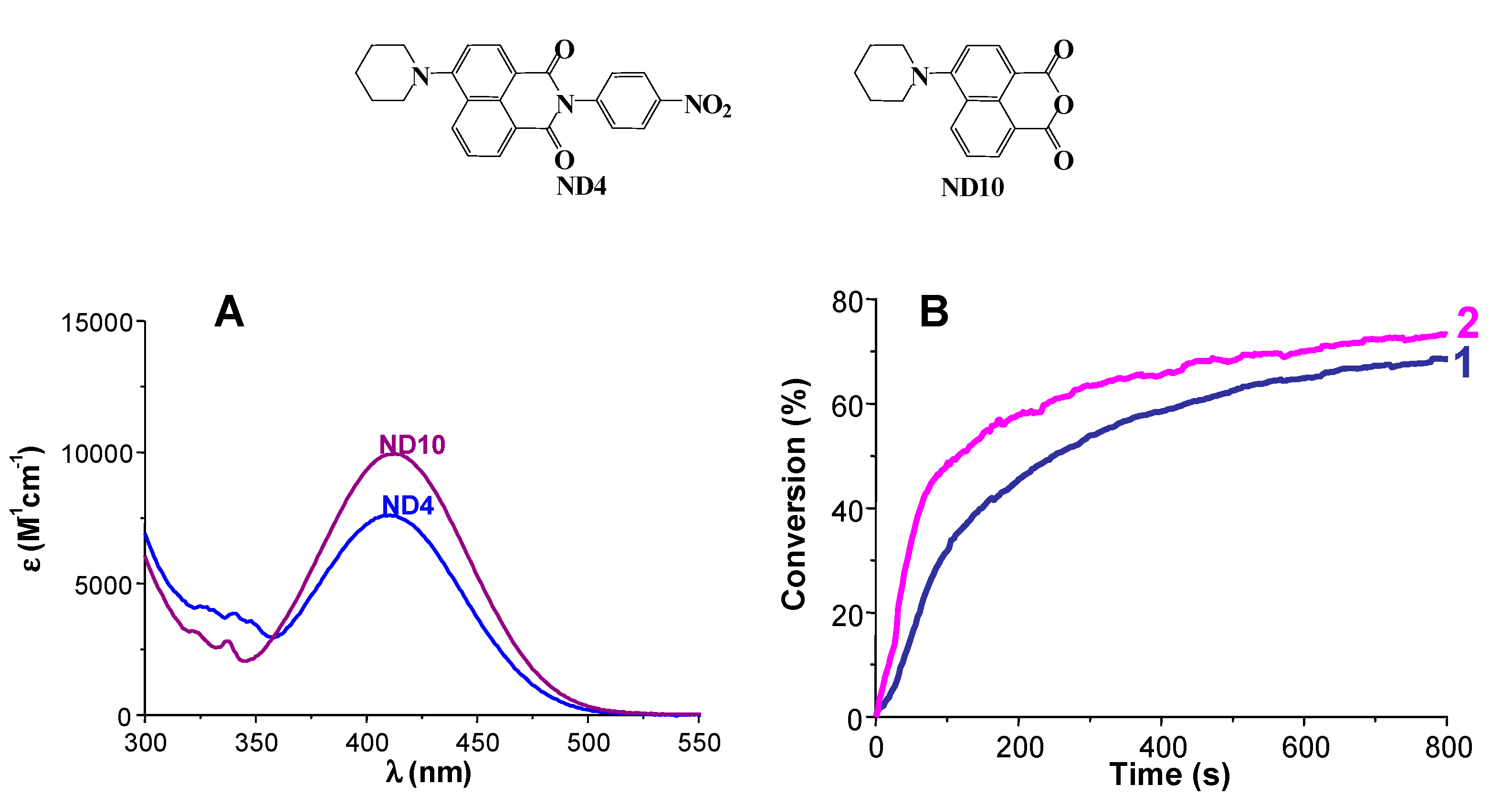
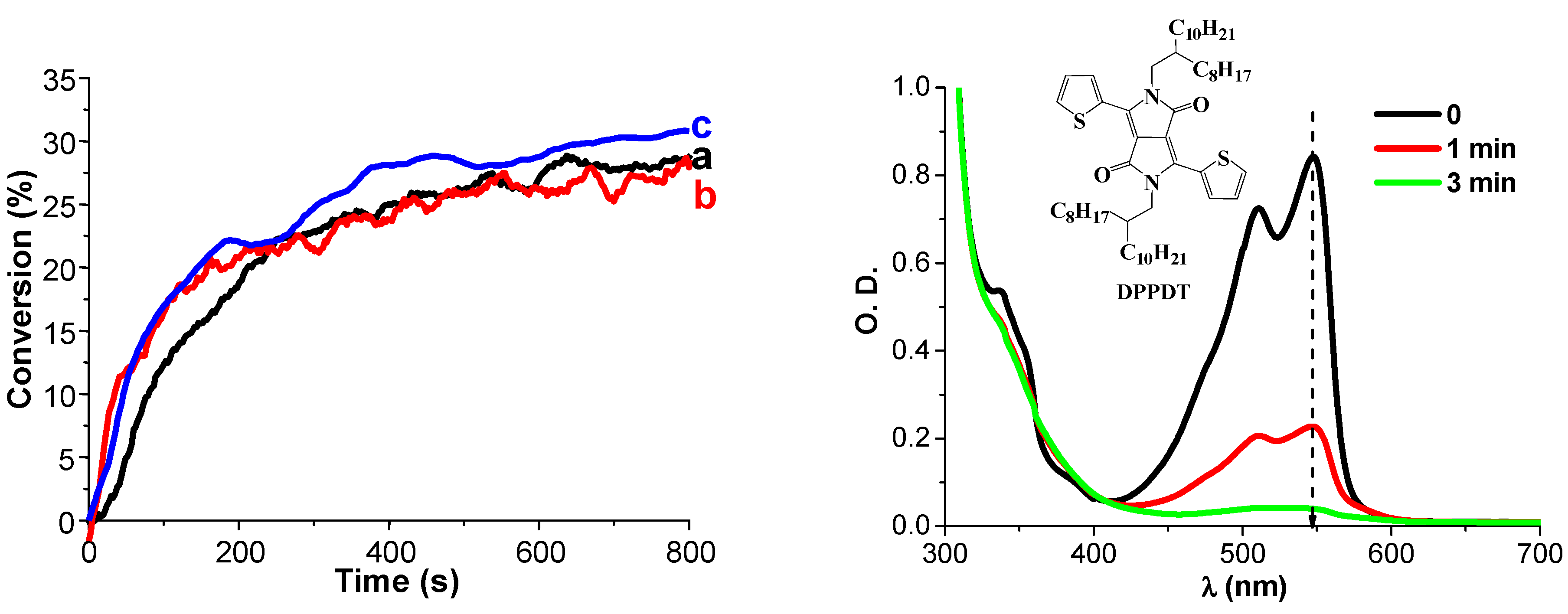
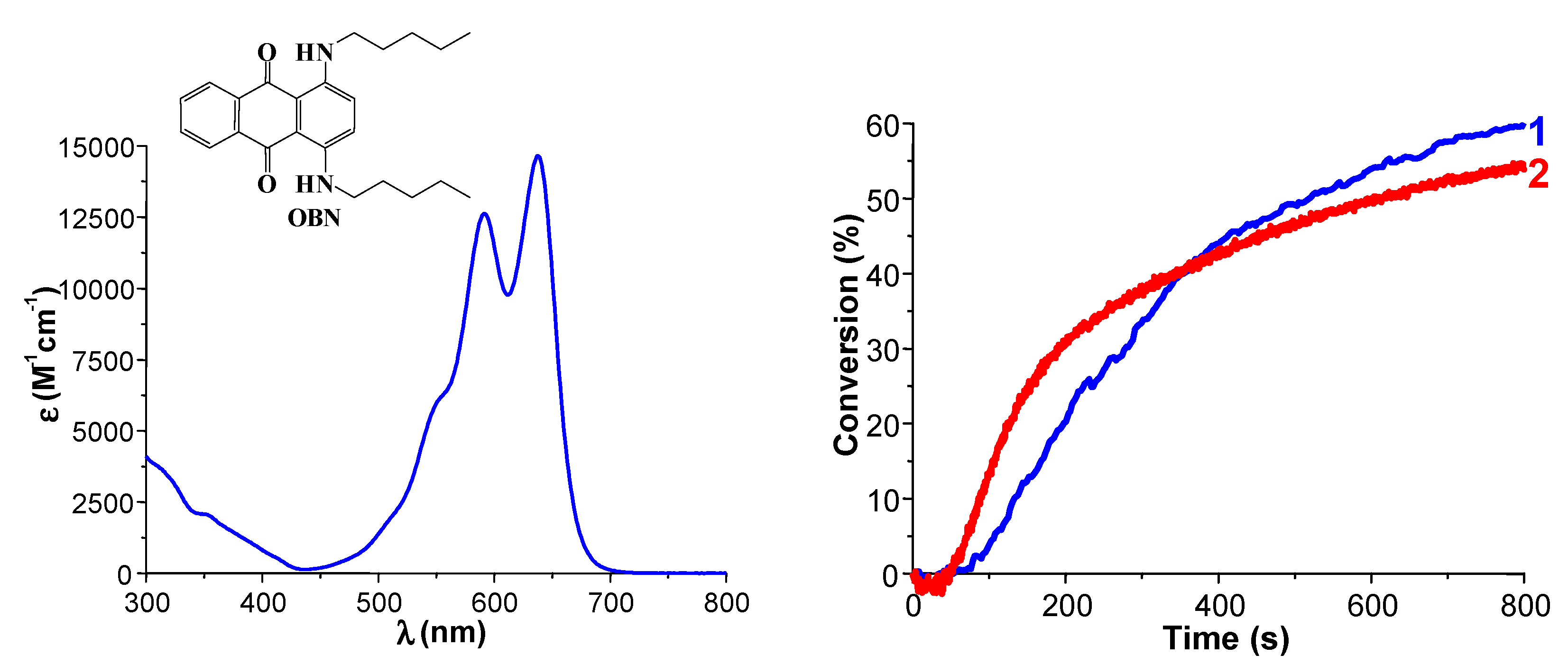
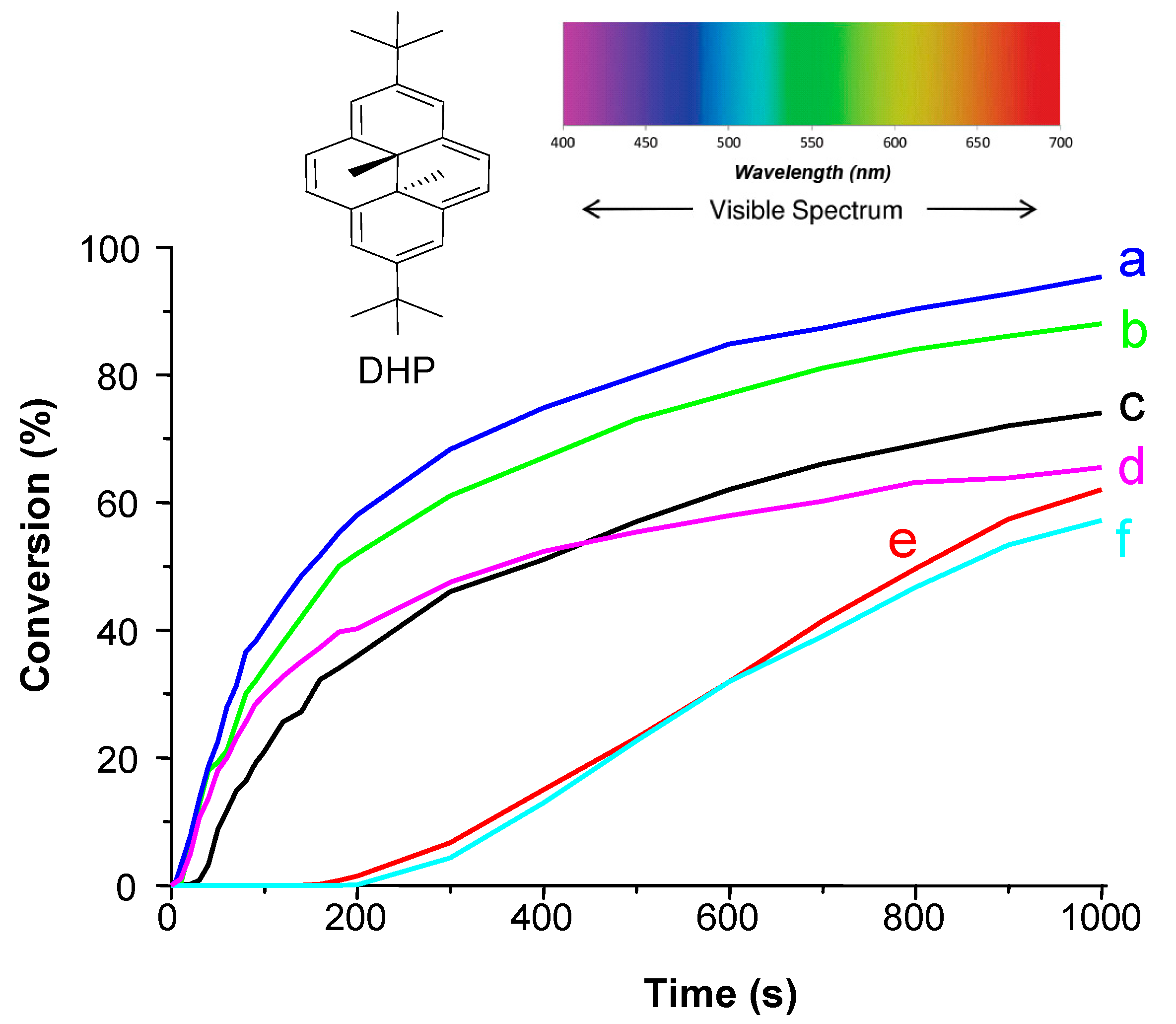
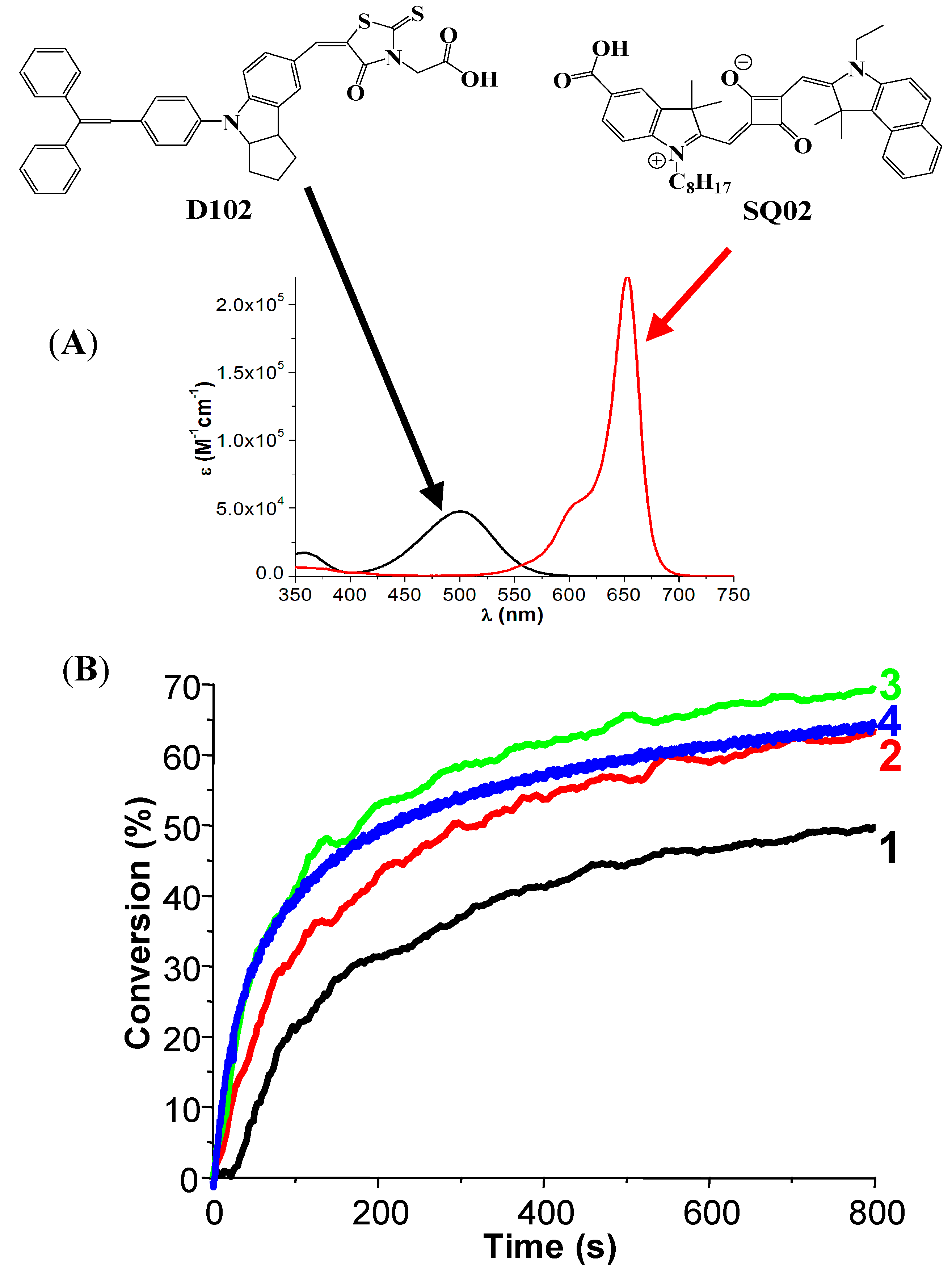
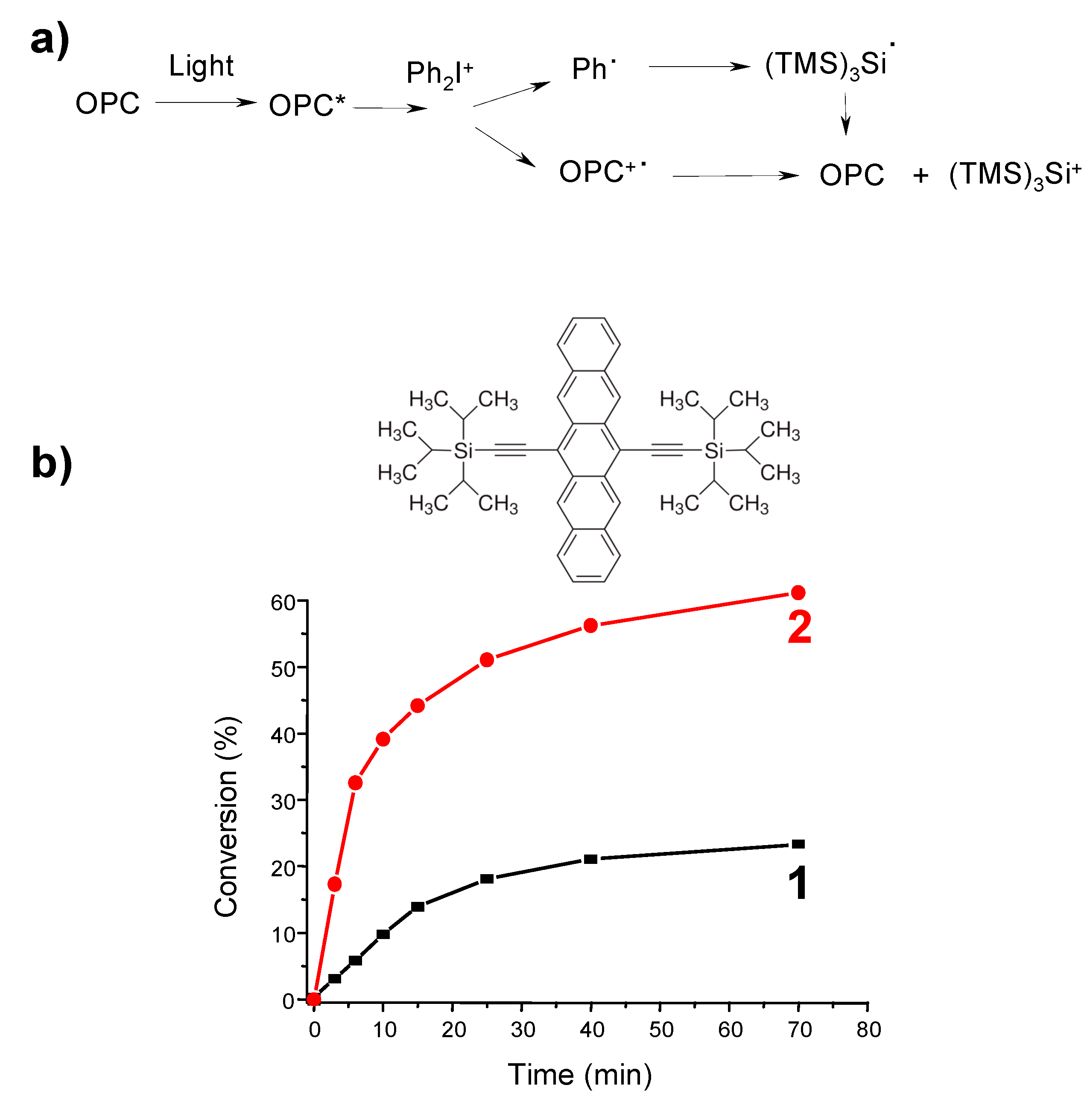
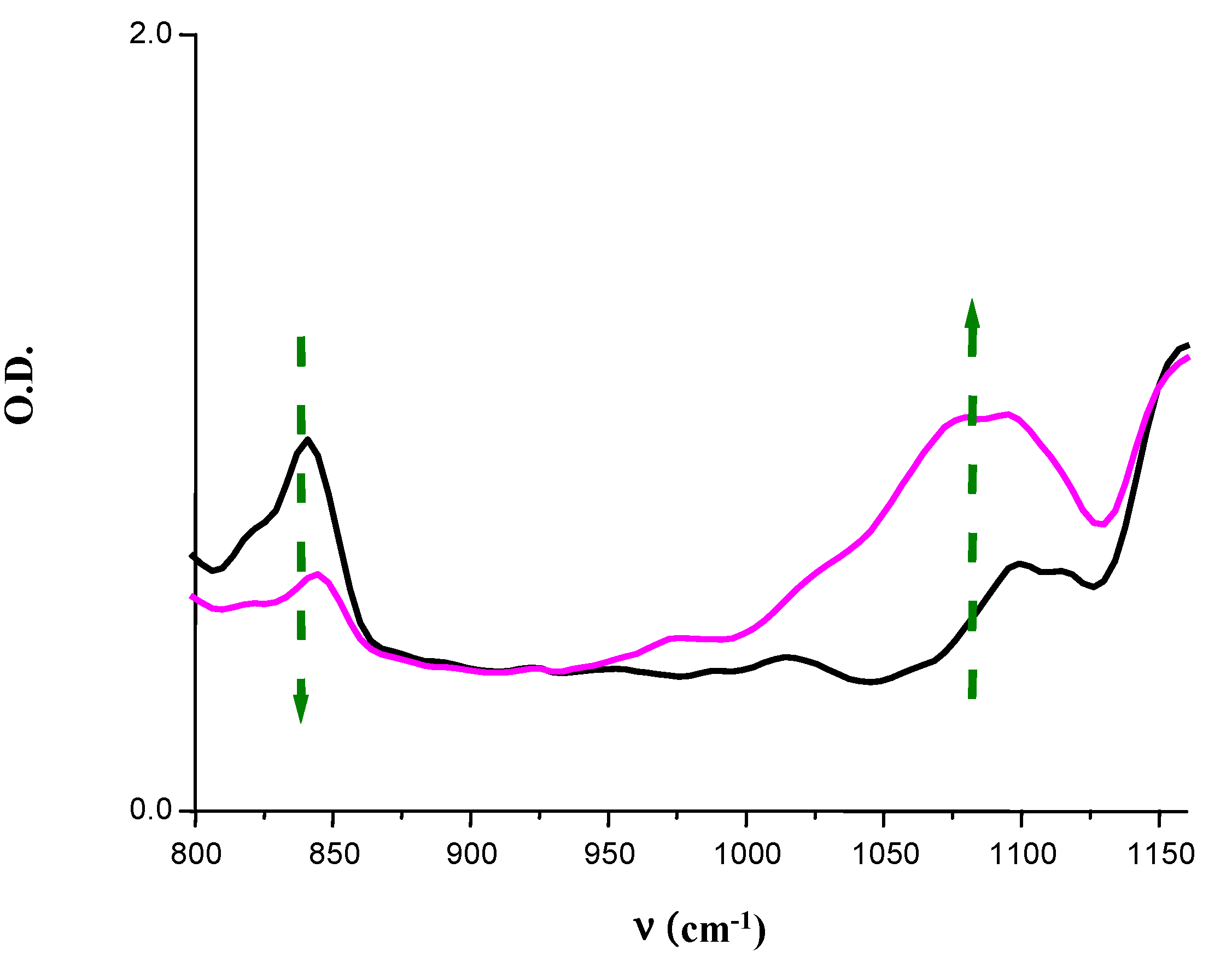
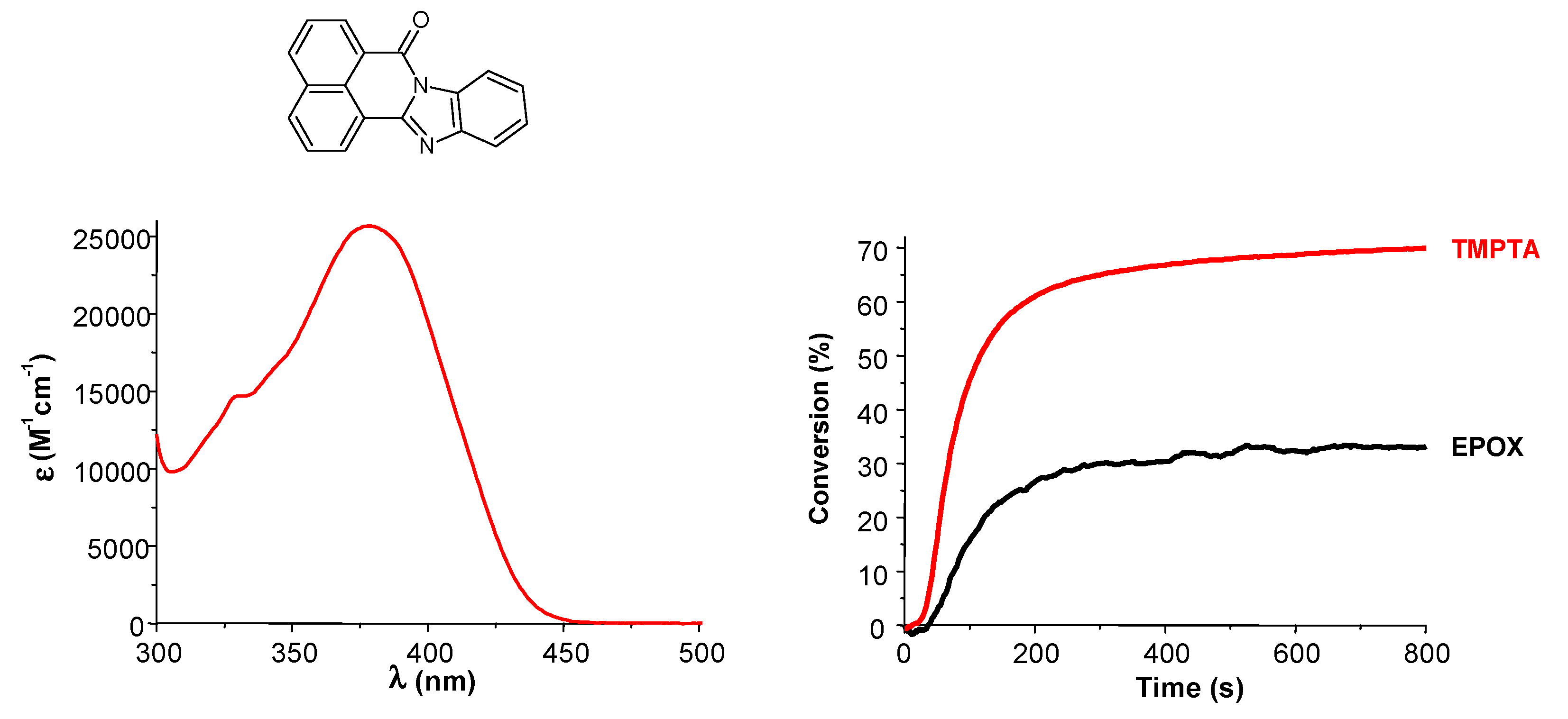
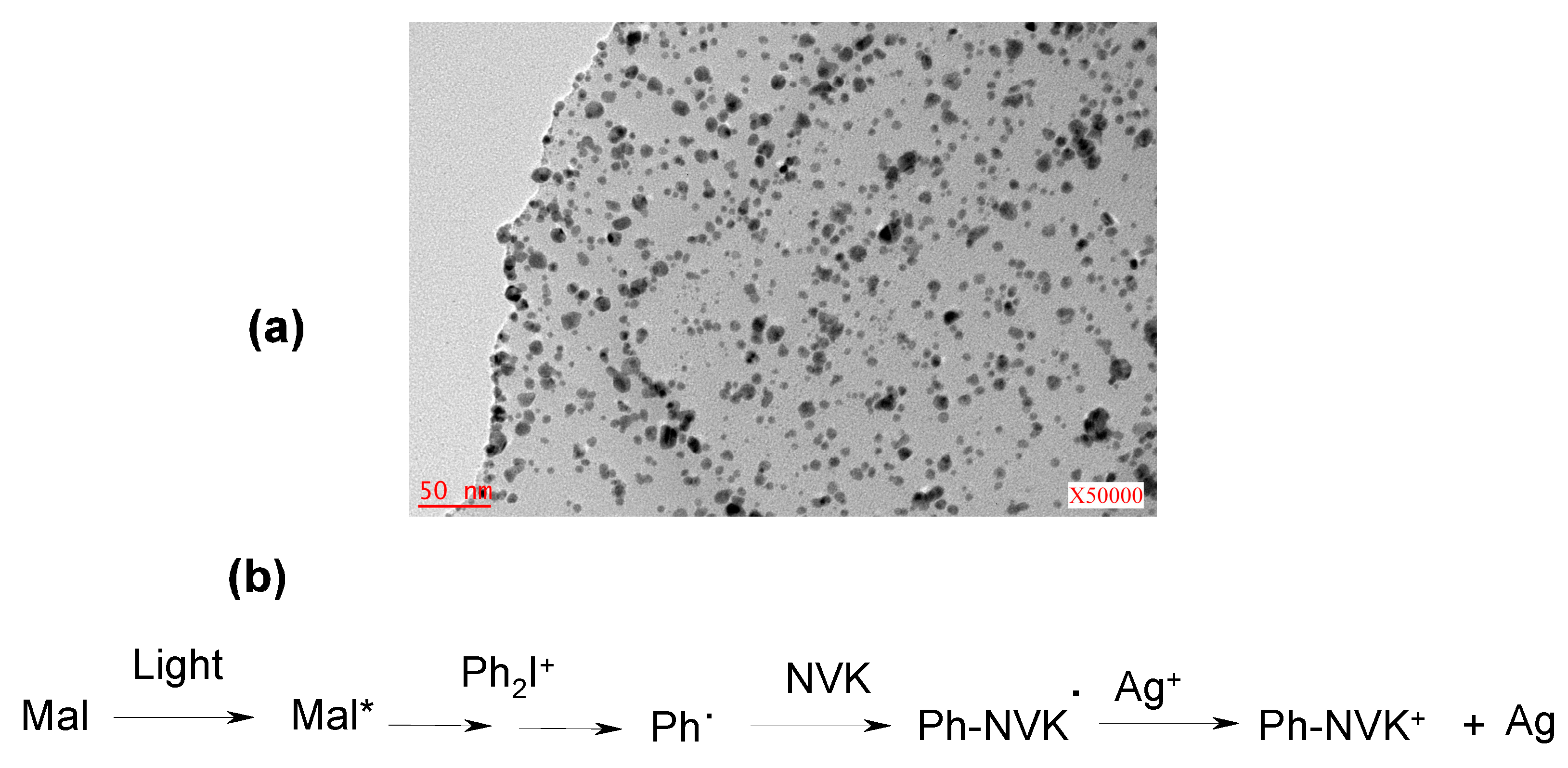
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Matyjaszewski, K.; Gnanou, Y.; Leibler, L. (Eds.) Wiley-VCH: Weinheim, Germany, 2007; Volume 1–4.
- Polymer Science: A Comprehensive Reference; Matyjaszewski, K.; Möller, M. (Eds.) Elsevier: Weinheim, Germany, 2012.
- Penczek, S.; Duda, A.; Kubisa, P.; Slombowski, S. Ionic and Coordination Ring Opening Polymerization in Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Matyjaszewski, K., Gnanou, Y., Leibler, L., Eds.; Wiley-VCH: Weinheim, Germany, 2007; Volume 1–4, pp. 103–160. [Google Scholar]
- Photoinitiated Polymerization; Belfied, K.D.; Crivello, J.V. (Eds.) ACS Symposium Series 847; ACS: Washington, DC, USA, 2003.
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Davidson, S. Exploring the Science, Technology and Application of UV and EB Curing; Sita Technology Ltd: London, UK, 1999. [Google Scholar]
- Neckers, D.C. UV and EB at the Millenium; Sita Technology: London, UK, 1999. [Google Scholar]
- Radiation Curing in Polymer Science and Technology; Fouassier, J.P.; Rabek, J.F. (Eds.) Chapman & Hall: London, UK, 1993.
- Fouassier, J.P. Photoinitiation, Photopolymerization, Photocuring; Hanser: Münich, Germany, 1995. [Google Scholar]
- Pappas, S.P. UV-Curing: Science and Technology; Tech. Mark. Corp. Stamford, 1986; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Photopolymerization: Fundamentals and Applications; Scranton, A.B.; Bowman, A.; Peiffer, R.W. ACS Symposium Series 673; ACS: Washington, DC, USA, 1997. [Google Scholar]
- Chemistry & Technology of UV & EB Formulation for Coatings, Inks and Paints; Olldring, K.; Holman, R. (Eds.) Wiley and Sita: London, UK, 1997; Volume I–VIII.
- Drobny, J.G. Radiation Technology for Polymers; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Roffey, C.G. Photogeneration of Reactive Species for UV Curing; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Schnabel, W. Polymer and Light; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Pappas, S.P. Photoinitiation of cationic and concurrent radical cationic polymerization. Part V. Progr. Org. Coat. 1985, 13, 35–64. [Google Scholar] [CrossRef]
- Kahveci, M.U.; Gilmaz, A.G.; Yagci, Y. Photoinitiated cationic polymerization: Reactivity and mechanistic aspects. In Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: New York, NY, USA, 2010; pp. 421–478. [Google Scholar]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 65, 6245–6260. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Lalevée, J. Polymerization under UV Lights. In Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Matyjaszewski, K., Gnanou, Y., Leibler, L., Eds.; Wiley-VCH: Weinheim, Germany, 2007; Volume 1, pp. 643–672. [Google Scholar]
- Crivello, J.V. Advances in the design of photoinitiators, photosensitizers and monomers for photoinitiated cationic polymerization. Des. Monomers Polym. 2002, 5, 141–154. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Louradour, F.; Fouassier, J.P.; Lalevée, J. Photopolymerization reactions: On the way to a green and sustainable chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. Recent advances in sunlight induced polymerization. Polym. Chem. 2011, 2, 1107–1113. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. Dye Photosensitized Polymerization Reactions: Novel Perspectives, RSC Photochemistry Reports; Albini, A., Fasani, E., Eds.; Photochemistry: London, UK, 2015; Volume 42, pp. 215–232. [Google Scholar]
- Fouassier, J.P.; Lalevée, J. Three-component photoinitiating systems: Towards innovative tailor made high performance combinations. RSC Adv. 2012, 2, 2621–2629. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Photoinitiating systems: Recent progress in visible light induced cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J.; Morlet-Savary, F. Dyes as photoinitiators or photosensitizers of polymerization reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef]
- Crivello, J.V. Newer Aspects of Photoinitiated Cationic Polymerization. In Ionic Polymerizations and Related Processes; Puskas, J.E., Michel, A., Barghi, S., Eds.; NATO Sci. Series 359, NATO: Weinheim, Germany, 1999; pp. 45–60. [Google Scholar]
- Crivello, J.V. Cationic polymerization: Iodonium and sulfonium salt photo initiators. Adv. Polym. Sci. 1984, 62, 1–48. [Google Scholar]
- Yağci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538. [Google Scholar] [CrossRef]
- Crivello, J.V. Photopolymerization. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Weinheim, Germany, 2012; Volume 4 (ring-opening polymerization and special polymerization processes), pp. 919–955. [Google Scholar]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium salts: A new class of photoinitiators for cationic polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Photoinitiated cationic polymerization with triarylsulfonium salts. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 977–999. [Google Scholar] [CrossRef]
- Timpe, H.J.; Jockush, S.; Korner, K. Dye sensitized photopolymerization. In Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Ed.; Elsevier: Barking, UK, 1993; Volume 2, pp. 575–602. [Google Scholar]
- Crivello, J.V. Latest developments in the chemistry of onium salts. In Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, pp. 435–472. [Google Scholar]
- Crivello, J.V.; Dietliker, K. Photoinitiators for Free Radical Cationic and Anionic Photopolymerization; Bradley, G., Ed.; Wiley: Weinheim, Germany, 1999; pp. 125–141. [Google Scholar]
- Hacker, N.P. New reactions of cationic photo initiators. In Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, pp. 473–504. [Google Scholar]
- Sahyun, M.R.; De Voe, R.J.; Olofson, P.M. Photochemistry of onium salt decomposition. In Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Ed.; Elsevier: Barking, UK, 1993; Volume 2, pp. 505–529. [Google Scholar]
- Schnabel, W.; Bottcher, A.; Hasebe, K.; Hizal, G.; Yagci, Y.; Stellberg, P. Initiation of cationic polymerization via oxidation of free radicals using pyridinium salts. Polymer 1991, 32, 2289. [Google Scholar] [CrossRef]
- Aydogan, B.; Gacal, B.; Yildirim, A.; Yonet, N.; Yuksel, Y.; Yagci, Y. Wavelength tunability in photoinitiated cationic polymerization. In Photochemistry and UV Curing; Fouassier, J.P., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 187–201. [Google Scholar]
- Colak, D.; Yurteri, S.; Kiskan, B.; Yagci, Y. Addition-fragmentation type reactions in photoinitiated cationic polymerization. In Photochemistry and UV Curing; Fouassier, J.P., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 175–186. [Google Scholar]
- Aydogan, B.; Gundogan, A.S.; Ozturk, T.; Yagci, Y. A Dithienothiophene derivative as a long-wavelength photosensitizer for onium salt photoinitiated cationic polymerization. Macromolecules 2008, 41, 3468–3471. [Google Scholar] [CrossRef]
- Aydogan, B.; Yagci, Y.; Toppare, L.; Jockusch, S.; Turro, N.J. Photoinduced electron transfer reactions of highly conjugated thiophenes for initiation of cationic polymerization and conjugated polymer formation. Macromolecules 2012, 45, 7829–7834. [Google Scholar] [CrossRef]
- Beyazit, S.; Aydogan, B.; Osken, I.; Ozturk, T.; Yagci, Y. Long wavelength photoinitiated free radical polymerization using conjugated thiophene derivatives in the presence of onium salts. Polym. Chem. 2011, 2, 1185–1189. [Google Scholar] [CrossRef]
- Bulut, U.; Gunbas, G.E.; Toppare, L. A quinoxaline derivative as a long wavelength photosensitizer for diaryliodonium salts. J. Polym. Sci. Part A Polym Chem. 2010, 48, 209–213. [Google Scholar] [CrossRef]
- Crivello, J.V. Radical-Promoted Visible light photoinitiated cationic polymerization of epoxides. J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 474–483. [Google Scholar] [CrossRef]
- Crivello, J.V. A new visible light sensitive photoinitiator system for the cationic polymerization of epoxides. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 866–875. [Google Scholar] [CrossRef]
- Podsiadły, R. 12H-Quinoxalino[2,3-b][1,4]benzothiazine derivatives as novel visible photosensitizers in cationic photopolymerization. J. Photochem. Photobiol. A 2009, 208, 147–153. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Moszner, N.; Yagci, Y. Visible light initiated free radical promoted cationic polymerization using acylgermane based photoinitiator in the presence of onium salts. Macromolecules 2008, 41, 6714–6718. [Google Scholar] [CrossRef]
- Tozuka, M.; Igarashi, T.; Sakurai, T. 1-(Arylmethyloxy)-9,10-anthraquinones: Photoinitiators for Radical and Cationic Polymerizations. Polym. J. 2009, 41, 709–714. [Google Scholar] [CrossRef]
- Yilmaz, G.; Acik, G.; Yagci, Y. Counteranion sensitization approach to photoinitiated free radical polymerization. Macromolecules 2012, 45, 2219–2224. [Google Scholar] [CrossRef]
- Semih, E.; Gorkem, Y.; Colak, D.G.; Demet; Cianga, I.; Yagci, Y. Chemistry of poly(phenylene vinylene)s as sensitizers for visible light induced cationic polymerization. Macromolecules 2014, 47, 7296–7302. [Google Scholar] [CrossRef]
- Bi, Y.; Neckers, D.C. A visible light initiating system for free radical promoted cationic polymerization. Macromolecules 1994, 27, 3683–3693. [Google Scholar] [CrossRef]
- Hua, Y.; Jiang, F.; Crivello, J.V. Photosensitized onium-salt induced cationic polymerization with hydroxymethylated polynuclear aromatic hydrocarbons. Chem. Mater. 2002, 14, 2369–2377. [Google Scholar] [CrossRef]
- Crivello, J.V.; Jiang, M. Anthracene electron transfer photosensitizers for onium salt induced cationic photopolymerizations. J. Photochem. Photobiol. A Chem. 2003, 159, 173–188. [Google Scholar] [CrossRef]
- Sangermano, M.; Crivello, J.V. Visible and Long-Wavelength Cationic Photopolymerization; ACS Symposium Series 847 (Photoinitiated Polymerization); ACS: Washington, DC, USA, 2003; pp. 242–252. [Google Scholar]
- Hua, Y.; Crivello, J.V. Photosensitization of Onium Salt Initiated Cationic Photopolymerizations by Carbazole Monomers, Polymers, and Oligomers; ACS Symposium Series 847 (Photoinitiated Polymerization); ACS: Washington, DC, USA, 2003; pp. 219–230. [Google Scholar]
- Crivello, J.V. Synergistic Free Radical Effects in Photoinitiated Cationic Polymerization; ACS Symposium Series 847 (Photoinitiated Polymerization); ACS: Washington, DC, USA, 2003; pp. 178–186. [Google Scholar]
- Gomurashvili, Z.; Crivello, J.V. Monomeric and polymeric phenothiazine photosensitizers for photoinitiated cationic polymerization. Macromolecules 2002, 35, 2962–2969. [Google Scholar] [CrossRef]
- Yilmaz, G.; Beyazit, S.; Yagci, Y. Visible light induced free radical promoted cationic polymerization using thioxanthone derivatives. J. Polym. Sci. Part A Polym Chem. 2011, 49, 1591–1596. [Google Scholar] [CrossRef]
- Crivello, J.V.; Sangermano, M. Visible and long-wavelength photoinitiated cationic polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 343–356. [Google Scholar] [CrossRef]
- Crivello, J.V.; Kong, S. Long-wavelength-absorbing dialkylphenacylsulfonium salt photoinitiators: Synthesis and photoinduced cationic polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1433–1442. [Google Scholar] [CrossRef]
- Toba, Y. Anthracene-sensitized polymerization of vinyl ethers by onium tetrakis(pentafluorophenyl)borate initiators. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 982–987. [Google Scholar] [CrossRef]
- Hartwig, A.; Harder, A.; Lühring, A.; Schröder, H. (9-Oxo-9H-fluoren-2-yl)-phenyl-iodonium hexafluoroantimonate(V)—A photoinitiator for the cationic polymerisation of epoxides. Eur. Polym. J. 2001, 37, 1449–1455. [Google Scholar] [CrossRef]
- Wang, T.; Ma, L.J.; Wan, P.Y.; Liu, J.P.; Wang, F. A study of the photoactivities and thermomechanical properties of epoxy resins using novel [cyclopentadien-Fe-arene]+PF6− photoinitiators. J. Photochem. Photobiol. A 2004, 163, 77–86. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Indian turmeric and its use in cationic photopolymerizations. Macromol. Symp. 2006, 240, 1–11. [Google Scholar] [CrossRef]
- Norcini, G.; Casiraghi, A.; Visconti, M.; Li, G. Bassi, Sulfonium Salts as Photo Initiators for Radiation Curable Systems. EP 1417198 B1, 8 May 2009. [Google Scholar]
- Crivello, J.V.; Ma, J.; Jiang, F. Synthesis and photoactivity of novel 5-arylthianthrenium salt cationic photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3465–3480. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Zhang, H.; Wang, T. A novel ferrocenium salt as visible light photoinitiator for cationic and radical photopolymerization. Prog. Org. Coat. 2010, 68, 234–239. [Google Scholar] [CrossRef]
- Kreutzer, J.; Demir, K.D.; Yagci, Y. Synthesis and characterization of a double photochromic initiator for cationic polymerization. Eur. Polym. J. 2011, 47, 792–799. [Google Scholar] [CrossRef]
- Crivello, J.V.; Aldersley, M.F. Supramolecular diaryliodonium salt-crown ether complexes as cationic photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 801–814. [Google Scholar] [CrossRef]
- Corakci, B.; Hacioglu, S.O.; Toppare, L.; Bulut, U. Long wavelength photosensitizers in photoinitiated cationic polymerization: The effect of quinoxaline derivatives on photopolymerization. Polymer 2013, 54, 3182–3187. [Google Scholar] [CrossRef]
- Bulut, U.; Yilmaz, S.; Yigitsoy, B.; Toppare, L. Long Wavelength Photosensitizers for Diaryliodonium Salts Based on the 2-Benzyl-2H-benzo[d][1,2,3]triazole Skeleton. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 445–449. [Google Scholar] [CrossRef]
- Podsiadły, R.; Maruszewska, A.; Michalski, R.; Marcinek, A.; Kolińska, J. Naphthoylenebenzimidazolone dyes as electron transfer photosensitizers for iodonium salt induced cationic photopolymerizations. Dyes Pigm. 2012, 95, 252–259. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Zaim, O.; Yagci, Y. Diethoxy-azobis(pyridinium) Salt as photoinitiator for cationic polymerization: Towards wavelength tunability by cis–trans isomerization. Macromol. Rapid Commun. 2008, 29, 892–896. [Google Scholar] [CrossRef]
- Aydogan, B.; Gunbas, G.E.; Durmus, A.; Toppare, L.; Yagci, Y. Highly conjugated thiophene derivatives as new visible light sensitive photoinitiators for cationic polymerization. Macromolecules 2009, 43, 101–106. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Asmussen, S.V.; Sangermano, M.; Vallo, C.I. Visible light polymerization of epoxy monomers using an iodonium salt with camphorquinone/ethyl-4-dimethyl aminobenzoate. Polym. Int. 2013, 62, 1368–1376. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Kukut, M.; Moszner, N.; Yagci, Y. Sequential photodecomposition of bisacylgermane type photoinitiator: Synthesis of block copolymers by combination of free radical promoted cationic and free radical polymerization mechanisms. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4793–4799. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Yilmaz, G.; Tasleden, M.A.; Aydogan, B.; Koz, B.; Yagci, Y. N-alkoxy pyridinium salt photoinitiators. In Basics and Applications of Photopolymerization Reactions; Fouassier, J.P., Allonas, X., Eds.; Research Signpost: Trivandrum, India, 2010; pp. 7–22. [Google Scholar]
- Liu, G.; Zhu, X.; Xu, B.; Qian, X.; Song, G.; Nie, J. Cationic photopolymerization of bisphenol A diglycidyl ether epoxy under 385 nm. J. Appl. Polym. Sci. 2013, 130, 3698–3703. [Google Scholar] [CrossRef]
- Uchida, N.; Nakano, H.; Igarashi, T.; Sakurai, T. Nonsalt 1-(arylmethyloxy)pyrene photoinitiators capable of initiating cationic polymerization. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Taskin, O.S.; Erel-Goktepe, I.; Khan, M.A.A.; Pispas, S.; Yagci, Y. Polystyrene-b-poly(2-vinyl phenacyl pyridinium) salts as photoinitiators for free radical and cationic polymerizations and their photoinduced molecular associations. J. Photochem. Photobiol. A Chem. 2014, 285, 30–36. [Google Scholar] [CrossRef]
- Tunc, D.; Yagci, Y. Thioxanthone-ethylcarbazole as a soluble visible light photoinitiator for free radical and free radical promoted cationic polymerizations. Polym. Chem. 2011, 2, 2557–2563. [Google Scholar] [CrossRef]
- Yilmaz, G.; Iskin, B.; Yilmaz, F.; Yagci, Y. Visible Light-Induced Cationic Polymerization Using Fullerenes. ACS Macro Lett. 2012, 1, 1212–1215. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Guo, D.; Wang, L.; Nie, J. Photopolymerization kinetics of a disulfone cationic photoinitiator. J. Photochem. Photobiol. A Chem. 2012, 232, 57–63. [Google Scholar] [CrossRef]
- Muller, U.; Utterodt, A.; Morke, W.; Deubzer, B.; Herzig, C. Diazonium Salts as Cationic Photoinitiators: Radical and Cationic Aspects; ACS Symposium Series 847 (Photoinitiated Polymerization); ACS: Washington, DC, USA, 2003; pp. 202–212. [Google Scholar]
- Milanesi, S.; Fagnoni, M.; Albini, A. (Sensitized) photolysis of diazonium salts as a mild general method for the generation of aryl cations. Chemoselectivity of the singlet and triplet 4-substituted phenyl cations. J. Org. Chem. 2005, 70, 603–610. [Google Scholar]
- Cunningham, A.F.; Desobry, V. Metal based photo initiators. In Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, pp. 323–374. [Google Scholar]
- Ivan, M.G.; Scaiano, J.C. Photoimaging and lithographic processes in polymers. In Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: New York, NY, USA, 2010; pp. 479–508. [Google Scholar]
- Castellanos, F.; Fouassier, J.P.; Priou, C.; Cavezzan, J. Synthesis, reactivity, and properties of new diaryliodonium salts as photoinitiators for the cationic polymerization of epoxy silicones. J. Appl. Polym. Sci. 1996, 60, 705–713. [Google Scholar] [CrossRef]
- Golaz, B.; Michaud, V.; Leterrier, Y.; Månson, J.-E. UV intensity, temperature and dark-curing effects in cationic photo-polymerization of a cycloaliphatic epoxy resin. Polymer 2012, 53, 2038–2048. [Google Scholar] [CrossRef]
- Park, H.J.; Ryu, C.Y.; Crivello, J.V. Photoinitiated cationic polymerization of limonene 1,2-oxide and α-pinene oxide. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 109–117. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J.P. Oxygen mediated and wavelength tunable cationic photopolymerization reactions under air and low intensity: A new concept. Prog. Org. Coat. 2011, 70, 23–31. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Design of chromophores for photoinitiators of polymerization: Brief survey and recent achievements. In New Developments in Chromophore Research; Nova Science Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
- Lalevée, J.; El Roz, M.; Allonas, X.; Fouassier, J.P. On the silyl radical chemistry in photopolymerization reactions. In Organosilanes: Properties, Performance and Applications; Wyman, E., Skief, M.C., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2009; Chapter 6. [Google Scholar]
- Lalevée, J.; Fouassier, J.P. Overview of radical initiation. In Handbook of Radical Chemistry & Biology; Studer, A., Chatgilialoglou, C., Eds.; Wiley: Weinheim, Germany, 2012; Volume 1, Chapter 2. [Google Scholar]
- Telitel, S.; Blanchard, N.; Schweizer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. BODIPY derivatives and boranil as new photoinitiating systems of cationic polymerization exhibiting a tunable absorption in the 400–600 nm spectral range. Polymer 2013, 54, 2071–2076. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevee, J. Design of new type I and type II photoinitiators possessing highly coupled pyrene-ketone moieties. Polym. Chem. 2013, 4, 2313–2324. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P. Light-Harvesting Organic Photoinitiators of Polymerization. Macromol. Rapid Commun. 2013, 34, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Clément, J.-L.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.-P.; Lalevée, J. New cleavable photoinitiator architecture with huge molar extinction coefficients for polymerization in the 340–450 nm range. Macromolecules 2013, 46, 736–746. [Google Scholar] [CrossRef]
- Zhang, J.; Frigoli, M.; Dumur, F.; Xiao, P.; Ronchi, L.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Design of novel photoinitiators for radical and cationic photopolymerizations under UV and visible LEDs (385, 395, 405 and 455 nm). Macromolecules 2014. [Google Scholar] [CrossRef]
- Tehfe, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Blue-to-red light sensitive push-pull structured photoinitiators: indanedione derivatives for radical and cationic photopolymerization reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Telitel, S.; Lalevée, J.; Blanchard, N.; Kavalli, T.; Tehfe, M.-A.; Schweizer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. Photopolymerization of cationic monomers and acrylate/divinylether blends under visible light using pyrromethene dyes. Macromolecules 2012, 45, 6864–6868. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Blue light sensitive dyes for various photopolymerization reactions: Naphthalimide and naphthalic anhydride derivatives. Macromolecules 2014, 47, 601–608. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Green bulb light source induced epoxy cationic polymerization under air using tris(2,2'-bipyridine)ruthenium(II) and silyl radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Peter, M.; Dumur, F.; Gigmes, D.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Subtle ligand effects in oxidative photocatalysis with iridium complexes: Application to photopolymerization. Chem. Eur. J. 2011, 17, 15027–15031. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Tehfe, M.A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.P. Iridium photocatalysts in free radical photopolymerization under visible lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Photopolymerization of N-Vinylcarbazole using visible-light harvesting iridium complexes as photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Green light sensitive diketopyrrolopyrrole derivatives used in versatile photoinitiating systems for photopolymerizations. Polym. Chem. 2014, 5, 2293–2300. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Blanchard, N.; Fouassier, J.P. Tunable organophotocatalysts for polymerization reactions under visible lights. Macromolecules 2012, 45, 1746–1752. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. A breakthrough toward long wavelength cationic photopolymerization: Initiating systems based on violanthrone derivatives and silyl radicals. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Red-light-induced cationic photopolymerization: Perylene derivatives as efficient photoinitiators. Macromol. Rapid Commun. 2013, 34, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dumur, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Cationic and thiol-ene photopolymerization upon red lights using anthraquinone derivatives as photoinitiators. Macromolecules 2013, 46, 6744–6750. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Vilà, N.; Graff, B.; Mayer, C.R.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. A multicolor photoinitiator for cationic polymerization and interpenetrated polymer network synthesis: 2,7-Di-tert-butyldimethyldihydropyrene. Macromol. Rapid Commun. 2013, 34, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dumur, F.; Bui, T.T.; Goubard, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Panchromatic photopolymerizable cationic films using indoline and squaraine dyes based photoinitiating systems. ACS Macro Lett. 2013, 2, 736–740. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: Sunlight induced cationic polymerization of renewable epoxy monomer under air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Novel attempts for the design of photoinitiators at 385–405 nm: Search around the naphtalene scaffold. Macromolecules 2014. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Telitel, S.; Vidal, L.; Xiao, P.; Versace, D.-L.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; et al. Photoinitiating systems of polymerization and in situ incorporation of metal nanoparticles into polymer matrices upon exposure to visible light: Push-pull malonate and malononitrile based dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalevée, J.; Mokbel, H.; Fouassier, J.-P. Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources. Molecules 2015, 20, 7201-7221. https://doi.org/10.3390/molecules20047201
Lalevée J, Mokbel H, Fouassier J-P. Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources. Molecules. 2015; 20(4):7201-7221. https://doi.org/10.3390/molecules20047201
Chicago/Turabian StyleLalevée, Jacques, Haifaa Mokbel, and Jean-Pierre Fouassier. 2015. "Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources" Molecules 20, no. 4: 7201-7221. https://doi.org/10.3390/molecules20047201
APA StyleLalevée, J., Mokbel, H., & Fouassier, J.-P. (2015). Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources. Molecules, 20(4), 7201-7221. https://doi.org/10.3390/molecules20047201





