Understanding the Physicochemical Properties of Mitragynine, a Principal Alkaloid of Mitragyna speciosa, for Preclinical Evaluation
Abstract
:1. Introduction
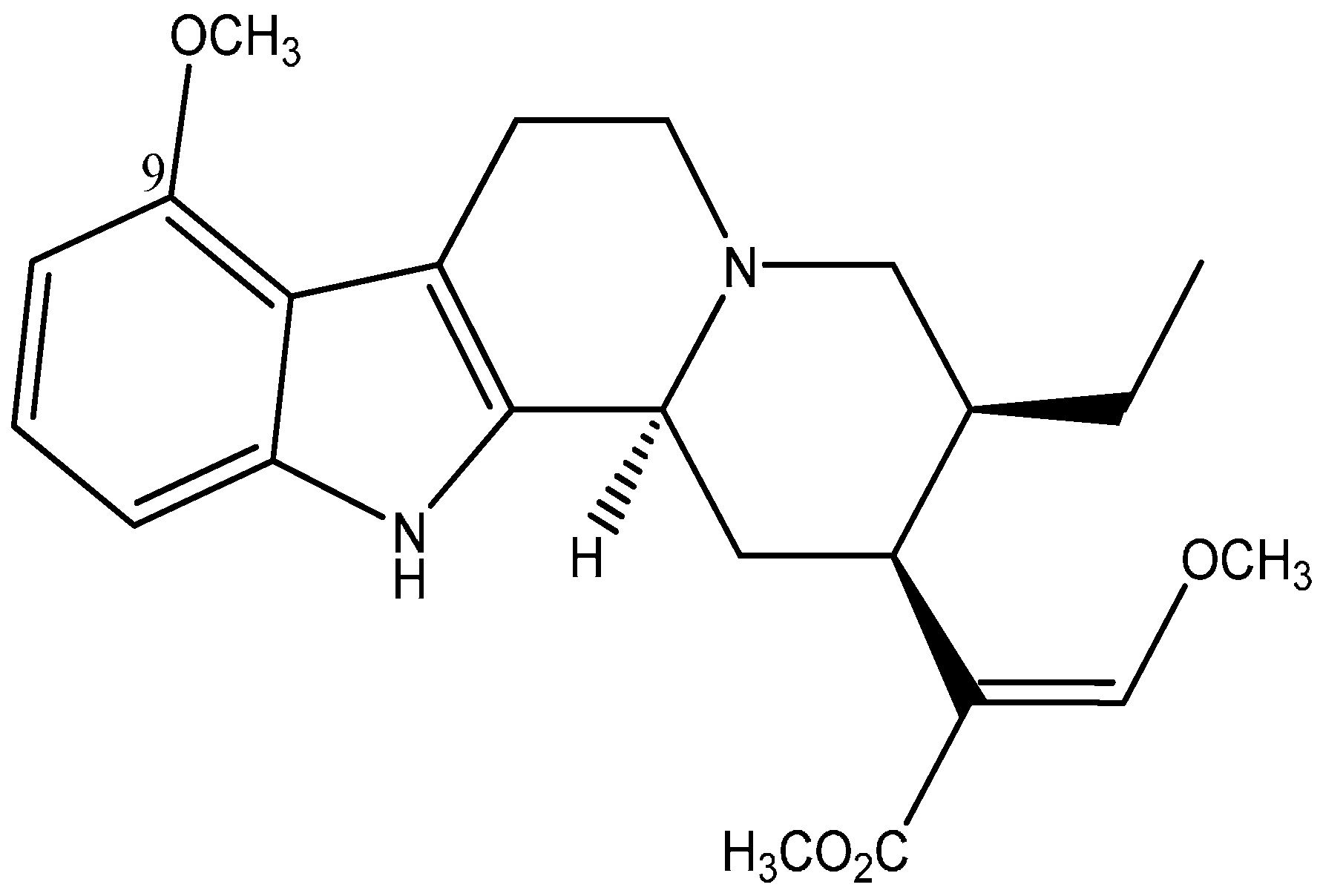
2. Results and Discussion
2.1. Results
| Target pH | UV Spectrophotometer Method | Microplate Spectrophotometer Method | ||||
|---|---|---|---|---|---|---|
| Actual pH achieved | Absorbance (d) | pKa | Actual pH achieved | Absorbance (d) | pKa | |
| 7.6 | 7.52 | 0.8436 | 8.0219 | 7.55 | 0.4993 | 8.0860 |
| 7.8 | 7.78 | 0.8186 | 8.1617 | 7.76 | 0.4823 | 8.1124 |
| 8.0 | 7.96 | 0.7786 | 8.1726 | 7.94 | 0.4527 | 8.0256 |
| 8.2 | 8.13 | 0.7367 | 8.1810 | 8.15 | 0.4350 | 8.0870 |
| 8.4 | 8.34 | 0.6677 | 8.1281 | 8.32 | 0.4170 | 8.1015 |
| 8.6 | 8.45 | 0.6232 | 8.0489 | 8.50 | 0.3910 | 8.0257 |
| 8.8 | 8.71 | 0.6148 | 8.2692 | 8.66 | 0.3877 | 8.1477 |
| 9.0 | 8.92 | 0.5347 | 7.9303 | 8.80 | 0.3707 | 8.0567 |
| Dm | 0.4917 (pH 12) | 0.3393 (pH 12) | ||||
| Di | 0.9544 (pH 5) | 0.5460 (pH 5) | ||||
| pKa (Mean ± SD) | 8.11 ± 0.11 | 8.08 ± 0.04 | ||||
| Instrument | Day | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| UV Spectrophotometer | 8.18 (0.10) | 8.11 (0.05) | 8.15 (0.12) |
| Microplate Spectrophotometer | 8.18 (0.13) | 8.10 (0.07) | 8.12 (0.10) |
| Solvent Layer | Mitragynine Content (µg/mL) at Different Buffer pH | |||
|---|---|---|---|---|
| pH 4 ** | pH 7 ** | pH 9 ** | Water * | |
| Octanol | 17.97 | 20.54 | 18.45 | 20.74 |
| Buffer | 2.98 | 0.38 | 0.51 | 0.42 |
| Calculated n-octanol/water partition coefficient | 0.78 | 1.73 | 1.56 | 1.70 |
| Time (min) | SGF | RD (%) | SIF | |
|---|---|---|---|---|
| Concentration Found (µg/mL) | Concentration Found (µg/mL) | RD (%) | ||
| 0 | 18.72 ± 0.05 | - | 6.70 ± 0.60 | - |
| 10 | 18.68 ± 0.23 | −0.21 | 6.69 ± 0.63 | −0.07 |
| 20 | 14.98 ± 0.88 | −20.00 | 6.56 ± 0.67 | 2.06 |
| 30 | 14.54 ± 0.58 | −22.5 | 6.99 ± 0.21 | 4.43 |
| 40 | 14.19 ± 0.37 | −22.31 | 7.03 ± 0.13 | 4.94 |
| 50 | 14.11 ± 0.22 | −24.19 | 6.93 ± 0.13 | 3.49 |
| 60 | 13.94 ± 0.20 | −25.53 | 6.86 ± 0.01 | 2.49 |
| 120 | - | - | 6.78 ± 0.79 | 1.31 |
| 180 | - | - | 6.93 ± 0.77 | 3.46 |
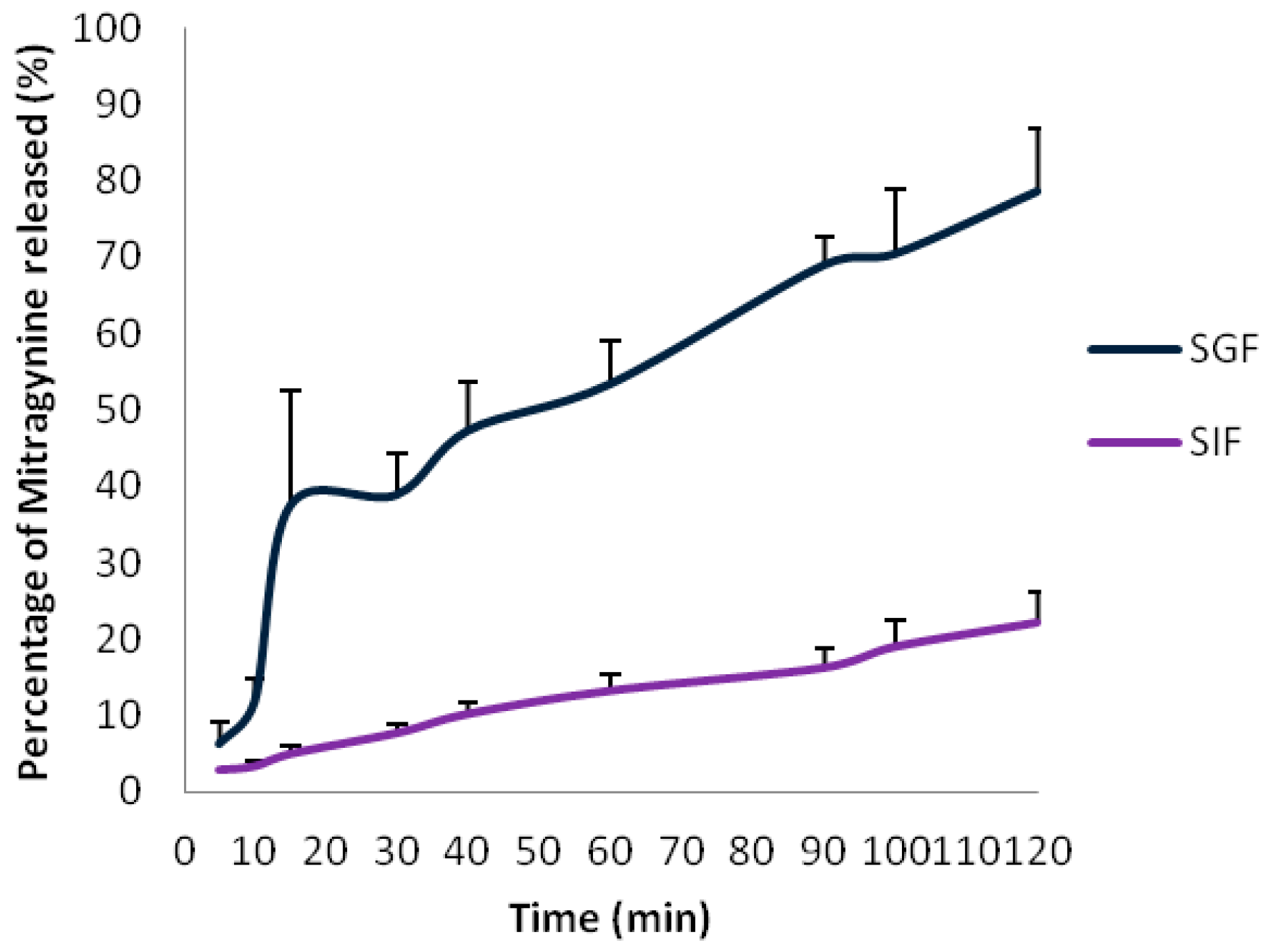
2.2. Discussion
3. Experimental Section
3.1. Experiments
3.1.1. Chemicals and Reagents
3.1.2. Extraction and Isolation of Mitragynine
3.1.3. Spectrophotometry
3.1.4. HPLC
3.1.5. pKa
3.1.6. Conventional UV Method
3.1.7. Microplate Method
3.1.8. Solubility and Stability
3.1.9. Partition Coefficient
3.2. Mitragynine Stability in Simulated Gastrointestinal Fluids
3.3. In Vitro Release Test of Mitragynine from Capsules Filled with Mitragynine Pure Standard
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burkhill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Crown Agents for the Colonies: London, UK, 1935; pp. 1480–1483. [Google Scholar]
- Takayama, H. Chemistry and pharmacology of analgesic Indole alkaloids from the rubiaceous plants, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Shellard, E.J. Ethnopharmacology of kratom and the Mitragyna alkaloids. J. Ethnopharmacol. 1989, 25, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Vicknasingam, B.; Narayanan, S.; Beng, G.T.; Mansor, S.M. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of Peninsular Malaysia and implications for drug substitution therapy. Int. J. Drug Policy 2010, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Müller, C.P.; Vicknasingam, B.K. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol. Depend. 2014, 139, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.H.M.; Suhaimi, F.W.; Vadivelu, R.K.; Hassan, Z.; Rümler, A.; Rotter, A.; Amato, D.; Dringenberg, H.C.; Mansor, S.M.; Navaratnam, V.; et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict. Biol. 2014. [Google Scholar] [CrossRef]
- Schmidt, M.; Sharma, A.; Schifano, F.; Feinmann, C. Legal highs on the net—Evaluation of UK-based websites, products and product information. Forensic Sci. Int. 2011, 206, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Mizowaki, M.; Suchitra, T.; Takayama, H.; Sakai, S.; Aimi, N.; Watanabe, H. Antinociceptive action of mitragynine in mice: Evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996, 59, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Sabetghadam, A.; Navaratnam, V.; Mansor, S.M. Dose–response relationship, acute toxicity, and therapeutic index between the alkaloid extract of Mitragyna speciosa and its main active compound mitragynine in mice. Drug Dev. Res. 2013, 74, 23–30. [Google Scholar] [CrossRef]
- Idid, S.Z.; Saad, L.B.; Yaacob, H.; Shahimi, M. Evaluation of Analgesia Induced by Mitragynine, Morphine and Paracetamol on Mice. ASEAN Rev. Biodivers. Environ. Conserv. 1998, pp. 1–7. Available online: http://entheology.com/wp-content/uploads/kratom-research/1998_std004.pdf (accessed on 4 March 2015).
- Parthasarathy, S.; Ramanathan, S.; Ismail, S.; Adenan, M.I.; Mansor, S.M.; Murugaiyah, V. Determination of mitragynine in plasma with solid-phase extraction and rapid HPLC–UV analysis, and its application to a pharmacokinetic study in rat. Anal. Bioanal. Chem. 2010, 397, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, N.V.; Moretti, R.A.; Furr, E.B., III; McCurdy, C.R.; Lanchote, V.L. Determination of mitragynine in rat plasma by LC–MS/MS: Application to pharmacokinetics. J. Chromatogr. B 2009, 877, 2593–2597. [Google Scholar] [CrossRef]
- Janchawee, B.; Keawpradub, N.; Cittrakarn, S.; Prasettho, S.; Wararatananurak, P.; Sawangjareon, K. A high-performance liquid chromatographic method for determination of mitragynine in serum and its application to a pharmacokinetic study in rats. Biomed. Chromatogr. 2007, 21, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Vuppala, P.K.; Boddu, S.P.; Furr, E.B.; McCurdy, C.R.; Avery, B.A. A Very Simple, Sensitive, High-Throughput Method for the Quantification of Mitragynine in Rat Plasma Using UPLC-MS and Its Application to an Intravenous Pharmacokinetic Study. Chromatographia 2011, 74, 703–710. [Google Scholar] [CrossRef]
- Macko, E.; Weisbach, J.A.; Douglas, B. Some observations on the pharmacology of mitragynine. Arch. Int. Pharmacodyn. Ther. 1972, 198, 145–161. [Google Scholar] [PubMed]
- Hassan, Z.; Mustapha, M.; Visweswaran, N.; Yusoff, N.H.M.; Suhaimi, F.W.; Rajakumar, V.; Vicknasingam, B.K.; Davide, A.; von Horsten, S.; Ismail, N.I.W.; et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar]
- Albert, A.; Serjeant, E.P. The Determination of Ionisation Constants; Chapman and Hall Ltd: London, UK, 1962; pp. 44–64. [Google Scholar]
- Predicted Properties: Biological Chemical Density Lipinski and Related Spectra Structure-Related Thermal. Calculated Using Advanced Chemistry Development (ACD/Laboratories, Toronto, ON, Canada) software, version 11.02. Available online: https://scifinder.cas.org/ (accessed on 5 March 2015).
- Bhagav, P.; Deshpande, P.; Pandey, S.; Chandran, S. Development and Validation of Stability Indicating UV Spectrophotometric Method for the Estimation of Brimonidine Tartrate in Pure Form, Formulations and Preformulation Studies. Pharm. Lett. 2010, 2, 106–122. [Google Scholar]
- Singh, S.; Sharda, N.; Mahajan, L. Spectrophotometric determination of pKa of nimesulide. Int. J. Pharm. 1999, 176, 261–264. [Google Scholar] [CrossRef]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2013, 5, 376–389. [Google Scholar] [CrossRef]
- Chan, K.B.; Pakiam, C.; Rahim, R.A. Psychoactive plant abuse: The identification of mitragynine in ketum and in ketum preparations. Bull. Narc. 2005, LVII, 249–256. [Google Scholar]
- Sabetghadam, A.; Ramanathan, S.; Mansor, S.M. The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharmacogn. Res. 2010, 2, 181–185. [Google Scholar]
- Parthasarathy, S.; Ramanathan, S.; Murugaiyah, V.; Hamdan, M.R.; Said, M.I.; Lai, C.S.; Mansor, S.M. A simple HPLC–DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for the application in forensic investigation. Forensic Sci. Int. 2013, 226, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A. Absorption and Drug Development; John Wiley& Sons Inc.: Hoboken, NJ, USA, 2003; pp. 42–66. [Google Scholar]
- Barthe, L.; Woodley, J.; Houin, G. Gastrointestinal absorption of drugs: Methods and studies. Fundam. Clin. Pharmacol. 1999, 13, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.S. The effect of mitragynine on man. Br. J. Med. Psychol. 1932, 12, 41–58. [Google Scholar] [CrossRef]
- Havemann-Reineck, U. Kratom and alcohol dependence: Clinical symptoms, withdrawal treatment and pharmacological mechanism—A case report. Eur. Psychiatry 2011, 26 (Suppl. S1). [Google Scholar] [CrossRef]
- Watanabe, K.; Yano, S.; Horie, S.; Yamamoto, L.T.; Takayama, H.; Aimi, N.; Sakai, S.; Ponglux, D.; Tongroach, P.; Shan, J.; et al. Pharmacological properties of some structurally related indole alkaloids contained in the Asian herbal medicines, hirsutine and mitragynine, with special reference to their Ca2+ antagonistic and opioid-like effects. In Pharmacological Research on Traditional Herbal Medicines; Watanabe, H., Shibuya, T., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 163–177. [Google Scholar]
- Manda, V.K.; Avula, B.; Ali, Z.; Khan, I.A.; Walker, LA.; Khan, S.I. Evaluation of In vitro Absorption, Distribution, Metabolism, and Excretion (ADME) Properties of Mitragynine, 7-Hydroxymitragynine, and Mitraphylline. Planta Med. 2014, 80, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2014, 65, 315–499. [Google Scholar] [CrossRef]
- Goh, T.; Mohamad, R.H.; Mohammad, J.S.; Mohd, N.M.; Sharif, M.M. A simple and cost effective isolation and purification protocol of mitragynine from Mitragyna speciosa Korth (Ketum) Leaves. MJAS 2011, 15, 54–60. [Google Scholar]
- Low, B.S.; Ng, B.H.; Choy, W.P.; Yuen, K.H.; Chan, K.L. Bioavailability and pharmacokinetic studies of eurycomanone from Eurycoma longifolia. Planta Med. 2005, 71, 803–807. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeia XXVIII and National Formulary XVIII; The US Pharmacopoeial Convention: Rockville, MD, USA, 2005; pp. 2854–2855.
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanathan, S.; Parthasarathy, S.; Murugaiyah, V.; Magosso, E.; Tan, S.C.; Mansor, S.M. Understanding the Physicochemical Properties of Mitragynine, a Principal Alkaloid of Mitragyna speciosa, for Preclinical Evaluation. Molecules 2015, 20, 4915-4927. https://doi.org/10.3390/molecules20034915
Ramanathan S, Parthasarathy S, Murugaiyah V, Magosso E, Tan SC, Mansor SM. Understanding the Physicochemical Properties of Mitragynine, a Principal Alkaloid of Mitragyna speciosa, for Preclinical Evaluation. Molecules. 2015; 20(3):4915-4927. https://doi.org/10.3390/molecules20034915
Chicago/Turabian StyleRamanathan, Surash, Suhanya Parthasarathy, Vikneswaran Murugaiyah, Enrico Magosso, Soo Choon Tan, and Sharif Mahsufi Mansor. 2015. "Understanding the Physicochemical Properties of Mitragynine, a Principal Alkaloid of Mitragyna speciosa, for Preclinical Evaluation" Molecules 20, no. 3: 4915-4927. https://doi.org/10.3390/molecules20034915
APA StyleRamanathan, S., Parthasarathy, S., Murugaiyah, V., Magosso, E., Tan, S. C., & Mansor, S. M. (2015). Understanding the Physicochemical Properties of Mitragynine, a Principal Alkaloid of Mitragyna speciosa, for Preclinical Evaluation. Molecules, 20(3), 4915-4927. https://doi.org/10.3390/molecules20034915






