Lectins with Potential for Anti-Cancer Therapy
Abstract
:1. Introduction
| Lectin | Cancer Cell Lines Affected | Effector Mechanism(s) |
|---|---|---|
| Galectins [8,9,10,11,12] | Epidermal keratinocytes, 1299 lung cells, fibroblast cells, thyroid cells, colon cells, prostate cells | T-cell binding, specific integrin binding, Ca2+-calpaincaspase-1 pathway |
| C-Type Lectins [13,14] | SW1116 colorectal cells | Le glycan recognition, perforin granzyme pathway, TRAIL and FAS ligand binding |
| Annexins [15,16,17,18,19,20] | Melanoma cells, lung cells | NF-κB signal transduction pathway, Ras-Raf-MAPK pathway, p53 apoptotic pathway |
| Sialic acid binding Haliotis discus discus lectin (HddSBL) [21,22] | Hep3B hepatocellular cells, SW480 colorectal cells, A549 and H1299 lung cancer cell line cells | Bcl-2 down-regulation |
| Polygonatum odoratum lectin (POL) [23,24,25,26] | A549 lung cells, L929 murine fibrosarcoma cells | Akt-mTOR pathway, Fas mediating apoptotic pathway, TNFα enhancement |
| Mistletoe lectin [7,27,28,29,30,31,32,33] | Hepatocarcinoma cells, breast cancer cells, NALM-6 acute lymphoblastic leukemia cells, glioblastoma cells, hepatomacarcinoma cells, peripheral blood mononuclear cells, A253 epidermoid cells | Wnt signaling, miR-135a & b, NK-mediated cell lysis, interleukin mRNA activation |
| Concanavalin A (ConA) [34,35,36,37,38,39,40] | A375 and B16 melanoma cells, fibroblast 3T3 cells, colorectal cancer cells | Mitochondrial apoptotic pathway, caspase induction |
2. Galectin
3. C-Type Lectins
4. Annexin
5. Other Animal Lectins
6. Marine Animal Lectins
6.1. Sialic Acid Binding Haliotis Discus Discus Lectin (HddSBL)
6.2. Fucose-Binding Dicentrarchus Labrax Lectin (DIFBL)
6.3. Rhamnose-Binding Strongylocentrotus Purpuratus Lectin (SpRBL)
7. Other Plant Lectins
7.1. Polygonatum Odoratum Lectin (POL)
7.2. Mistletoe (Viscum Album) Lectin
7.4. Soybean (Glycine Max) Lectin
7.5. Clematis Montana Lectin
7.6. Sclerotium Rolfsii Lectin
8. Cellular Targeting of Lectins
| Lectin | Cellular Target |
|---|---|
| Galectins [11,60,61] | Galectin 1: α5β1 integrin |
| Galectin 3: oncogenic K-Ras protein | |
| Galectin 9: antigens presented on T-cell, Ca2+ levels, calpain and caspase-1 | |
| C-Type Lectins [62] | Myeloid C-type lectin receptors |
| Annexins [63] | Bax and caspase-3 |
| Sialic acid binding Haliotis discus discus lectin (HddSBL) [22] | Bcl-2 |
| Polygonatum odoratum lectin (POL) [26] | Bcl-3 and LC3 |
| Mistletoe Lectin [30,64] | Caspase-8, caspase-9, caspase-3, Bcl-2, and telomerase activity |
| Concanavalin A (ConA) [36,65] | Surface glycoproteins such as mannose sugars, matrix metalloproteinase, cytochrome c, and caspase-3 and -9 |
9. Lectins for Apoptosis-Induced Chemotherapy
10. Autophagy: Another Possibility for Lectin-Mediated Cancer Therapy
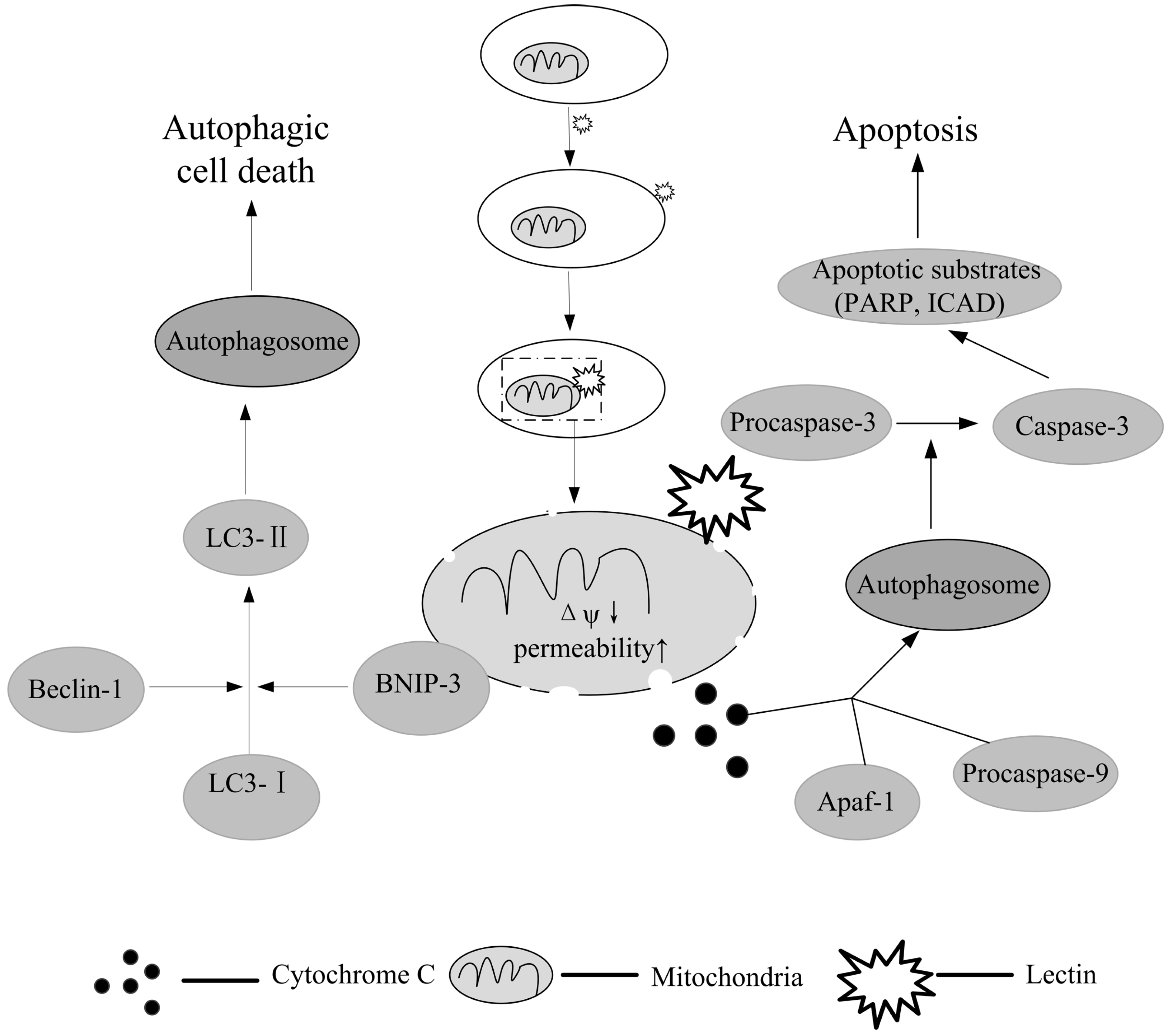
11. Conclusions
Author Contributions
Conflicts of Interest
References
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Section 11.4, Lectins Are Specific Carbohydrate-Binding Proteins. In Biochemistry, 5th ed.; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Section 4.1, The purification of proteins is an essential first step in understanding their function. In Biochemistry, 5th ed.; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Goldberg, R.B.; Hoschek, G.; Vodkin, L.O. An insertion sequence blocks the expression of a soybean lectin gene. Cell 1983, 33, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rao, M.V.; Tweardy, D.J.; Prakash, M.; Galili, U.; Gorelik, E. Lectin-induced apoptosis of tumour cells. Glycobiology 1993, 3, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Hollville, E.; Martin, S.J. Measuring apoptosis by microscopy and flow cytometry. Methods 2003, 61, 90–97. [Google Scholar] [CrossRef]
- Fu, L.L.; Zhao, X.; Xu, H.L.; Wen, X.; Wang, S.Y.; Liu, B.; Bao, J.K.; Wei, Y.Q. Identification of microRNA-regulated autophagic pathways in plant lectin-induced cancer cell death. Cell Prolif. 2012, 45, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Zhang, H.D.; Zhi, R.; Yuan, S.J. Down-regulation of some miRNAs by degrading their precursors contributes to anti-cancer effect of mistletoe lectin-I. Br. J. Pharmacol. 2011, 162, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Raz, A. Biological modulation by lectins and their ligands in tumor progression and metastasis. AntiCancer Agents Med. Chem. 2008, 8, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Hadari, Y.R.; Arbel-Goren, R.; Levy, Y.; Amsterdam, A.; Alon, R.; Zakut, R.; Zick, Y. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J. Cell Sci. 2000, 113, 2385–2397. [Google Scholar] [PubMed]
- Fischer, C.; Sanchez-Ruderisch, H.; Welzel, M.; Wiedenmann, B.; Sakai, T.; André, S.; Gabius, H.-J.; Khachigian, L.; Detjen, K.; Rosewicz, S. Galectin-1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 2005, 9, 37266–37277. [Google Scholar] [CrossRef]
- Bernerd, F.; Sarasin, A.; Magnaldo, T. Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 11329–11334. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Ma, B.Y.; Murai, R.; Nakamura, N.; Baba, M.; Kawasaki, N.; Hodohara, K.; Asano, S.; Kawasaki, T. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells. J. Immunol. 2008, 180, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Lundqvist, A.; Childs, R.W. Bringing natural killer cells to the clinic: Ex vivo manipulation. Cytotherapy 2008, 10, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Q.; Peng, H.; Zhang, W. Animal lectins: potential antitumor therapeutic targets in apoptosis. Appl. Biochem. Biotechnol. 2012, 168, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Zhao, W.; Rigas, B. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: Anticancer effects in vitro and in vivo. Cancer Res. 2010, 70, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signalling pathway in cancer therapy. Expert Opin. Ther. Target 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Fleet, A.; Ashworth, R.; Kubista, H.; Edwards, H.; Bolsover, S.; Mobbs, P.; Moss, S.E. Inhibition of EGF-dependent calcium influx by annexin VI is splice form-specific. Biochem. Biophys. Res. Commun. 1999, 260, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Francia, G.; Mitchell, S.D.; Moss, S.E.; Hanby, A.M.; Marshall, J.F.; Hart, I.R. Identification by differential display of annexin-VI, a gene differentially expressed during melanoma progression. Cancer Res. 1996, 56, 3855–3858. [Google Scholar] [PubMed]
- Srivastava, M.; Bubendorf, L.; Srikantan, V.; Fossom, L.; Nolan, L.; Glasman, M.; Leighton, X.; Fehrle, W.; Pittaluga, S.; Raffeld, M.; et al. ANX7, a candidate tumor suppressor gene for prostate cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 4575–4580. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Whang, I.; Lee, J. A novel C-type lectin from abalone, Haliotis discus discus, agglutinates Vibrio alginolyticus. Dev. Comp. Immunol. 2008, 32, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, L.; Duan, X.; Cui, L.; Luo, J.; Li, G. Adenovirus carrying gene coding Haliotis discus discus sialic acid binding lectin induces cancer cell apoptosis. Mar. Drugs 2014, 127, 3994–4004. [Google Scholar] [CrossRef]
- Wu, L.; Bao, J.K. Anti-tumor and anti-viral activities of Galanthus nivalis agglutinin (GNA)-related lectins. Glycoconj. J. 2013, 30, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, J.; Lu, B.; Shi, Z.; Wang, H.; Zhang, B.; Zhao, K.; Qi, W.; Bao, J.; Wang, Y. Molecular switch role of Akt in Polygonatum odoratum lectin-induced apoptosis and autophagy in human non-small cell lung cancer A549 cells. PLoS One 2014, 9, e101526. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, B.; Min, M.W.; Bian, H.J.; Chen, L.F.; Liu, Q.; Bao, J.K. Induction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cells. Biochim. Biophys. Acta 2009, 1790, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Chen, Y.; Wang, X.Y.; Lu, R.F.; Zhang, S.Y.; Tian, M.; Xie, T.; Liu, B.; He, G. Polygonatum odoratum lectin induces apoptosis and autophagy via targeting EGFR-mediated Ras-Raf-MEK-ERK pathway in human MCF-7 breast cancer cells. Phytomedicine 2014, 21, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Park, W.B. Effects of Korean mistletoe lectin (Viscum album coloratum) on proliferation and cytokine expression in human peripheral blood mononuclear cells and T-lymphocytes. Arch. Pharmacal Res. 2007, 30, 1252–1264. [Google Scholar] [CrossRef]
- Podlech, O.; Harter, P.N.; Mittelbronn, M.; Poschel, S.; Naumann, U. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid. Based Complement. Altern. Med. 2012, 2012, 501796. [Google Scholar]
- Hong, C.E.; Park, A.K.; Lyu, S.Y. Synergistic anticancer effects of lectin and doxorubicin in breast cancer cells. Mol. Cell Biochem. 2014, 94, 225–235. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Choi, S.H.; Park, W.B. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharmacal Res. 2002, 25, 93–101. [Google Scholar] [CrossRef]
- Seifert, G.; Jesse, P.; Laengler, A.; Tobias Reindl, T.; Lüth, M.; Lobitz, S.; Henze, G.; Prokopa, A.; Lode, H.N. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 2008, 264, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Zwierzina, H.; Bergmann, L.; Fiebig, H.; Aamdal, S.; Schoffski, P.; Witthohn, K.; Lentzen, H. The preclinical and clinical activity of aviscumine: A potential anticancer drug. Eur. J. Cancer 2011, 47, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, U.; Feldhaus, S.; Mengs, U. Recombinant mistletoe lectin (rML) is successful in treating human ovarian cancer cells transplanted into severe combined immunodeficient (SCID) mice. Cancer Lett. 2000, 150, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.Y.; Chang, C.P. Induction of autophagy by Concanavalin A and its application in anti-tumor therapy. Autophagy 2007, 4, 402–404. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.Y.; Bian, H.J.; Min, M.W.; Chen, L.F.; Bao, J.K. Antiproliferative activity and apoptosis-inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 2009, 482, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Min, M.W.; Bao, J.K. Induction of apoptosis by Concanavalin A and its molecular mechanisms in cancer cells. Autophagy 2009, 5, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.Y.; Chang, C.P. Lectin of Concanavalin A as an anti-hepatoma therapeutic agent. J. Biomed. Sci. 2009, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Roy, R.; Annabi, B. Concanavalin-A-induced autophagy biomarkers requires membrane type-1 matrix metalloproteinase intracellular signaling in glioblastoma cells. Glycobiology 2012, 22, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Xu, H.L.; Liu, B.; Bao, J.K. Concanavalin A from an old protein to novel candidate anti-neoplastic drug. Curr. Mol. Pharmacol. 2010, 5, 123–128. [Google Scholar] [CrossRef]
- Roy, B.; Pattanaik, A.K.; Das, J.; Bhutia, S.K.; Behera, B.; Singh, P.; Maiti, T.K. Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem. Biol. Interact. 2014, 210, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nangia-Makker, P.; Balan, V.; Hogan, V.; Raz, A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010, 1, e101. [Google Scholar] [CrossRef] [PubMed]
- Pace, K.E.; Lee, C.; Stewart, P.L.; Baum, L.G. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J. Immunol. 1999, 163, 3801–3811. [Google Scholar] [PubMed]
- Matarrese, P.; Tinari, A.; Mormone, E.; Bianco, G.A.; Toscano, M.A.; Ascione, B.; Rabinovich, G.A.; Malorni, W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J. Biol. Chem. 2005, 280, 6969–6985. [Google Scholar] [CrossRef] [PubMed]
- Earl, L.A.; Bi, S.; Baum, L.G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 2010, 285, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.; Bratman, S.V.; Shultz, D.B.; von Eyben, R.; Chan, C.; Wang, Z.; Say, C.; Gupta, A.; Loo, B.W., Jr.; Giaccia, A.J.; et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin. Cancer Res. 2014, 20, 5558–5569. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.H.; Wang, S.Q.; Zhang, M.H.; Yu, F.B.; Zhang, L.; Yu, Z.X.; Kuang, Y. Knockdown of galectin-1 suppresses the growth and invasion of osteosarcoma cells through inhibition of the MAPK/ERK pathway. Oncol. Rep. 2014, 32, 1497–1504. [Google Scholar] [PubMed]
- Wells, V.; Mallucci, L. Phosphoinositide 3-kinase targeting by the beta galactoside binding protein cytokine negates akt gene expression and leads aggressive breast cancer cells to apoptotic death. Breast Cancer Res. 2009, 11, R2. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.G.; Cammarata, M.; Benenati, G.; Salerno, G.; Mangano, V.; Vizzini, A.; Parrinello, N. A serum fucose-binding lectin (DlFBL) from adult Dicentrarchus labrax is expressed in larva and juvenile tissues and contained in eggs. Cell Tissue Res. 2010, 341, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, X.; Duan, X.; Cui, L.; Li, G. Exogenous expression of marine lectins DlFBL and SpRBL induces cancer cell apoptosis possibly through PRMT5-E2F-1 pathway. Sci. Rep. 2014, 4, 4505. [Google Scholar] [PubMed]
- Jiang, Q.L.; Zhang, S.; Tian, M.; Zhang, S.Y.; Xie, T.; Chen, D.Y.; Chen, Y.J.; He, J.; Liu, J.; Ouyang, L.; et al. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015, 48, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Lei, H.Y. Autophagy induction in T cell-independent acute hepatitis induced by concanavalin A in SCID/NOD mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 817–826. [Google Scholar] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Behera, B.; Bhol, C.S.; Dey, S.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K.; et al. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Lv, H.; Wang, Y.; Liu, Y.H.; Li, C.Y.; Meng, L.; Chen, F.; Bao, J.K. Clematis montana lectin, a novel mannose-binding lectin from traditional Chinese medicine with antiviral and apoptosis-inducing activities. Peptides 2009, 30, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, B.; Qi, W.; Zhu, Y.; Zhao, Y.; Zhou, N.; Sun, R.; Bao, J.; Wu, C. Conformational study reveals amino acid residues essential for hemagglutinating and anti-proliferative activities of Clematis montana lectin. Acta Biochim. Biophys. Sin. (Shanghai) 2014, 46, 923–934. [Google Scholar] [CrossRef]
- Savanur, M.A.; Eligar, S.M.; Pujari, R.; Chen, C.; Mahajan, P.; Borges, A.; Shastry, P.; Ingle, A.; Kalraiya, R.D.; Swamy, B.M.; et al. Sclerotium rolfsii Lectin Induces Stronger Inhibition of Proliferation in Human Breast Cancer Cells than Normal Human Mammary Epithelial Cells by Induction of Cell Apoptosis. PLoS One 2014, 9, e110107. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2002, 2, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; An, N.; Zhao, S.; Li, X.; Bao, J.K.; Yue, B.S. In silico analysis of molecular mechanisms of legume lectin-induced apoptosis in cancer cells. Cell Prolif. 2013, 46, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Yang, M.C.; Liu, H.S.; Lin, Y.S.; Lei, H.Y. Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology 2007, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Shalom-Feuerstein, R.; Cooks, T.; Raz, A.; Kloog, Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005, 65, 7292–7300. [Google Scholar] [CrossRef] [PubMed]
- Kashio, Y.; Nakamura, K.; Abedin, M.J.; Seki, M.; Nishi, N.; Yoshida, N.; Nakamura, T.; Hirashima, M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J. Immunol. 2003, 170, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Sancho, D.; Reis e Sousa, C. Sensing of cell death by myeloid C-type lectin receptors. Curr. Opin. Immunol. 2013, 25, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Nogueira, C.R.; Tavares, L.P.; Soriani, F.M.; Lopes, F.; Russo, R.C.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Annexin A1 modulates natural and glucocorticoid induced resolution of inflammation by enhancing neutrophil apoptosis. J. Leukoc. Biol. 2012, 92, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Engels, I.H.; Voelter, W.; Schulze-Osthoff, K.; Wesselborg, S. Mistletoe lectin activates caspase-8/FLICE independently of death receptor signaling and enhances anticancer drug-induced apoptosis. Cancer Res. 1999, 59, 2083–2090. [Google Scholar] [PubMed]
- Amin, A.R.; Paul, R.K.; Thakur, V.S.; Agarwal, M.L. A novel role for p73 in the regulation of Akt-Foxo1a-Bim signaling and apoptosis induced by the plant lectin, Concanavalin A. Cancer Res. 2007, 67, 5617–5621. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A.; Raak, C.; Ostermann, T. Quality of life and related dimensions in cancer patients treated with mistletoes extract (Iscador): A meta-analysis. Evid. Based Complement. Altern. Med. 2012, 2012, 219402. [Google Scholar]
- Ernst, E.; Schmidt, K.; Steuer-Vogt, M.K. Mistletoe for cancer? A systematic review of randomised clinical trials. Int. J. Cancer 2003, 107, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Horneber, M.A.; Bueschel, G.; Huber, R.; Linde, K.; Rostock, M. Mistletoe therapy in oncology. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Ziegler, R.; Grossarth-Maticek, R. Individual patient data meta-analysis of survival and psychosomatic self-regulation from published prospective controlled cohort studies for long-term therapy of breast cancer patients with a mistletoe preparation (Iscador). Evid. Based Complement. Altern. Med. 2010, 7, 157–166. [Google Scholar] [CrossRef]
- Marvibaigi, M.; Supriyanto, E.; Amini, N.; Abdul Majid, F.A.; Jaganathan, S.K. Preclinical and clinical effects of mistletoe against breast cancer. BioMed Res. Int. 2014, 2014, 785479. [Google Scholar] [CrossRef] [PubMed]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur. J. Cancer 2013, 49, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Yook, J.H.; Eisenbraun, J.; Kim, B.S.; Huber, R. Quality of life, immunomodulation and safety of adjuvant mistletoe treatment in patients with gastric carcinoma—A randomized, controlled pilot study. BMC Complement. Altern. Med. 2012, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Eisenbraun, J.; Scheer, R.; Kröz, M.; Schad, F.; Huber, R. Quality of life in breast cancer patients during chemotherapy and concurrent therapy with a mistletoe extract. Phytomedicine 2011, 18, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Friedel, W.E.; Matthes, H.; Bock, P.R.; Zänker, K.S. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: multicenter, controlled, observational cohort study. J. Soc. Integr. Oncol. 2009, 7, 137–145. [Google Scholar] [PubMed]
- Bar-Sela, G.; Wollner, M.; Hammer, L.; Agbarya, A.; Dudnik, E.; Haim, N. Mistletoe as complementary treatment in patients with advanced non-small-cell lung cancer treated with carboplatin-based combinations: A randomised phase II study. Eur. J. Cancer 2013, 49, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Beuth, J.; Schneider, B.; Schierholz, J.M. Impact of complementary treatment of breast cancer patients with standardized mistletoe extract during aftercare: A controlled multicenter comparative epidemiological cohort study. Anticancer Res. 2008, 28, 523–527. [Google Scholar] [PubMed]
- Miyagi, T.; Takehara, T.; Tatsumi, T.; Suzuki, T.; Jinushi, M.; Kanazawa, Y.; Hiramatsu, N.; Kanto, T.; Tsuji, S.; Hori, M.; et al. Concanavalin A injection activates intrahepatic innate immune cells to provoke an antitumor effect in murine liver. Hepatology 2004, 40, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Bevier, M.; Huhn, S.; Sainz, J.; Lascorz, J.; Pardini, B.; Naccarati, A.; Vodickova, L.; Novotny, J.; Hemminki, K.; et al. Genetic variants in C-type lectin genes are associated with colorectal cancer susceptibility and clinical outcome. Int. J. Cancer 2013, 133, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Newlaczyl, A.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Hippert, M.M.; O’Toole, P.S.; Thorburn, A. Autophagy in cancer: Good, bad, or both? Cancer Res. 2006, 66, 9349–9351. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. In Autophagosome and Phagosome (Methods in Molecular Biology, Book 445); Deretic, V., Ed.; Humana Press: New York, NY, USA, 2008; pp. 77–88. [Google Scholar]
- Levine, B. Cell biology: Autophagy and cancer. Nature 2007, 446, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Karantza-Wadsworth, V. Role and regulation of autophagy in cancer. Biochim. Biophys. Acta 2009, 1793, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS One 2014, 9, e102535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ney, P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Ghislat, G.; Aguado, C.; Knecht, E. Annexin A5 stimulates autophagy and inhibits endocytosis. J. Cell Sci. 2012, 125, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cheng, Y.; Zhang, B.; Bian, H.J.; Bao, J.K. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS–p38–p53 pathway. Cancer Lett. 2009, 75, 54–60. [Google Scholar] [CrossRef]
- Liu, H.; He, Z.; Simon, H.U. Protective role of autophagy and autophagy-related protein 5 in early tumorigenesis. J. Mol. Med. (Berl) 2015, 93, 159–164. [Google Scholar] [CrossRef]
- Gaafar, R.; Abdel Rahman, A.R.; Aboulkasem, F.; El Bastawisy, A. Mistletoe preparation (Viscum Fraxini-2) as palliative treatment for malignant pleural effusion: A feasibility study with comparison to bleomycin. Ecancermedicalscience 2014, 8, 424. [Google Scholar] [PubMed]
- Mansky, P.J.; Wallerstedt, D.B.; Sannes, T.S.; Stagl, J.; Johnson, L.L.; Blackman, M.R.; Grem, J.L.; Swain, S.M.; Monahan, B.P. NCCAM/NCI Phase 1 Study of mistletoe extract and gemcitabine in patients with advanced solid tumors. Evid. Based Complement. Altern. Med. 2013, 2013, 964592. [Google Scholar]
- Kirsch, A.; Hajto, T. Case reports of sarcoma patients with optimized lectin-oriented mistletoe extract therapy. J. Altern. Complement. Med. 2011, 17, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Grugel, R.; Kiene, H. Safety of higher dosages of Viscum album L. in animals and humans—Systematic review of immune changes and safety parameters. BMC Complement. Altern. Med. 2011, 11, 72. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with Potential for Anti-Cancer Therapy. Molecules 2015, 20, 3791-3810. https://doi.org/10.3390/molecules20033791
Yau T, Dan X, Ng CCW, Ng TB. Lectins with Potential for Anti-Cancer Therapy. Molecules. 2015; 20(3):3791-3810. https://doi.org/10.3390/molecules20033791
Chicago/Turabian StyleYau, Tammy, Xiuli Dan, Charlene Cheuk Wing Ng, and Tzi Bun Ng. 2015. "Lectins with Potential for Anti-Cancer Therapy" Molecules 20, no. 3: 3791-3810. https://doi.org/10.3390/molecules20033791
APA StyleYau, T., Dan, X., Ng, C. C. W., & Ng, T. B. (2015). Lectins with Potential for Anti-Cancer Therapy. Molecules, 20(3), 3791-3810. https://doi.org/10.3390/molecules20033791





