Toxicity of Amorphigenin from the Seeds of Amorpha fruticosa against the Larvae of Culex pipiens pallens (Diptera: Culicidae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Amorphigenin
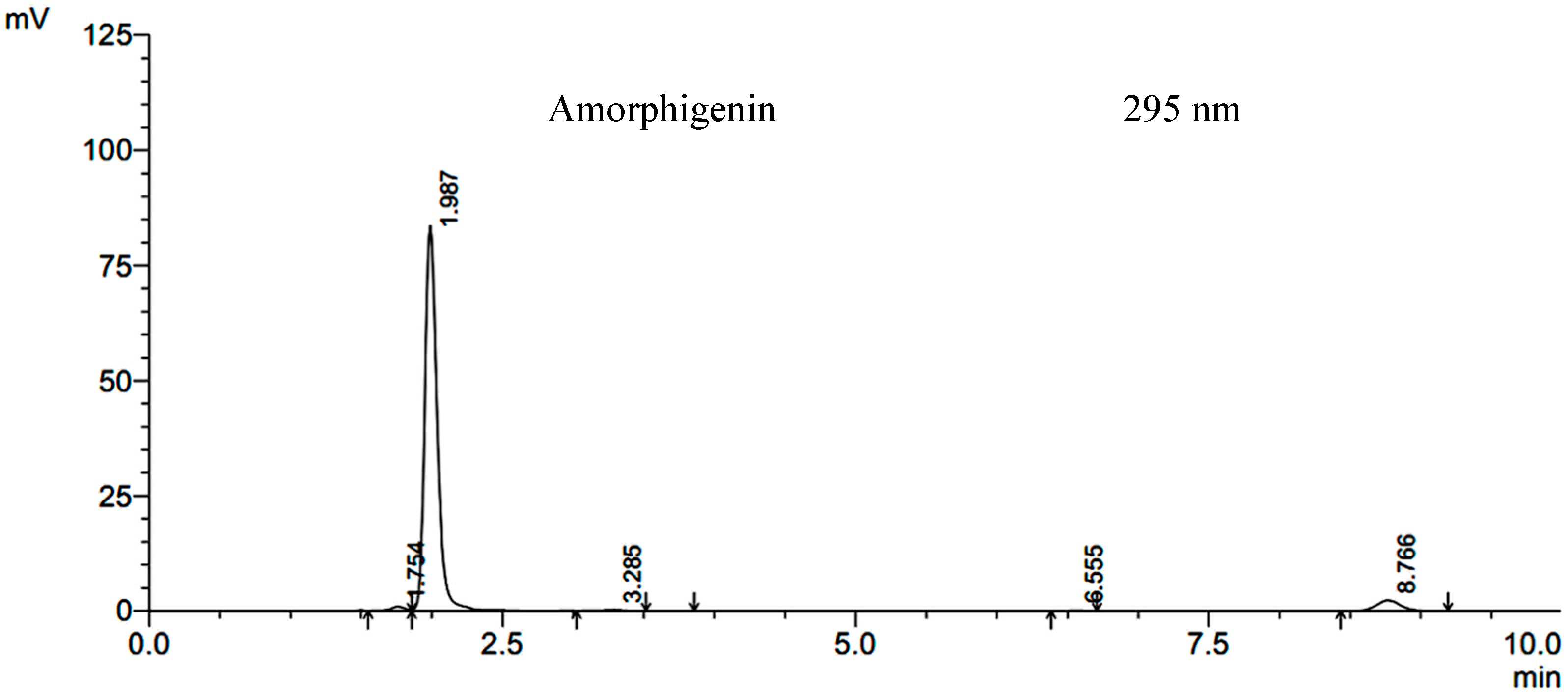

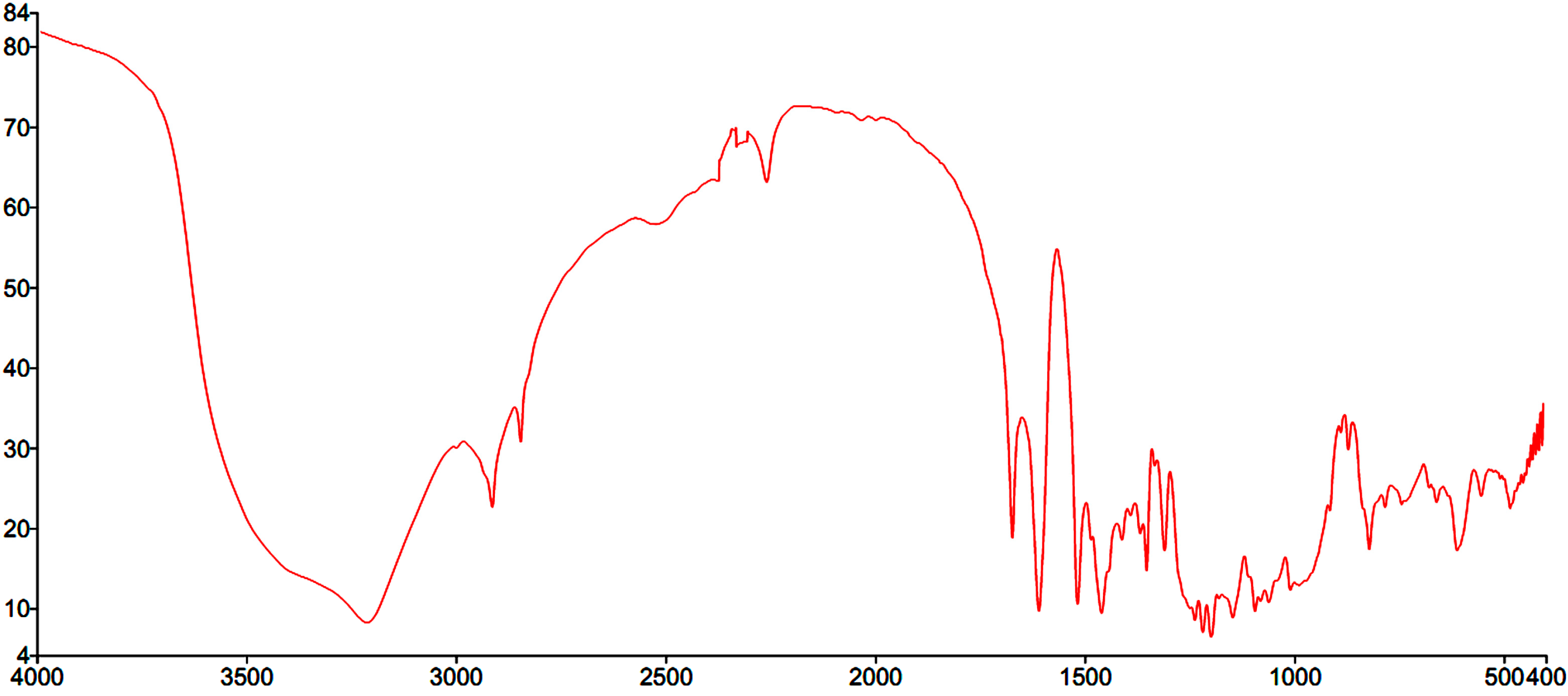

2.2. Effect of Extracts and Amorphigenin on Larval Mortality
| Material | Solvents | % Mortality a(mg/L) ± SE |
|---|---|---|
| Seeds of Amorpha fruticosa | Petroleum ether | 92.33 ± 0.1949 |
| Ethyl acetate | 69.77 ± 1.0632 | |
| Chloroform | 87.89 ± 0.9112 | |
| Acetone | 91.26 ± 0.3515 | |
| Ethanol | 94.21 ± 0.2631 |
| Treatment | LC50 (mg/L) | LCL-UCL a (mg/L) | LC90 (mg/L) | LCL-UCL (mg/L) | Regression Equation | R value |
|---|---|---|---|---|---|---|
| Petroleum ether | 33.02 | 19.73–55.27 | 128.45 | 76.74–215.01 | Y = 1.7008 + 2.1723X | 0.995 |
| Ethyl acetate | 86.16 | 49.78–149.15 | 339.78 | 196.30–588.15 | Y = 0.8375 + 2.1508X | 0.995 |
| Chloroform | 36.43 | 25.68–51.67 | 171.78 | 121.11–243.64 | Y = 2.0287 + 1.9029X | 0.999 |
| Acetone | 34.23 | 17.18–68.20 | 138.19 | 69.35–275.38 | Y = 1.7558 + 2.1144X | 0.992 |
| Ethanol | 22.69 | 11.45–44.96 | 105.78 | 53.39–209.58 | Y = 2.4009 + 1.9169X | 0.979 |
| Amorphigenin | 4.29 | 3.22–5.72 | 11.27 | 8.46–15.01 | Y = 3.0665 + 3.0576X | 0.993 |
| Rotenone | 4.69 | 3.58–6.15 | 12.20 | 9.31–15.99 | Y = 2.9259 + 3.0887X | 0.999 |
| Treatment a | PE | EAC | MT | DMK |
|---|---|---|---|---|
| EAC | 0.681 b | - | - | - |
| MT | 1.018 | 1.538 | - | - |
| DMK | 1.050 | 1.587 | 1.032 | - |
| EA | 1.206 | 1.949 | 1.267 | 1.228 |
2.3. Effect of Amorphigenin in Vivo on Inhibition of Mitochondrial Complex I

2.4. Effect of Amorphigenin on Protein Production

2.5. Discussion
3. Experimental Section
3.1. Plant Materials
3.2. Mosquito Culture
3.3. Preparation of Plant Extracts
3.4. Isolation and Purification of Active Ingredients
3.5. Larvicidal Bioassay
3.6. Assay of Mitochondrial Complex I Activity
3.7. Protein Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, Z.; Li, H.; Simons, F.E.R. Immunoblot analysis of salivary allergens in 10 mosquito species with worldwide distribution and the human ige responses to these allergens. J. Allergy Clin. Immunol. 1998, 101, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Ma, L.; Sun, L.X.; Zhu, C.L. Biotic characteristics in the deltamethrin-susceptible and resistant strains of Culex pipiens pallens (diptera: Culicidae) in china. Appl. Entomol. Zool. 2002, 37, 305–308. [Google Scholar] [CrossRef]
- Jiang, S.F.; Zhao, T.Y.; Zhang, Y.M.; Dong, Y.D.; Guo, X.X. RT-PCR detection of west nile virus in mosquitoes and leghorn chicken infected experimentally. Acta Parasitol. Med. Entomol. Sin. 2006, 13, 21–24. [Google Scholar]
- Hemingway, J.; Beaty, B.J.; Rowland, M.; Scott, T.W.; Sharp, B.L. The innovative vector control consortium: Improved control of mosquito-borne diseases. Trends Parasitol. 2006, 22, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Lin, L.F.; Qiao, C.L.; Xu, Y.; Marquine, M.; Weill, M.; Raymond, M. Insecticide resistance in chinese populations of the Culex pipiens complex through esterase overproduction. Entomol. Exp. Appl. 2006, 120, 211–220. [Google Scholar] [CrossRef]
- Cui, F.; Raymond, M.; Berthomieu, A.; Alout, H.; Weill, M.; Qiao, C.-L. Recent emergence of insensitive acetylcholinesterase in chinese populations of the mosquito Culex pipiens (diptera: Culicidae). J. Med. Entomol. 2006, 43, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Tan, Y.; Qiao, C.-L. Filariasis vector in China: Insecticide resistance and population structure of mosquito Culex pipiens complex. Pest Manag. Sci. 2007, 63, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Chang, K.S.; Lee, W.J.; Ahn, Y.J. Monitoring of insecticide resistance in field-collected populations of Culex pipiens pallens (diptera: Culicidae). J. Asia-Pac. Entomol. 2007, 10, 257–261. [Google Scholar] [CrossRef]
- Shin, E.H.; Kim, N.J.; Kim, H.K.; Park, C.; Lee, D.K.; Ahn, Y.J.; Chang, K.S. Resistance of field-collected populations of Culex pipiens pallens (diptera: Culicidae) to insecticides in the republic of korea. J. Asia-Pac. Entomol. 2012, 15, 1–4. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A.; Conti, B. Eco-friendly control strategies against the asian tiger mosquito, Aedes albopictus (diptera: Culicidae): Repellency and toxic activity of plant essential oils and extracts. Pharmacologyonline 2014, 1, 44–51. [Google Scholar]
- Sukumar, K.; Perich, M.J.; Boobar, L.R. Botanical derivatives in mosquito control: A review. J. Am. Mosq. Control Assoc. 1991, 7, 210–237. [Google Scholar] [PubMed]
- Shaalan, E.A.-S.; Canyon, D.; Younes, M.W.F.; Abdel-Wahab, H.; Mansour, A.-H. A review of botanical phytochemicals with mosquitocidal potential. Environ. Int. 2005, 31, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Wachira, S.; Omar, S.; Jacob, J.; Wahome, M.; Alborn, H.; Spring, D.; Masiga, D.; Torto, B. Toxicity of six plant extracts and two pyridone alkaloids from Ricinus communis against the malaria vector Anopheles gambiae. Parasites Vectors 2014, 7, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Moraes, D. Essential oils and their compounds as Aedes aegypti L. (diptera: Culicidae) larvicides: Review. Parasitol. Res. 2014, 113, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, L.-C.; Hao, W.-B.; Tang, M.; Wan, S.-Q. Larvicidal activity of lansiumamide b from the seeds of Clausena lansium against Aedes albopictus (diptera: Culicidae). Parasitol. Res. 2013, 112, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Park, I.-K.; Kim, E.-H.; Lee, H.-S.; Ahn, Y.-J. Larvicidal activity of medicinal plant extracts against Aedes aegypti, Ochlerotatus togoi, and Culex pipiens pallens (diptera: Culicidae). J. Asia-Pac. Entomol. 2004, 7, 227–232. [Google Scholar] [CrossRef]
- Park, I.-K.; Lee, S.-G.; Shin, S.-C.; Park, J.-D.; Ahn, Y.-J. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. J. Agric. Food Chem. 2002, 50, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Jeon, J.-H.; Lee, H.-S. Larvicidal activity of the active constituent isolated from Tabebuia avellanedae bark and structurally related derivatives against three mosquito species. J. Agric. Food Chem. 2013, 61, 10741–10745. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-K.; Shin, S.-C.; Kim, C.-S.; Lee, H.-J.; Choi, W.-S.; Ahn, Y.-J. Larvicidal activity of lignans identified in Phryma leptostachya var. Asiatica roots against three mosquito species. J. Agric. Food Chem. 2005, 53, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Jang, Y.-S.; Ahn, Y.-J.; Lee, D.-K.; Lee, H.-S. Larvicidal activity of australian and mexican plant extracts against Aedes aegypti and Culex pipiens pallens (diptera: Culicidae). J. Asia-Pac. Entomol. 2002, 5, 227–231. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, J.-R.; Wang, M.; Shu, S.; Ahn, Y.-J. Larvicidal activity of Cnidium monnieri fruit coumarins and structurally related compounds against insecticide-susceptible and insecticide-resistant Culex pipiens pallens and Aedes aegypti. Pest Manag. Sci. 2012, 68, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Baek, B.R.; Yang, Y.C.; Kim, M.K.; Lee, H.S. Larvicidal activity of leguminous seeds and grains against Aedes aegypti and Culex pipiens pallens. J. Am. Mosq. Control Assoc. 2002, 18, 210–213. [Google Scholar] [PubMed]
- Yang, Y.-C.; Lim, M.-Y.; Lee, H.-S. Emodin isolated from Cassia obtusifolia (leguminosae) seed shows larvicidal activity against three mosquito species. J. Agric. Food Chem. 2003, 51, 7629–7631. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Flamini, G.; Fiore, G.; Cioni, P.; Conti, B. Larvicidal and repellent activity of the essential oil of Coriandrum sativum l. (apiaceae) fruits against the filariasis vector Aedes albopictus skuse (diptera: Culicidae). Parasitol. Res. 2013, 112, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Benelli, G.; Flamini, G.; Cioni, P.; Profeti, R.; Ceccarini, L.; Macchia, M.; Canale, A. Larvicidal and repellent activity of Hyptis suaveolens (lamiaceae) essential oil against the mosquito Aedes albopictus skuse (diptera: Culicidae). Parasitol. Res. 2012, 110, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Leonardi, M.; Pistelli, L.; Profeti, R.; Ouerghemmi, I.; Benelli, G. Larvicidal and repellent activity of essential oils from wild and cultivated Ruta chalepensis l. (rutaceae) against Aedes albopictus skuse (diptera: Culicidae), an arbovirus vector. Parasitol. Res. 2013, 112, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Benelli, G.; Leonardi, M.; Afifi, F.; Cervelli, C.; Profeti, R.; Pistelli, L.; Canale, A. Repellent effect of Salvia dorisiana, S. longifolia, and S. Sclarea (Lamiaceae) essential oils against the mosquito Aedes albopictus skuse (diptera: Culicidae). Parasitol. Res. 2012, 111, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Bedini, S.; Cosci, F.; Toniolo, C.; Conti, B.; Nicoletti, M. Larvicidal and ovideterrent properties of neem oil and fractions against the filariasis vector Aedes albopictus (diptera: Culicidae): A bioactivity survey across production sites. Parasitol. Res. 2015, 114, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Conti, B.; Garreffa, R.; Nicoletti, M. Shedding light on bioactivity of botanical by-products: Neem cake compounds deter oviposition of the arbovirus vector Aedes albopictus (diptera: Culicidae) in the field. Parasitol. Res. 2014, 113, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Khandagle, A.; Tare, V.; Raut, K.; Morey, R. Bioactivity of essential oils of Zingiber officinalis and Achyranthes aspera against mosquitoes. Parasitol. Res. 2011, 109, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-S.; Chang, H.-T.; Chang, S.-T.; Tsai, K.-H.; Chen, W.-J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahuman, A.; Gopalakrishnan, G.; Venkatesan, P.; Geetha, K. Isolation and identification of mosquito larvicidal compound from Abutilon indicum (linn.) sweet. Parasitol. Res. 2008, 102, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-guided fractionation and gc/ms fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2008, 57, 464–470. [Google Scholar] [CrossRef]
- Park, H.-M.; Park, I.-K. Larvicidal activity of Amyris balsamifera, Daucus carota and Pogostemon cablin essential oils and their components against Culex pipiens pallens. J. Asia-Pac. Entomol. 2012, 15, 631–634. [Google Scholar] [CrossRef]
- Jang, Y.S.; Jeon, J.H.; Lee, H.S. Mosquito larvicidal activity of active constituent derived from Chamaecyparis obtusa leaves against 3 mosquito species. J. Am. Mosq. Control Assoc. 2005, 21, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, Y.; Wu, L.; Zhang, X. Isolation and insecticidal activity of sesquiterpenes alkaloids from Tripterygium wilfordii hook f. Ind. Crops Prod. 2014, 52, 642–648. [Google Scholar] [CrossRef]
- Xiao, X.-M.; Hu, Z.-N.; Shi, B.-J.; Wei, S.-P.; Wu, W.-J. Larvicidal activity of lignans from Phryma leptostachya l. Against Culex pipiens pallens. Parasitol. Res. 2012, 110, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-Q.; Yue, Y.-D.; Peng, Z.-H.; Hua, R.-M.; Tang, F. Evaluation of extracts from bamboo for biological activity against Culex pipiens pallens. Insect Sci. 2004, 11, 267–273. [Google Scholar] [CrossRef]
- Lee, S.E. Mosquito larvicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum. J. Am. Mosq. Control Assoc. 2000, 16, 245–247. [Google Scholar] [PubMed]
- Sun, L.; Dong, H.; Guo, C.; Qian, J.; Sun, J.; Ma, L.; Zhu, C. Larvicidal activity of extracts of Ginkgo biloba exocarp for three different strains of Culex pipiens pallens. J. Med. Entomol. 2006, 43, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-J.; Kim, N.-J.; Byun, S.-G.; Cho, J.-E.; Chung, K. Larvicidal activity of Kaempferia galanga rhizome phenylpropanoids towards three mosquito species. Pest Manag. Sci. 2008, 64, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Chang, K.S.; Park, C.; Ahn, Y.-J. Larvicidal activity of Asarum heterotropoides root constituents against insecticide-susceptible and -resistant Culex pipiens pallens and Aedes aegypti and Ochlerotatus togoi. J. Agric. Food Chem. 2010, 58, 10001–10006. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Kim, J.R.; Kim, S.I.; Kwon, H.W.; Ahn, Y.J. Enhanced toxicity of binary mixtures of larvicidal constituents from Asarum heterotropoides root to Culex pipiens pallens (diptera: Culicidae). J. Med. Entomol. 2012, 49, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Allen, O.N.; Allen, E.K. The Leguminosae: A Source Book of Characteristics, Uses, and Nodulation; University of Wisconsin Press: Madison, WI, USA, 1981; p. 812. [Google Scholar]
- Qu, X.; Diao, Y.; Zhang, Z.; Wang, S.; Jia, Y. Evaluation of anti-bacterial and wound healing activity of the fruits of Amorpha fruticosa L. Afr. J. Tradit. Complement. Altern Med. 2013, 10, 458–468. [Google Scholar] [PubMed]
- Brett, C.H. Repellent properties of extract of Amorpha fruticosa. J. Econ. Entomol. 1946, 39, 810–810. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.H. Insecticidal properties of the indigobush (Amorpha fruticosa). J. Agric. Res. 1946, 73, 81–96. [Google Scholar] [PubMed]
- Ji, M.; Liu, C.; Li, X.; Liu, D.; Wu, D.; Wang, Y. Insecticidal and antifeeding activity of seeds of Amorpha fruticosa against Schizaphis graminum. Jiangsu Agric. Sci. 2011, 39, 208–210. [Google Scholar]
- Sariaslani, F.S.; Rosazza, J.P. Microbial transformations of natural antitumor agents: Products of rotenone and dihydrorotenone transformation by Cunninghamella blakesleeana. Appl. Environ. Microbiol. 1983, 45, 616–621. [Google Scholar] [PubMed]
- Abe, F.; Donnelly, D.M.X.; Moretti, C.; Polonsky, J. Isoflavanoid constituents from Dalbergia monetaria. Phytochemistry 1985, 24, 1071–1076. [Google Scholar] [CrossRef]
- Kondratenko, E.S.; Kasymov, A.U.; Abubakirov, N.K. Structure of amorphigenin. Chem. Nat. Compd. 1967, 3, 260–262. [Google Scholar] [CrossRef]
- Kasymov, A.U.; Kondratenko, E.S.; Abubakirov, N.K. Stepwise hydrolysis of amorphin. Chem. Nat. Compd. 1970, 6, 482–482. [Google Scholar] [CrossRef]
- Crombie, L.; Dewick, P.M.; Whiting, D.A. Biosynthesis of rotenoids. Chalcone, isoflavone, and rotenoid stages in the formation of amorphigenin by Amorpha fruticosa seedlings. J. Chem. Soc. Perkin Trans. 1 1973, 1285–1294. [Google Scholar] [CrossRef]
- Kadyrova, F.R.; Shamsutdinov, M.R.I.; Shakirov, T.T. The isolation of fruticin from the seeds of Amorpha fruticosa. Chem. Nat. Compd. 1973, 9, 107–107. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.K.; Chang, J.J.; McPhail, A.T.; McPhail, D.R.; Terada, H.; Konoshima, T.; Kokumai, M.; Kozuka, M.; Estes, J.R.; et al. Antitumor agents, 138. Rotenoids and isoflavones as cytotoxic constitutents from Amorpha fruticosa. J. Nat. Prod. 1993, 56, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Terada, H.; Kokumai, M.; Kozuka, M.; Tokuda, H.; Estes, J.R.; Li, L.; Wang, H.K.; Lee, K.H. Studies on inhibitors of skin tumor promotion, xii. Rotenoids from Amorpha fruticosa. J. Nat. Prod. 1993, 56, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kwak, H.B.; Choi, E.Y.; Kim, H.S.; Kim, M.H.; Kim, S.H.; Choi, M.K.; Chun, C.H.; Oh, J.; Kim, J.J. Amorphigenin inhibits osteoclast differentiation by suppressing c-fos and nuclear factor of activated t cells. Anat. Cell Biol. 2010, 43, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kloutek, E.; Popov, A.; Drenska, D.; Uzunov, P. Experimental research on the hepatoprotective activity of flavonoids isolated from Amorpha fructiosa. Eksp. Med. Morfol. 1985, 24, 50–54. [Google Scholar] [PubMed]
- Kim, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Yuk, H.J.; Cho, J.K.; Kim, J.Y.; Kim, K.D.; Lee, W.S.; Park, K.H. Flavanones and rotenoids from the roots of Amorpha fruticosa L. That inhibit bacterial neuraminidase. Food Chem. Toxicol. 2011, 49, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Darrouzet, E.; Issartel, J.-P.; Lunardi, J.; Dupuis, A. The 49-kda subunit of NADH-ubiquinone oxidoreductase (complex I) is involved in the binding of piericidin and rotenone, two quinone-related inhibitors. FEBS Lett. 1998, 431, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, R.M.; Ahammadsahib, K.I.; Gadelhak, G.; McLaughlin, J.L. New inhibitors of complex I of the mitochondrial electron transport chain with activity as pesticides. Biochem. Soc. Trans. 1994, 22, 230–233. [Google Scholar] [PubMed]
- Earley, F.G.; Ragan, C.I. Photoaffinity labelling of mitochondrial NADH dehydrogenase with arylazidoamorphigenin, an analogue of rotenone. Biochem. J. 1984, 224, 525–534. [Google Scholar] [PubMed]
- Rai, M.; Carpinella, M.C. Pesticides based on plant essential oils: from traditional practice to commercialization. In Naturally Occurring Bioactive Compounds; Elsevier Science Ltd: Amsterdam, The Netherlands, 2006; p. 178. [Google Scholar]
- Kishore, N.; Mishra, B.; Tiwari, V.; Tripathi, V.; Lall, N. Natural products as leads to potential mosquitocides. Phytochem. Rev. 2014, 13, 587–627. [Google Scholar] [CrossRef]
- De Oliveira, C.F.R.; Luz, L.A.; Paiva, P.M.G.; Coelho, L.C.B.B.; Marangoni, S.; Macedo, M.L.R. Evaluation of seed coagulant Moringa oleifera Lectin (cmol) as a bioinsecticidal tool with potential for the control of insects. Process Biochem. 2011, 46, 498–504. [Google Scholar] [CrossRef]
- Al-Anizi, A.A.; Hellyer, M.T.; Zhang, D. Toxicity assessment and modelling of Moringa oleifera seeds in water purification by whole cell bioreporter. Water Res. 2014, 56, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Flamini, G.; Cioni, P.; Ceccarini, L.; Macchia, M.; Benelli, G. Mosquitocidal essential oils: Are they safe against non-target aquatic organisms? Parasitol. Res. 2014, 113, 251–259. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Instructions for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticides; WHO/VBC/81.807, Ed.; WHO: Geneva, Switzerland, 1981. [Google Scholar]
- Mahmoudvand, M.; Abbasipour, H.; Garjan, A.; Bandani, A. Sublethal effects of indoxacarb on the diamondback moth, Plutella xylostella (L.) (lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 2011, 46, 75–80. [Google Scholar] [CrossRef]
- Piri, F.; SAhrAGArd, A.; GhAdAMyAri, M. Sublethal effects of spinosad on some biochemical and biological parameters of Glyphodes pyloalis walker (lepidoptera: Pyralidae). Plant Prot. Sci. 2014, 50, 135–144. [Google Scholar]
- Akbar, S.M.; Sharma, H.C.; Jayalakshmi, S.K.; Sreeramulu, K. Methylparathion- and carbofuran-induced mitochondrial dysfunction and oxidative stress in Helicoverpa armigera (noctuidae: Lepidoptera). Pestic. Biochem. Physiol. 2012, 103, 31–37. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Briggs, H.L.; Saborido, A.A.; Bindoff, L.A.; Turnbull, D.M. An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem. Med. Metab. Biol. 1994, 51, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jin-Clark, Y.; Anderson, T.; Zhu, K. Effect of alachlor and metolachlor on toxicity of chlorpyrifos and major detoxification enzymes in the aquatic midge, Chironomus tentans (diptera: Chironomidae). Arch. Environ. Contam. Toxicol. 2008, 54, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds amorphigenin are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Li, X.; Gu, Z.; Qin, P.; Ji, M. Toxicity of Amorphigenin from the Seeds of Amorpha fruticosa against the Larvae of Culex pipiens pallens (Diptera: Culicidae). Molecules 2015, 20, 3238-3254. https://doi.org/10.3390/molecules20023238
Liang Y, Li X, Gu Z, Qin P, Ji M. Toxicity of Amorphigenin from the Seeds of Amorpha fruticosa against the Larvae of Culex pipiens pallens (Diptera: Culicidae). Molecules. 2015; 20(2):3238-3254. https://doi.org/10.3390/molecules20023238
Chicago/Turabian StyleLiang, Yaping, Xiuwei Li, Zumin Gu, Peiwen Qin, and Mingshan Ji. 2015. "Toxicity of Amorphigenin from the Seeds of Amorpha fruticosa against the Larvae of Culex pipiens pallens (Diptera: Culicidae)" Molecules 20, no. 2: 3238-3254. https://doi.org/10.3390/molecules20023238
APA StyleLiang, Y., Li, X., Gu, Z., Qin, P., & Ji, M. (2015). Toxicity of Amorphigenin from the Seeds of Amorpha fruticosa against the Larvae of Culex pipiens pallens (Diptera: Culicidae). Molecules, 20(2), 3238-3254. https://doi.org/10.3390/molecules20023238





