1. Introduction
Phenolic acids (PAs) have varied biological activity in the human body. They help to scavenge free radicals, chelate metal ions, induce changes in enzyme activity and protein availability. It was also confirmed that PAs prevent coronary heart disease, cancer, inflammation and diabetes. Hydroxycinnamic acid and its derivatives (e.g., coumaric, caffeic, ferulic and sinapic acids) are important pharmaceuticals for high blood pressure, stroke prevention and possess antitumor activity [
1,
2,
3].
There are three isomers of coumaric acid,
i.e.
o-,
m- and
p-coumaric acid, that differ in the position of the hydroxyl group substitution on the phenyl group. In Nature the most widespread is the
para-isomer [
4]. Coumaric acids can be found in cereals (barley, rye, corn, oats, rice, wheat), fruits (berries, grapes, apples, currants), legumes (beans, peas), nuts (hazelnut, pecan, peanut, walnut), vegetables (celery, tomato, garlic, carotene), oilseeds (flax, mustard), red wine, beer and tea [
3,
5,
6].
Phenolic acids are responsible for the sour and bitter taste of some food products of plant origin. It was found that the characteristic flavor of products made from flour obtained from maize embryos is the result of the presence of certain PAs, namely: ferulic and
o-and
p-coumaric acids [
7]. Coumaric acid shows lower antioxidant activity than ferulic acid. This is related to the presence of only one hydroxyl group in coumaric acid. In order to increase the antioxidant activity compared to the low-density lipoprotein (LDL) fraction it should be subjected to esterification with tartaric acid. Several papers report on the antioxidative properties of coumaric acids [
8,
9]. Moreover, cinnamic acid and its derivatives:
p-coumaric, ferulic and sinapic acid, are important copolymer building blocks of lignin [
10].
Cinnamic acid is a precursor for lignin biosynthesis, being transformed into 4-coumaric acid (4-hydroxycinnamic acid), ferulic acid (4-hydroxy-3-methoxycinnamic acid), and sinapic acid (4-hydroxy-3,5-dimethoxycinnamic acid) before its incorporation into a lignin polymer.
Pannala
et al. [
11] showed that hydroxycinnamates can interact with peroxynitrite depending on the nature of the ring substitutions and the positions of the hydroxyl groups. Studies on the potency of PAs to inhibit tyrosine nitration by peroxynitrite demonstrated that PAs’ activity decreases in the order: caffeic acid→chlorogenic acid→ferulic acid→
p-coumaric acid→
o-coumaric acid→
m-coumaric acid.
p-Coumaric acid inhibits LDL peroxidation, is shown to be antimutagenic, antigenotoxic, and antimicrobial, inhibits cellular melanogenesis, plays a role in immune regulation in humans and reduces the risk of stomach cancer [
8]. This acid is an instance of the PAs commonly used as additives in chemical, food, health, cosmetic and pharmaceutical industries.
In our earlier works, the influence of various metals and halogens on the electronic structure of the benzoic, salicylic, nicotinic and isonicotinic acids were studied [
12,
13,
14,
15]. Those ligands were treated as models to study enzymes and other biologically important molecules. We studied the relationship between certain parameters (
i.e., ionic potential, electronegativity, atomic mass of the metal or ring substituent) and electronic charge distribution in the whole molecule. In case of those ligands the decrease in the ionic potential of the metal brings about the decrease in the uniform charge distribution in the aromatic ring. In the case of halogens, the ionic potential was even more important than the polarity of the C-X bond. The question arises then if the same or other parameters influence the electronic charge distribution in the case of alkali metal
o-coumarates.
The second aim of this work is to continue earlier research [
16,
17,
18,
19] on a correlation between the molecular structure and electronic charge distribution of phenolic compounds and their biological activity. In one of our papers [
20] the influence of lithium, sodium, potassium, rubidium and cesium on the electronic system of the
p-coumaric (4-hydroxycinnamic) acid was investigated. Experimental and theoretical FT-IR, FT-Raman,
1H- and
13C-NMR spectra of
p-coumaric acid and its salts were registered and analyzed.
In the present paper another isomer trans o-coumaric acid and its lithium, sodium, potassium, rubidium and cesium complexes were synthesized and studied by spectroscopic methods (FT-IR, FT-Raman, UV-VIS, 1H- and 13C-NMR). A logical series of metals was selected, (different radius but the same degree of oxidation). The alkali metals taken to this study meet the following criteria (which are important from the point of view of further possible applications): (1) possibility of practical application because of the good solubility of the alkali metal compounds in water and polar solvents; (2) as small as possible harmfulness to the human body and the natural environment; (3) availability, stability and ease of preparation.
UV-VIS spectroscopy was applied to study/establish the changes in π→π* transition energies and draw conclusions on delocalization energy changes. The antimicrobial activity of the studied compounds was tested against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus vulgaris, Bacillus subtilis and, Candida albicans, which allowed us to draw some correlations between physicochemical parameters and microbial properties of trans o-coumaric acid and alkali metal o-coumarates. Elementary analysis for all synthesized complexes was performed in order to confirm the chemical composition of the tested compounds. The theoretically predicted values were compared with the experimentally measured data and the results were also discussed. Optimized geometrical structures, atomic charges, infrared and NMR spectra of trans o-coumaric acid and Li, Na and K trans o-coumarates were calculated by the B3LYP/6-311++G** method.
2. Results and Discussion
2.1. Calculated Geometrical Structure
Optimized geometrical structures were calculated using B3LYP/6-311++G** quantum chemical method [
21]. The distances between atoms in
trans o-coumaric acid and alkali metal
o-coumarate molecules and the angles between bonds were calculated and the values are presented in
Table 1. The atom numbering is shown in
Figure 1. Changes in the bond distances and angles between bonds suggest that alkali metal cations influence the molecular structure of
trans o-coumaric acid. The bond lengths of C1-C9, C7-C8, C7-O1 and C7-O2 do not change in the series of salts, although they are different from the corresponding values calculated for the 2-hydroxycinnamic acid molecule. Large differences between bonds are also noticed for C7-O2 (it increases by: 0.065 Å (Li); 0.056 Å (Na); 0.058 Å (K)). A slightly lower extension of the C7-C8 bond between carbon atoms of the carboxylic acid and C1-C9 is observed. The bond lengths of C7-O1 in the acid is higher than in the salts (0.006–0.014 Å). The values of the bond lengths in aromatic ring are almost the same in acid and salts.
Table 1.
Distances between atoms (Å), bonds angles (°) and geometric aromaticity indices calculated for trans o-coumaric acid and lithium, sodium and potassium o-coumarates.
Table 1.
Distances between atoms (Å), bonds angles (°) and geometric aromaticity indices calculated for trans o-coumaric acid and lithium, sodium and potassium o-coumarates.
| | Acid | Li | Na | K |
|---|
| Calculated | Experimental [22] | Calculated |
|---|
| Distances [Å] |
| C1-C2 a | 1.412 | 1.401 | 1.411 | 1.412 | 1.411 |
| C2-C3 | 1.396 | 1.389 | 1.396 | 1.397 | 1.396 |
| C3-C4 | 1.389 | 1.368 | 1.390 | 1.391 | 1.391 |
| C4-C5 | 1.396 | 1.379 | 1.396 | 1.397 | 1.395 |
| C5-C6 | 1.387 | 1.379 | 1.388 | 1.389 | 1.389 |
| C6-C1 | 1.406 | 1.394 | 1.405 | 1.407 | 1.405 |
| C1-C9 | 1.460 | 1.466 | 1.464 | 1.466 | 1.466 |
| C7-C8 | 1.471 | 1.460 | 1.481 | 1.343 | 1.497 |
| C8-C9 | 1.344 | 1.337 | 1.343 | 1.501 | 1.341 |
| C2-O3 | 1.365 | 1.353 | 1.368 | 1.369 | 1.370 |
| C7-O1 | 1.364 | 1.329 | 1.278 | 1.270 | 1.272 |
| C7-O2 | 1.211 | 1.229 | 1.276 | 1.266 | 1.268 |
| O3-H2 | 0.963 | 0.790 | 0.963 | 0.965 | 0.962 |
| C3-H3 | 1.086 | 0.904 | 1.086 | 1.086 | 1.087 |
| C4-H4 | 1.084 | 0.989 | 1.084 | 1.084 | 1.084 |
| C5-H5 | 1.083 | 0.917 | 1.083 | 1.083 | 1.084 |
| C6-H6 | 1.083 | 1.082 | 1.083 | 1.083 | 1.083 |
| C8-H7 | 1.083 | 1.059 | 1.084 | 1.085 | 1.085 |
| C9-H8 | 1.085 | 0.613 | 1.084 | 1.084 | 1.084 |
| O1-H1(M) | 0.968 | 0.987 | 1.854 | 2.372 | 2.516 |
| O2-Me | - | - | 1.850 | 2.365 | 2.509 |
| Angles [°] |
| C1-C2-C3 | 120.867 | 120.590 | 121.014 | 121.063 | 121.145 |
| C2-C3-C4 | 120.181 | 119.880 | 120.253 | 120.152 | 120.338 |
| C3-C4-C5 | 120.066 | 120.410 | 119.881 | 119.962 | 119.699 |
| C4-C5-C6 | 119.522 | 120.310 | 119.565 | 119.578 | 119.609 |
| C5-C6-C1 | 121.962 | 120.590 | 122.110 | 122.015 | 122.262 |
| C6-C1-C2 | 117.402 | 118.210 | 117.177 | 117.230 | 116.947 |
| C2-C1-C9 | 119.370 | 118.660 | 119.606 | 119.585 | 119.781 |
| C1-C9-C8 | 126.973 | 126.160 | 126.882 | 127.421 | 126.824 |
| C7-C8-C9 | 120.355 | 120.130 | 121.977 | 122.953 | 122.615 |
| C8-C7-O1 | 111.123 | 112.460 | 118.273 | 116.528 | 116.496 |
| C8-C7-O2 | 126.998 | 126.060 | 121.069 | 119.740 | 119.511 |
| C7-C8-H7 | 116.222 | 116.610 | 114.692 | 114.930 | 114.494 |
| C9-C8-H7 | 123.423 | 123.250 | 123.332 | 122.117 | 122.891 |
| C8-C9-H8 | 116.851 | 117.230 | 116.776 | 116.648 | 116.691 |
| C1-C9-H8 | 116.176 | 86.400 | 116.342 | 115.931 | 116.486 |
| C9-C1-C2 | 119.370 | 118.660 | 119.606 | 119.585 | 119.781 |
| C1-C2-O3 | 119.370 | 116.780 | 117.705 | 117.807 | 117.759 |
| C3-C2-O3 | 119.370 | 122.620 | 121.281 | 121.130 | 121.096 |
| C3-C4-H4 | 119.370 | 118.660 | 119.661 | 119.595 | 119.736 |
| C4-C5-H5 | 119.370 | 125.840 | 120.326 | 120.338 | 120.331 |
| C6-C5-H5 | 119.370 | 113.680 | 120.109 | 120.085 | 120.060 |
| C5-C6-H6 | 119.370 | 118.640 | 119.100 | 119.048 | 119.119 |
| C1-C6-H6 | 119.370 | 120.600 | 118.790 | 118.937 | 118.619 |
| C6-C1-C9 | 119.370 | 123.130 | 123.217 | 123.185 | 123.272 |
| C9-C8-C7 | 120.355 | 120.130 | 121.977 | 122.953 | 122.615 |
| C5-C4-H4 | 120.344 | 120.930 | 120.458 | 120.443 | 120.566 |
| C2-O3-H2 | 110.021 | 114.110 | 109.702 | 109.812 | 109.442 |
| C7-O1-H1(M) | 106.701 | 110.930 | 82.753 | 89.771 | 91.302 |
| O1-C7-O2 | 121.879 | 121.480 | 120.657 | 123.732 | 123.994 |
| C7-O2-M | - | - | 82.988 | 90.165 | 91.693 |
| O1-M-O2 | - | - | 73.601 | 56.332 | 53.011 |
| C1-C9-C8-C7 | −179.998 | −179.210 | 179.998 | −179.999 | 179.998 |
| C6-C1-C9-C8 | 0.011 | 7.290 | −0.023 | −0.030 | −0.023 |
| C9-C8-C7-O1 | −179.999 | 176.670 | 179.999 | 179.994 | 179.999 |
| C9-C8-C7-O2 | 0.002 | −2.790 | 0.004 | −0.004 | 0.004 |
Figure 1.
Structures of: trans o-coumaric acid (a) and alkali metal o-coumarates (M = Li, Na, K, Rb, Cs) (b).
Figure 1.
Structures of: trans o-coumaric acid (a) and alkali metal o-coumarates (M = Li, Na, K, Rb, Cs) (b).
In the case of angles, the increase in the order: acid < Li < Na < K was observed for C1-C2-C3 angles in the aromatic ring. The highest changes were noticed for the carboxylate group, as the C7-O1-H1(M) angle decreases by 23.948° (Li); 16.930° (Na); 15.399° (K). C8-C7-O1 angle increases by 7.150° (Li); 5.405° (Na); 5.372° (K), while the C8-C7-O2 angle decreases by 5.928° (Li); 7.257° (Na); 7.487° (K). In the aromatic ring only small angle changes were observed.
Dipole moments, energies and geometric aromaticity indices [
23,
24] for
trans o-coumaric acid and
o-coumarates were calculated and are presented in
Table 2. Comparing values of dipole moment for molecules of lithium, sodium and potassium salts of 2-hydroxycinnamic acid an increasing tendency can be seen along the series: Li (1.909 D) < H (5.046 D) < Na (5.488 D) < K (6.155 D). The energy values decrease in the series: H→Li→K→Na. The obtained results show that the degree of ionic bonding increases from H to K atom, because of an increase in symmetrization of the carboxylate ion and in the aromaticity of the molecule.
Table 2.
The calculated aromaticity indices, dipole moments (Debye) and total energy (Hartree, 1 hartree = 2625.5 kJ/mol) calculated using B3LYP/6-311++G** method for trans o-coumaric acid and o-coumarates lithium, sodium and potassium.
Table 2.
The calculated aromaticity indices, dipole moments (Debye) and total energy (Hartree, 1 hartree = 2625.5 kJ/mol) calculated using B3LYP/6-311++G** method for trans o-coumaric acid and o-coumarates lithium, sodium and potassium.
| | Acid | Li | Na | K |
|---|
| Geometric Aromaticity Indices |
| Aj | 0.991 | 0.992 | 0.992 | 0.993 |
| BAC | 0.900 | 0.910 | 0.906 | 0.915 |
| HOMA | 0.955 | 0.958 | 0.952 | 0.960 |
| GEO | 0.020 | 0.017 | 0.018 | 0.016 |
| EN | 0.025 | 0.025 | 0.030 | 0.025 |
| I6 | 92.652 | 93.182 | 93.023 | 93.482 |
| Dipole Moment |
| Debye (D) | 5.046 | 1.909 | 5.488 | 6.155 |
| Energy |
| Hartree | −573.632 | −580.615 | −735.372 | −1173.020 |
Geometric aromaticity indices: A
j—normalized function of the bond variance lengths; BAC—Bond Alternation Coefficient HOMA—Harmonic Oscillator Model of Aromaticity; and I
6—Bird’s index were calculated. These index values calculated for salts are almost the same as those obtained for
o-coumaric acid. Comparing the aromaticity indices for
trans o-coumaric acid with those for
p-coumaric acid (HOMA = 0.963; A
j = 0.992; BAC = 0.887; I
6 = 93.134) [
20] and cinnamic acid (HOMA = 0.968; A
j = 0.995; BAC = 0.915; I
6 = 94.430) [
25] show that the aromaticity of these compounds increases in the series:
trans o-coumaric acid→
p-coumaric acid→cinnamic acid (except BAC).
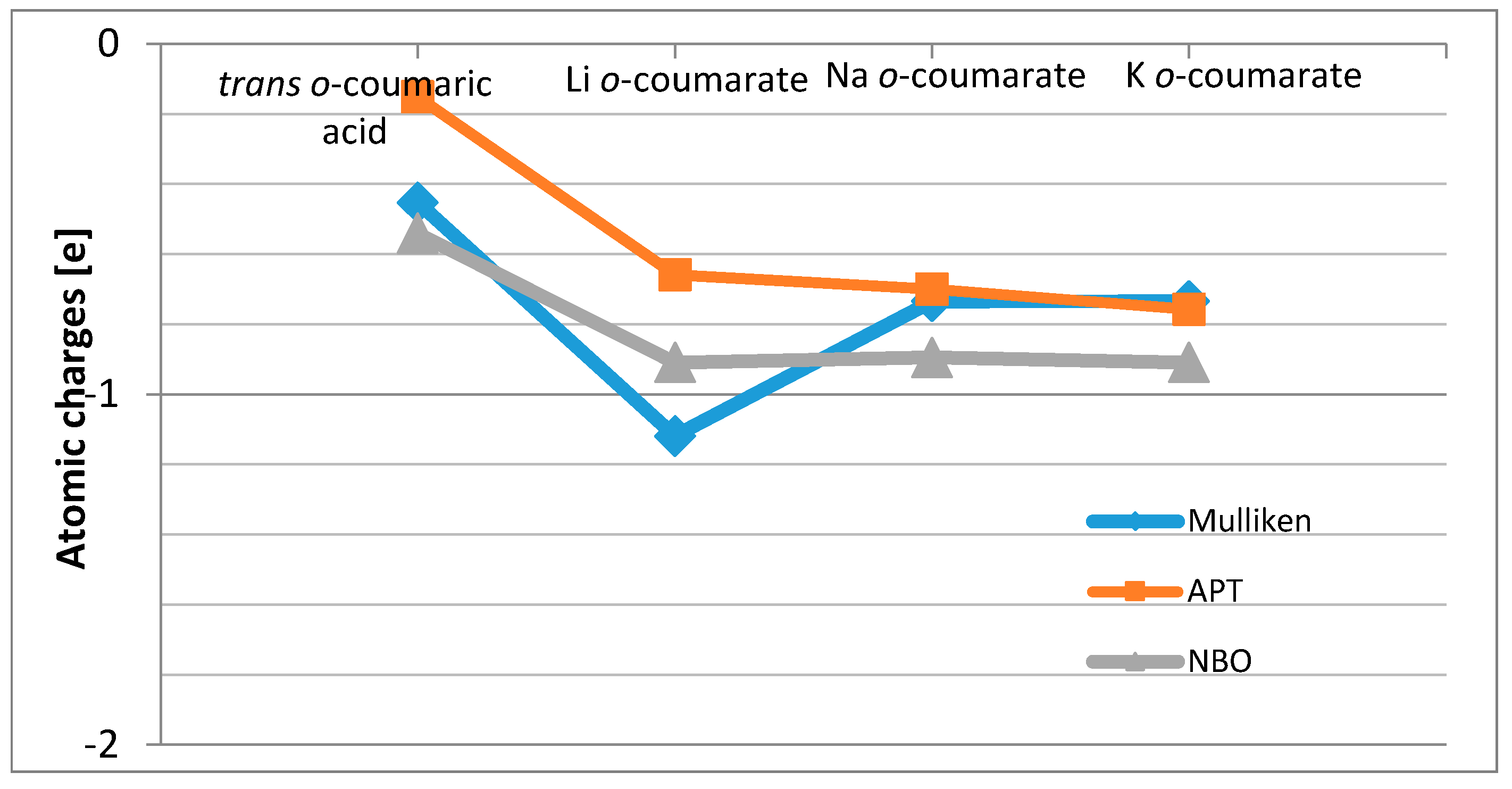
Mulliken, APT (atomic polar tensor) and NBO (natural bond orbital) atomic charges on the atoms of 2-hydroxycinnamic acid molecule and its alkali metal salts are gathered in
Table 3. The electron density increases around atoms C1 and C8 and at the same time decreases around atoms C2, C4, C6 and C9 in the following order: K
o-coumarate > Na
o-coumarate > Li
o-coumarate
> trans o-coumaric acid, according to APT and NBO calculations.
Table 3.
The atomic charges for trans o-coumaric acid as well as for lithium, sodium and potassium o-coumarates calculated in the B3LYP/6-311++G6** level.
Table 3.
The atomic charges for trans o-coumaric acid as well as for lithium, sodium and potassium o-coumarates calculated in the B3LYP/6-311++G6** level.
| Atom Position | Trans o-Coumaric Acid | Li o-Coumarate | Na o-Coumarate | K o-Coumarate |
|---|
| Mulliken | APT | NBO | Mulliken | APT | NBO | Mulliken | APT | NBO | Mulliken | APT | NBO |
|---|
| C1 a | 1.790 | −0.230 | −0.141 | 1.920 | −0.146 | −0.131 | 1.855 | −0.074 | −0.123 | 1.855 | −0.058 | −0.121 |
| C2 | 0.148 | 0.513 | 0.353 | 0.007 | 0.481 | 0.345 | −0.110 | 0.454 | 0.340 | −0.099 | 0.450 | 0.338 |
| C3 | −0.358 | −0.115 | −0.208 | −0.296 | −0.101 | −0.282 | −0.249 | −0.088 | −0.282 | −0.272 | −0.087 | −0.283 |
| C4 | −0.278 | 0.055 | −0.165 | −0.257 | 0.026 | −0.175 | −0.271 | 0.002 | −0.183 | −0.254 | −0.002 | −0.185 |
| C5 | −0.610 | −0.212 | −0.228 | −0.673 | −0.187 | −0.230 | −0.597 | −0.166 | −0.231 | −0.602 | −0.162 | −0.231 |
| C6 | −1.334 | 0.072 | −0.144 | −1.307 | 0.046 | −0.150 | −1.350 | 0.024 | −0.155 | −1.352 | 0.019 | −0.156 |
| C7 | 0.043 | 1.575 | 0.761 | −0.446 | 1.498 | 0.735 | 0.218 | 1.418 | 0.738 | 0.295 | 1.448 | 0.743 |
| C8 | −0.239 | −0.542 | −0.307 | 0.530 | −0.449 | −0.275 | −0.162 | −0.336 | −0.262 | −0.495 | −0.338 | −0.260 |
| C9 | −0.036 | 0.370 | −0.100 | −0.010 | 0.248 | −0.130 | 0.059 | 0.134 | −0.157 | −0.039 | 0.116 | −0.160 |
| H1 (Me) | 0.292 | 0.307 | 0.481 | 0.151 | 0.843 | 0.886 | 0.557 | 0.884 | 0.918 | 0.997 | 0.957 | 0.939 |
| H2 | 0.265 | 0.294 | 0.472 | 0.260 | 0.286 | 0.469 | 0.257 | 0.280 | 0.467 | 0.256 | 0.279 | 0.466 |
| H3 | 0.126 | 0.028 | 0.203 | 0.116 | 0.022 | 0.200 | 0.117 | 0.019 | 0.197 | 0.115 | 0.017 | 0.197 |
| H4 | 0.163 | 0.041 | 0.208 | 0.161 | 0.036 | 0.205 | 0.152 | 0.032 | 0.203 | 0.151 | 0.031 | 0.203 |
| H5 | 0.182 | 0.037 | 0.209 | 0.177 | 0.032 | 0.207 | 0.171 | 0.028 | 0.204 | 0.170 | 0.027 | 0.204 |
| H6 | 0.083 | 0.049 | 0.205 | 0.085 | 0.049 | 0.205 | 0.079 | 0.049 | 0.205 | 0.077 | 0.048 | 0.205 |
| H7 | 0.209 | 0.051 | 0.208 | 0.186 | 0.037 | 0.200 | 0.171 | 0.023 | 0.192 | 0.173 | 0.024 | 0.192 |
| H8 | 0.245 | 0.086 | 0.238 | 0.264 | 0.086 | 0.237 | 0.253 | 0.084 | 0.235 | 0.252 | 0.085 | 0.235 |
| O1 | −0.185 | −0.856 | −0.691 | −0.334 | −1.121 | −0.817 | −0.463 | −1.098 | −0.813 | −0.502 | −1.141 | −0.822 |
| O2 | −0.310 | −0.871 | −0.611 | −0.339 | −1.036 | −0.827 | −0.490 | −1.019 | −0.820 | −0.527 | −1.064 | −0.830 |
| O3 | −0.195 | −0.649 | −0.67 | −0.193 | −0.649 | −0.672 | −0.198 | −0.649 | −0.674 | −0.199 | −0.648 | −0.674 |
| Sum: Ring | −0.642 | 0.083 | −0.605 | −0.606 | 0.119 | −0.623 | −0.722 | 0.152 | −0.634 | −0.724 | 0.160 | −0.638 |
| C=C | −0.275 | −0.172 | −0.407 | 0.520 | −0.201 | −0.405 | −0.103 | −0.202 | −0.419 | −0.534 | −0.222 | −0.420 |
| COO− | −0.452 | −0.152 | −0.541 | −1.119 | −0.659 | −0.909 | −0.735 | −0.699 | −0.895 | −0.734 | −0.757 | −0.909 |
The highest changes of total charge independently on the method used were observed on carboxylate anion (
Figure 2).The theoretical wavenumbers of IR, Raman and chemical shifts in NMR spectra were obtained and compared with experimental spectra.
Figure 2.
The changes in Mulliken, APT and NBO total charges on carboxylate group in alkali metal o-coumarate molecules in comparison with trans o-coumaric acid.
Figure 2.
The changes in Mulliken, APT and NBO total charges on carboxylate group in alkali metal o-coumarate molecules in comparison with trans o-coumaric acid.
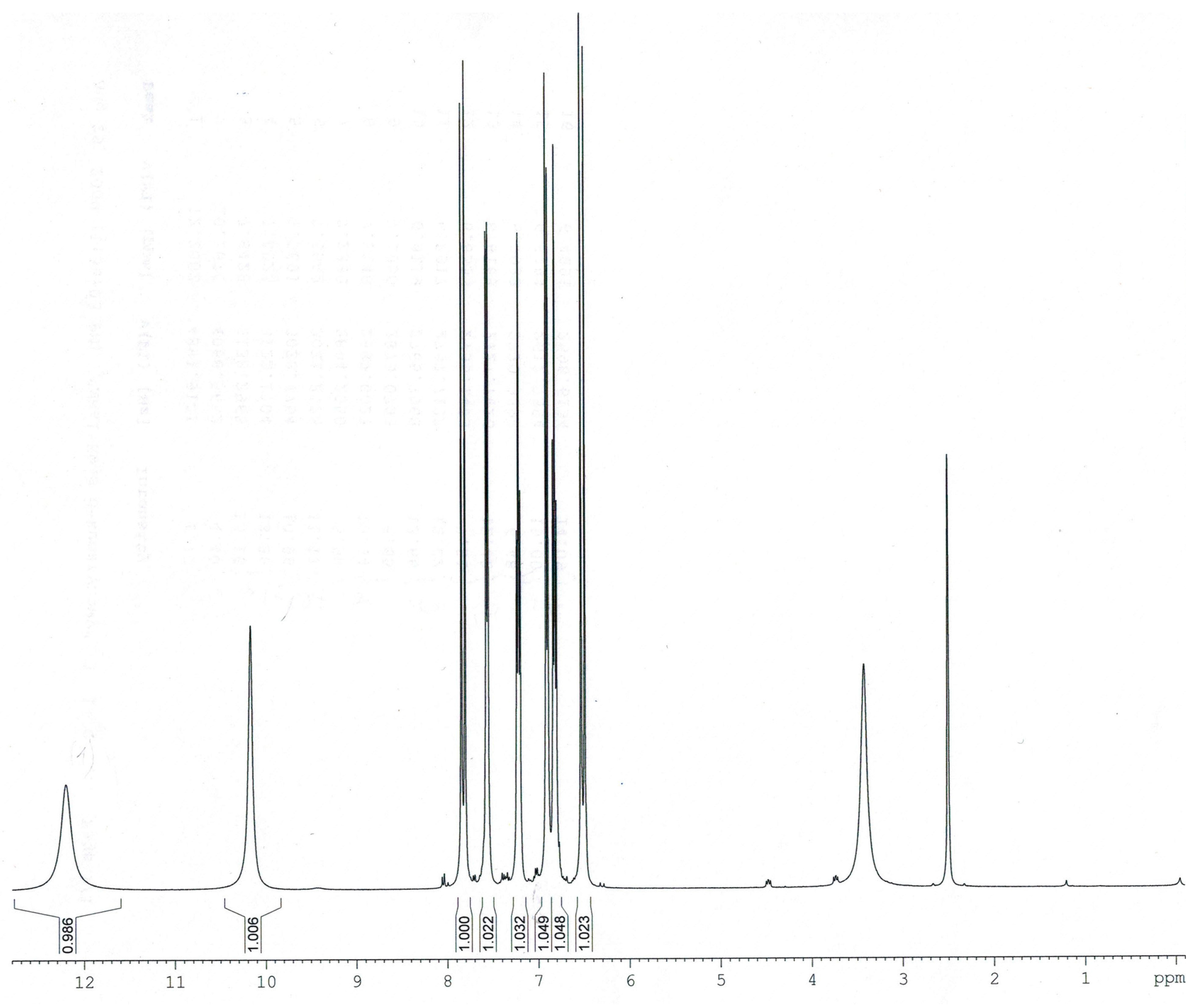
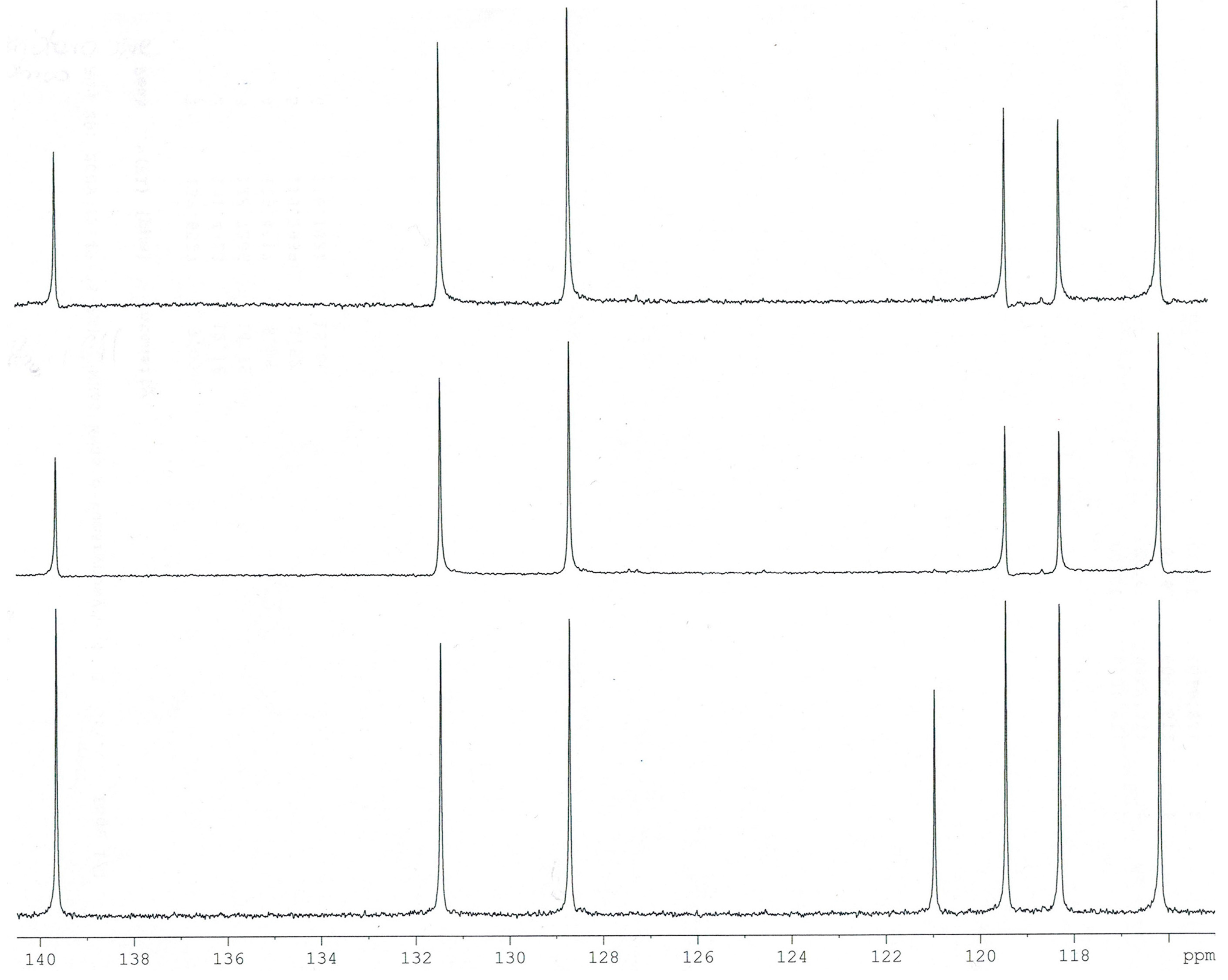
2.2. NMR Spectra
Experimental and calculated (B3LYP/6-311++G**) data for the chemical shifts in
1H- and
13C-NMR spectra obtained for
trans o-coumaric acid and
o-coumarates are gathered in
Table 4. The numbering of atoms is shown in
Figure 1. The experimental
1H-,
13C- and DEPT NMR spectra are shown in
Figure 3,
Figure 4 and
Figure 5. Only experimental data were obtained for rubidium and cesium
o-coumarates. A good correlation between experimental and calculated chemical shifts is obtained. Values of correlation coefficient R for carbon (
13C-NMR) are in the range of 0.9742 to 0.9918. For proton (
1H-NMR) the corresponding range is 0.9456–0.9695.
In the proton spectra of o-coumarates the signals from almost all protons are shifted downfield in comparison to appropriate signals in the spectrum of trans o-coumaric acid (except H3 and H7). This tendency suggests that introduction of metal cations causes the decrease in ring current intensity. Moreover the general tendency can be observed in the decrease of chemical shifts of particular protons in the series: Li→Na→K→Rb→Cs.
This order depicts the increase in the destabilization of the electronic system. In the carbon spectra of o-coumarates there is almost no regularity in the chemical shifts of carbons along the series of alkali metal o-coumarates. Only the values of chemical shifts of C5 and C6 atoms regularly increase in the series: acid→Li→Na→K→Rb→Cs.
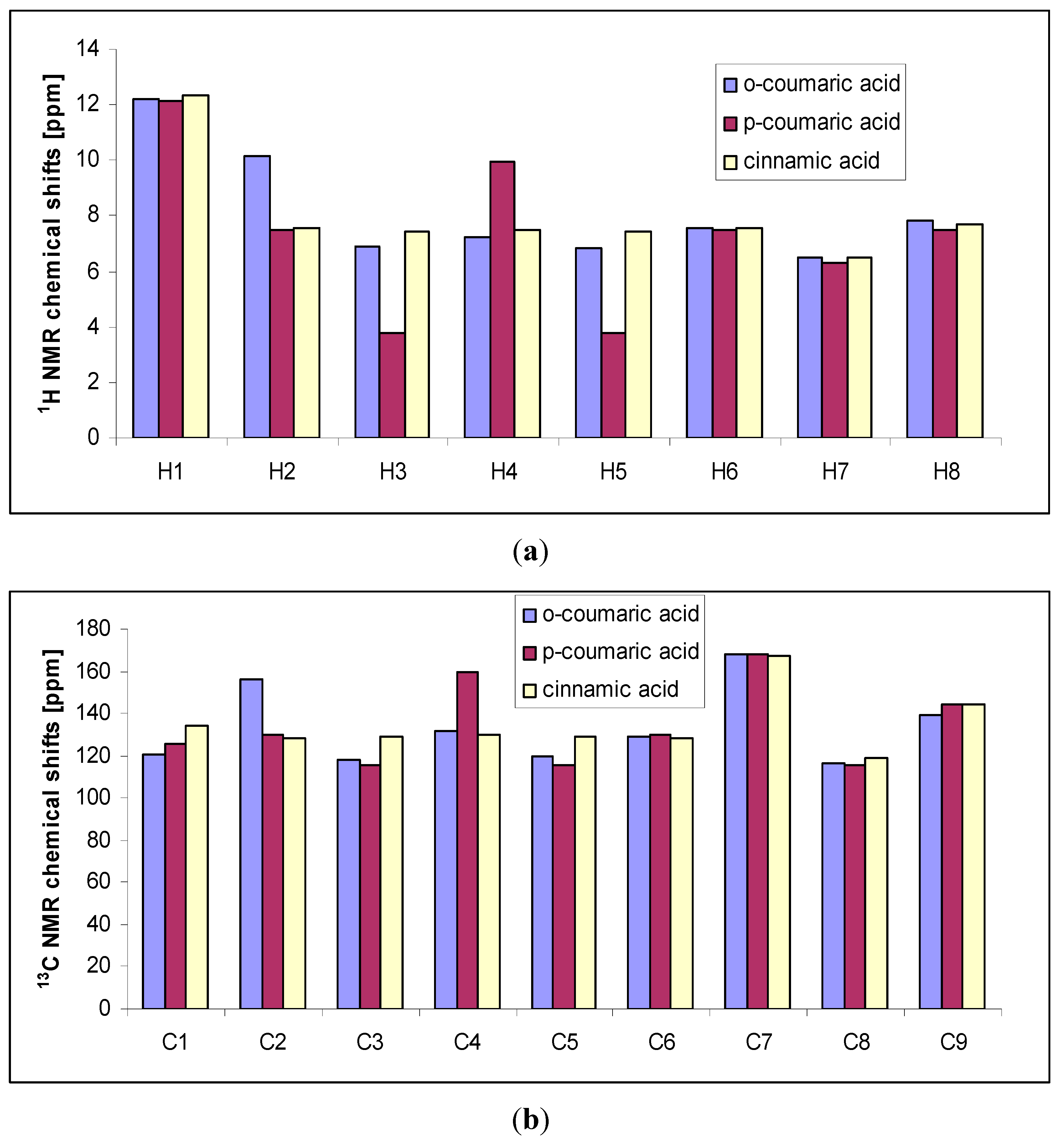
Chemical shift values of signals for
trans o-coumaric acid were compared with those for
p-coumaric acid [
20] and cinnamic acid [
25], and presented in
Figure 6. The signals from protons No. 2, 8 and carbon No. 2 are shifted downfield in comparison to the appropriate signals in the spectrum of
trans o-coumaric acid, whereas the signals from proton No. 4 and carbons No. 1 and 9 are shifted upfield. The values of chemical shifts of H1, H6, H7, H8, C6, C7, C8 and C9 were found almost the same.
Table 4.
Experimental and calculated (B3LYP/6-311++G**) chemical shifts [ppm] in 1H- and 13C-NMR spectra of DMSO solution of trans o-coumaric acid and o-coumarates.
Table 4.
Experimental and calculated (B3LYP/6-311++G**) chemical shifts [ppm] in 1H- and 13C-NMR spectra of DMSO solution of trans o-coumaric acid and o-coumarates.
| Atoms | transo-Coumaric acid | Li o-Coumarate | Na o-Coumarate | K o-Coumarate | Rb o-Coumarate | Cs o-Coumarate |
|---|
| Exp. | Theoret. | Exp. | Theoret. | Exp. | Theoret. | Exp. | Theoret. | Exp. | Exp. |
|---|
| H1 | 12.20 | 6.03 | - | - | - | - | - | - | - | - |
| H2 | 10.17 | 4.94 | - | 4.77 | - | 4.77 | - | 4.68 | - | - |
| H3 | 6.90 | 7.00 | 6.93 | 6.98 | 6.91 | 6.97 | 6.95 | 6.98 | 6.96 | 6.95 |
| H4 | 7.21 | 7.58 | 7.06 | 7.47 | 7.05 | 7.43 | 7.02 | 7.45 | 7.01 | 6.99 |
| H5 | 6.82 | 7.20 | 6.71 | 7.15 | 6.71 | 7.13 | 6.67 | 7.12 | 6.65 | 6.60 |
| H6 | 7.56 | 8.01 | 7.39 | 8.09 | 7.36 | 8.06 | 7.33 | 8.05 | 7.32 | 7.29 |
| H7 | 6.51 | 6.76 | 6.50 | 6.74 | 6.49 | 6.73 | 6.54 | 6.74 | 6.53 | 6.55 |
| H8 | 7.82 | 8.82 | 7.62 | 8.59 | 7.51 | 8.32 | 7.49 | 8.39 | 7.48 | 7.45 |
| C1 | 120.97 | 126.79 | 127.16 | 128.21 | 127.39 | 129.24 | 127.28 | 129.28 | 127.38 | 127.45 |
| C2 | 156.64 | 165.17 | 156.6 | 163.87 | 156.59 | 163.38 | 157.04 | 163.24 | 157.42 | 157.56 |
| C3 | 118.30 | 121.59 | 118.56 | 121.28 | 118.42 | 121.34 | 117.83 | 121.13 | 117.71 | 117.58 |
| C4 | 131.47 | 141.22 | 129.30 | 138.69 | 128.96 | 137.51 | 128.58 | 137.35 | 128.67 | 128.45 |
| C5 | 119.45 | 127.09 | 123.07 | 126.68 | 123.25 | 126.96 | 123.41 | 126.74 | 123.46 | 123.57 |
| C6 | 128.72 | 134.14 | 127.22 | 133.25 | 127.81 | 132.76 | 128.17 | 132.58 | 128.37 | 128. 49 |
| C7 | 168.13 | 175.50 | 172.18 | 191.67 | 171.99 | 184.30 | 171.24 | 185.76 | 171.31 | 171.19 |
| C8 | 116.18 | 117.72 | 116.31 | 127.45 | 116.28 | 130.99 | 116.37 | 132.13 | 116.49 | 116.52 |
| C9 | 139.65 | 150.54 | 133.48 | 143.54 | 132.75 | 140.77 | 132.51 | 140.22 | 132.61 | 132.48 |
Figure 3.
Experimental 13C-NMR spectrum of trans o-coumaric acid.
Figure 3.
Experimental 13C-NMR spectrum of trans o-coumaric acid.
Figure 4.
Experimental 1H-NMR spectrum of trans o-coumaric acid.
Figure 4.
Experimental 1H-NMR spectrum of trans o-coumaric acid.
Figure 5.
Experimental DEPT NMR spectrum of trans o-coumaric acid.
Figure 5.
Experimental DEPT NMR spectrum of trans o-coumaric acid.
Figure 6.
Experimental 1H- (a) and 13C-NMR (a) chemical shifts of trans o-coumaric acid, p-coumaric acid and cinnamic acid.
Figure 6.
Experimental 1H- (a) and 13C-NMR (a) chemical shifts of trans o-coumaric acid, p-coumaric acid and cinnamic acid.
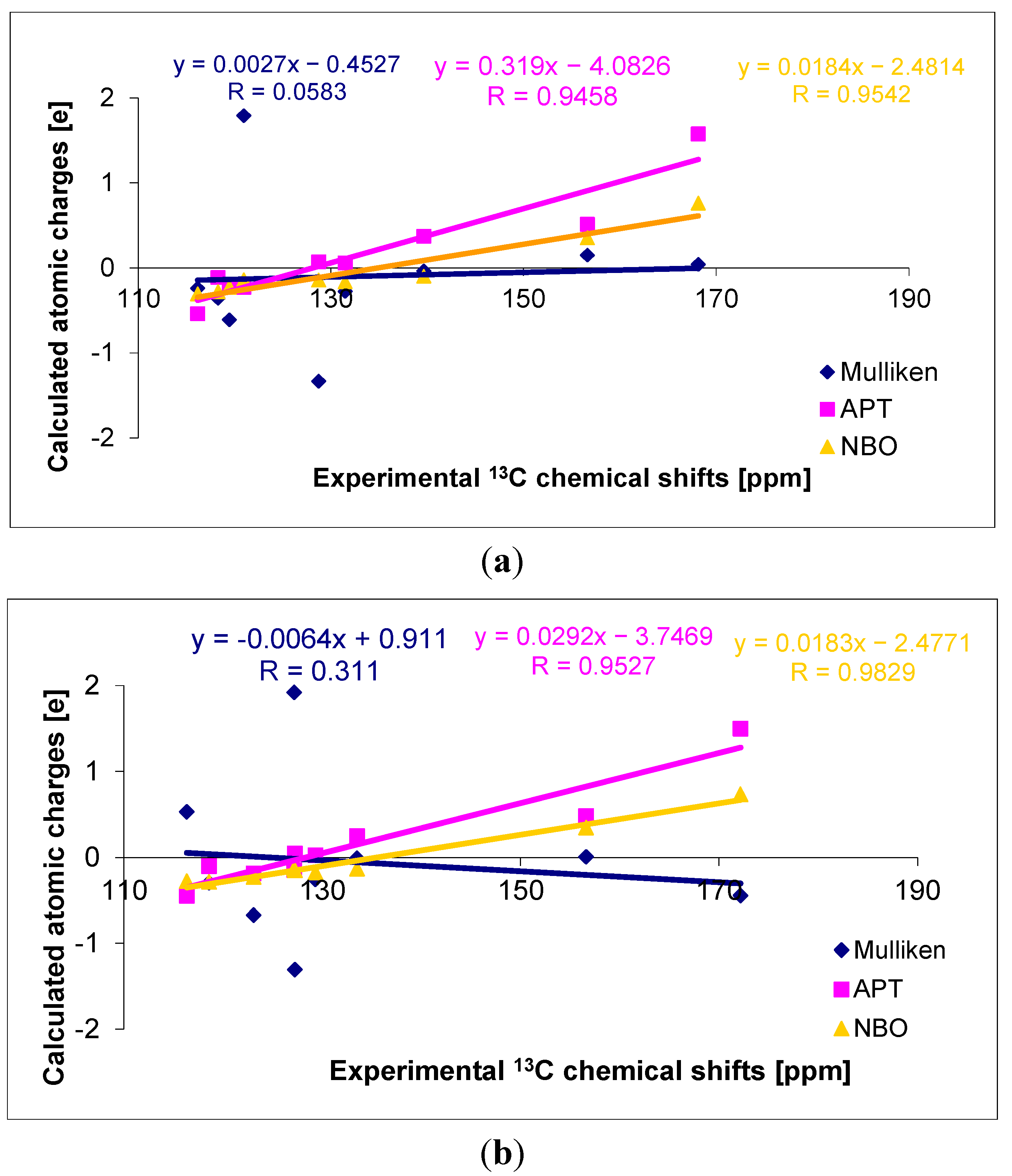
The relationship between calculated atomic charges spectra and experimental chemical shifts from the carbon for
trans o-coumaric acid and
o-coumarates was found and can be seen in
Figure 7. Namely, the increase of the atomic charge along with the increase of the chemical shift is observed. The highest correlation coefficients R were obtained for the linear correlation between the chemical shifts and the NBO (for acid: 0.9542; for Li salt: 0.9866; for Na salt: 0.9829; for K salt: 0.9806) and APT (for acid: 0.9458; for Li salt: 0.9527; for Na salt: 0.9555; for K salt: 0.9477) atomic charges. Whereas the lower ones were obtained for the Mulliken atomic charges (for acid: 0.0583; for Li salt: 0.1311; for Na salt: 0.1284; for K salt: 0.1934).
Figure 7.
The correlation between the calculated atomic charges on carbon atoms in trans o-coumaric acid and (a) and Na o-coumarate (b) molecules and experimental 13C-NMR chemical shifts.
Figure 7.
The correlation between the calculated atomic charges on carbon atoms in trans o-coumaric acid and (a) and Na o-coumarate (b) molecules and experimental 13C-NMR chemical shifts.
2.3. Vibrational Spectra
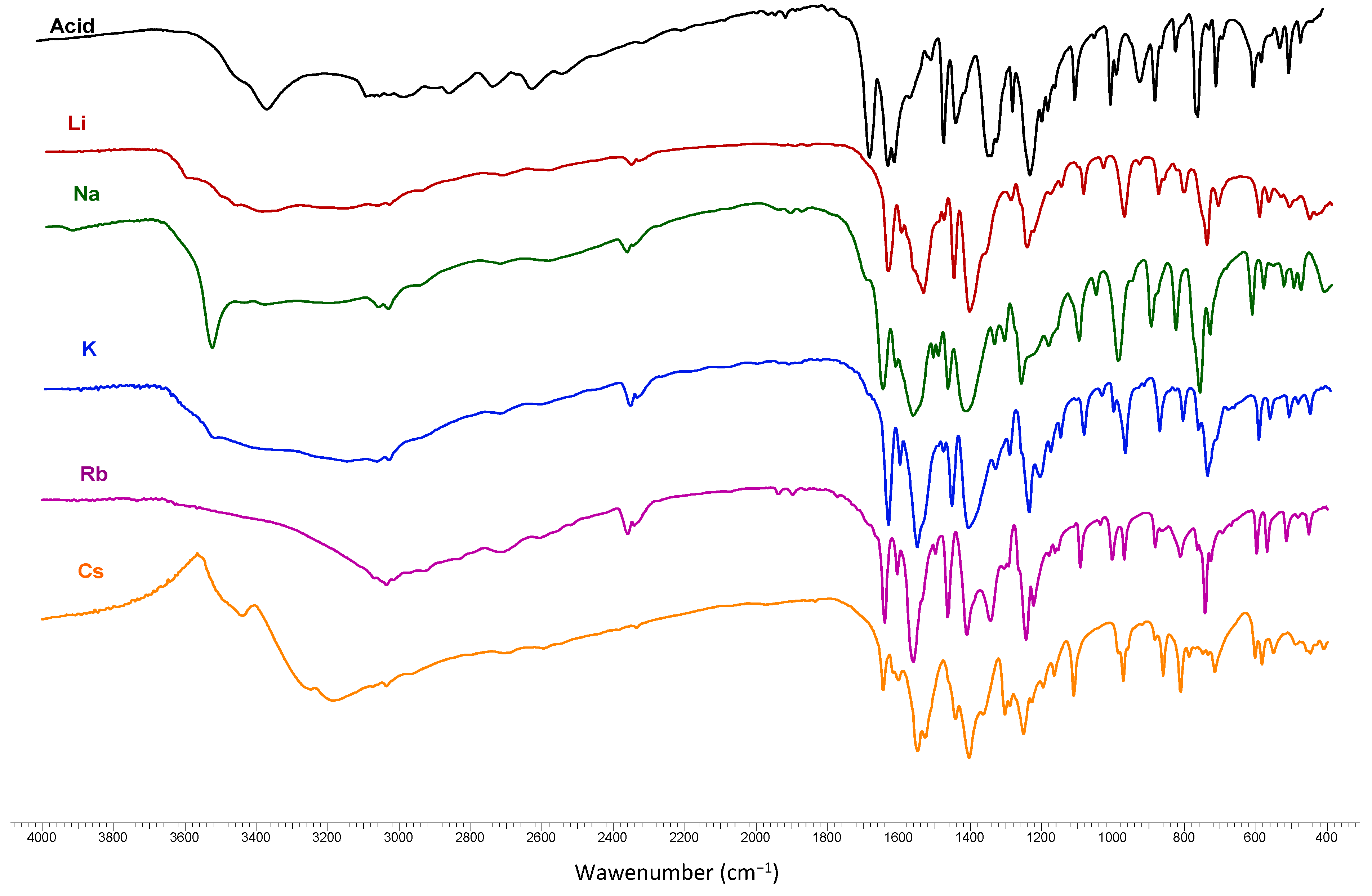
The IR spectra of
trans o-coumaric acid and lithium, sodium, potassium, rubidium and cesium
o-coumarates are presented in
Figure 8. The wavenumbers, intensities and assignments of bands occurring in the FT-IR and FT-Raman spectra of
trans o-coumaric acid and the synthesized lithium, sodium, potassium, rubidium and cesium
o-coumarates are presented in
Table 5 and
Table 6. Theoretical wavenumbers of the bands in vibrational spectra and also band intensities were obtained using B3LYP/6-311++G** method. The numeration of the normal vibrations of benzene ring was done according to the notation used by Varsányi [
26]. A good correlation between experimental and theoretical wavenumbers in the IR spectra of
trans o-coumaric acid as well as its salts were obtained; the correlation coefficients R for acid equal 0.9972; the corresponding value for lithium salt is 0.9976; for sodium salt −0.9975 and for potassium salt 0.9973.
While comparing the spectra obtained for alkali metal o-coumarates with that obtained for acid, there can be observed a disappearance of bands characteristic for stretching vibrations of carboxyl group νOH: 2847 cm−1 (IR), 3771 cm−1 (theoret.), νC=O: 1668 cm−1 (IR), 1665 cm−1 (Raman), 1781 cm−1 (theoret.) and deformation vibrations βOH: 1460 cm−1 (IR), 1446 cm−1 (Raman), 1279 cm−1 (theoret.). The medium intensity bands connected with carboxylic group also disappeared, namely νC-OH: 1169 cm−1 (IR), 1170 cm−1 (Raman), γCO (698 cm−1 and 688 cm−1 in IR and Raman, respectively).
Besides there are other bands characteristic only for spectra of salts concerning asymmetric and symmetric stretching vibrations νasCOO− and νsCOO−; in plane deformations βasCOO− and βsCOO− and out-of-plane bending γsCOO− of carboxylate group. The very strong bands assigned to the stretching vibrations of the carboxylate group are located in the range 1543–1380 cm−1 (IR), 1563–1377 cm−1 (Raman).
Figure 8.
The IR spectra of trans o-coumaric acid and lithium, sodium, potassium, rubidium and cesium o-coumarates.
Figure 8.
The IR spectra of trans o-coumaric acid and lithium, sodium, potassium, rubidium and cesium o-coumarates.
Table 5.
The wavenumbers (cm−1) and assignments of bands occurring in the experimental FT-IR, FT-Raman and calculated spectra of trans o-coumaric acid.
Table 5.
The wavenumbers (cm−1) and assignments of bands occurring in the experimental FT-IR, FT-Raman and calculated spectra of trans o-coumaric acid.
| FT-IR | FT-Raman | Assignment | No [26] |
|---|
| Exp. | Theoret. | Int. | Exp. |
|---|
| 3356 | s a | 3837 | 85.64 | | | ν b(OH)ar | |
| | | 3771 | 108.64 | | | ν(OH) | |
| | | 3200 | 13.48 | | | ν(CH)ar, ν(CH)C=C | |
| | | 3195 | 0.84 | | | ν(CH)ar | 2 |
| 3080 | m | 3185 | 9.85 | 3061 | vw | ν(CH)ar, ν(CH)C=C | 20a |
| 3055 | m | 3183 | 2.73 | 3045 | vw | ν(CH)ar, ν(CH)C=C | 20b |
| 2972 | m | 3175 | 2.59 | | | ν(CH)ar, ν(CH)C=C | 7b |
| | | 3152 | 12.85 | | | ν(CH)ar | |
| 2847–2616 | m | | | | | ν(OH) | |
| 1668 | s | 1781 | 372.28 | 1665 | vw | ν(C=O) | |
| 1616 | vs. | 1673 | 302.05 | 1627 | m | ν(CC)C=C | |
| 1599 | vs. | 1621 | 8.71 | 1604 | vs | ν(CC)ar | 8b |
| 1557 | m | 1644 | 94.51 | | | ν(CC)ar | 8a |
| 1497 | m | 1488 | 66.5 | 1509 | w | ν(CC)ar | 19b |
| 1460 | s | 1529 | 11.43 | | | ν(CC)ar | 19a |
| 1427 | s | 1377 | 193.31 | 1446 | w | β(OH), β(CH)ar | |
| 1337 | s | 1326 | 31.15 | 1333 | vw | β(CH)C=C, β(CH)ar | 3 |
| 1327 | s | 1359 | 35.02 | | | β(CH)C=C, β(CH)ar | 14 |
| 1267 | s | 1351 | 28.97 | 1266 | w | β(CH)C=C | |
| | | 1279 | 11.61 | | | β(OH) | |
| 1219 | vs | 1274 | 62.45 | 1225 | s | ν(C-OH)ar | |
| | | 1225 | 13.72 | | | βCH | |
| 1186 | s | 1191 | 73.89 | | | β(CH)ar | 9a |
| 1169 | s | 1131 | 639.08 | 1170 | w | ν(C-OH) | |
| 1150 | sh | 1108 | 49.47 | 1151 | w | β(CH)ar | |
| 1094 | m | 1184 | 6.15 | 1097 | vw | β(CH)ar | 18b |
| 1040 | w | 1065 | 5.58 | 1039 | w | β(CH)ar | 18a |
| | m | 1037 | 28.63 | 996 | vw | γ(CH)C=C | |
| 993 | | 967 | 10.48 | | | ν(CCO) | |
| 978 | m | 977 | 0.18 | 981 | vw | γ(CH)ar, | 5 |
| | | 895 | 20.88 | | | γ(CH)C=C | |
| 910 | m | | | | | γ(OH) | |
| 868 | m | 849 | 0.09 | 871 | vw | γ(CH)ar | 17b |
| 851 | w | | | 852 | vw | γ(CH)ar | |
| 810 | w | | | 809 | w | γ(CH) | |
| 754 | s | 810 | 12.21 | 758 | vw | γ(CH)ar | 10b |
| 748 | s | | | | | φ(CCC) | 4 |
| 698 | m | 769 | 49.02 | 688 | w | γ(C=O) | |
| | | 758 | 46.43 | | | γ(CH) | 11 |
| 681 | w | 709 | 10.9 | | | α(CCC) | |
| 592 | m | | | 595 | vw | β(CH) | |
| 571 | m | | | 563 | vw | α(CCC) | 6a |
| 519 | w | 658 | 10.99 | 511 | vw | β(CO), α(CCC) | 6b |
| | | 598 | 43.27 | | | β(CH) | |
| 494 | m | 462 | 0.92 | | | φ(CC) | 16a |
| | | 582 | 89.8 | | | γ(OH) | |
| | | 514 | 5.43 | | | α(CCC) | |
| | | 460 | 19.93 | | | β(CH) | 9b |
| 461 | w | | | 458 | vw | φ(CC) | 16b |
Table 6.
The wavenumbers (cm−1) and assignments of bands from the FT-IR and FT-Raman spectra of lithium, sodium, potassium, rubidium and cesium o-coumarates.
Table 6.
The wavenumbers (cm−1) and assignments of bands from the FT-IR and FT-Raman spectra of lithium, sodium, potassium, rubidium and cesium o-coumarates.
| Li o-Coumarate | Na o-Coumarate | K o-Coumarate | Rb o-Coumarate | Cs o-Coumarate | Assignment | No. [26] |
|---|
| FT-IR | FT-Raman | FT-IR | FT-Raman | FT-IR | FT-Raman | FT-IR | FT-Raman | FT-IR | FT-Raman |
|---|
| Exp. | Theoret. | Int. | Exp. | Exp. | Theoret. | Int. | Exp. | Exp. | Theoret. | Int. | Exp. | Exp. | Exp. | Exp. | Exp. |
|---|
| 3564 | W a | 3839 | 75.44 | | | 3526 | w | 3811 | 69.42 | | | 3519 | w | 3840 | 66.91 | | | | | | | 3433 | w | | | ν b(OH)ar | |
| | | 3197 | 15.93 | | | | | 3195 | 18.22 | | | | | 3194 | 18.26 | | | | | | | | | | | ν(CH)ar, ν(CH)C=C | |
| 3154 | w | 3191 | 0.15 | | | | | 3188 | 0.24 | | | | w | 3189 | 0.3 | | | | | | | | | | | ν(CH)ar | 2 |
| 3073 | w | 3186 | 8.64 | 3073 | w | 3071 | w | 3184 | 13.28 | 3070 | vw | 3070 | w | 3183 | 12.34 | 3075 | vw | 3071 | w | 3074 | vw | 3058 | w | 3061 | m | ν(CH)ar, ν(CH)C=C | 20a |
| 3040 | w | | | 3038 | vw | 3038 | w | | | 3045 | w | 3035 | w | | | 3042 | w | 3036 | w | 3037 | w | 3035 | w | | | ν(CH)ar, ν(CH)C=C | 20b |
| 2949 | w | 3172 | 4.45 | | | | | 3173 | 2.87 | | | | | 3171 | 3.09 | | | 2932 | w | | | | | 2932 | vw | ν(CH)ar, ν(CH)C=C | 7b |
| | | 3179 | 7.74 | | | | | 3165 | 13.65 | | | | | 3167 | 12.54 | | | | | | | | | | | ν(CH)C=C | |
| | | 3148 | 15.71 | | | | | 3153 | 15.89 | | | | | 3143 | 18.84 | | | | | | | | | | | ν(CH)ar | |
| 1641 | vs | 1681 | 162.62 | | | 1638 | vs | 1678 | 101.79 | 1639 | vs | | | 1683 | 88.66 | 1642 | vs | 1639 | vs | 1640 | vs | 1631 | vs | 1627 | vs | ν(CC)C=C | |
| 1605 | m | 1621 | 14.18 | 1645 | vs | 1605 | m | 1617 | 8.41 | 1608 | m | 1641 | vs | 1620 | 11.72 | 1608 | m | 1605 | m | 1607 | m | 1605 | m | 1605 | vs | ν(CC)ar | 8b |
| | | 1643 | 34.17 | 1607 | s | | | 1638 | 22.02 | | | 1605 | m | 1641 | 19.49 | | | | | | | | | | | ν(CC)ar | 8a |
| 1543 | vs | 1527 | 403.69 | 1546 | vw | 1557 | vs | 1568 | 460.22 | 1560 | w | 1557 | vs | 1559 | 411.25 | 1563 | m | 1560 | vs | 1555 | w | 1552 | vs | | | νas(COO−) | |
| 1485 | m | 1486 | 89.69 | | | 1483 | w | 1483 | 71.48 | 1470 | vw | 1483 | w | 1484 | 71.48 | 1499 | w | 1499 | w | 1500 | vw | 1470 | w | | | ν(CC)ar | 19b |
| 1458 | vs | 1530 | 63.41 | 1462 | w | 1460 | vs | 1525 | 30.82 | | | 1460 | vs | 1528 | 36.01 | 1469 | vw | 1464 | vs | 1467 | vw | 1456 | s | 1454 | w | ν(CC)ar | 19a |
| 1414 | vs | 1430 | 700.96 | 1429 | w | 1412 | vs | 1398 | 600.77 | 1427 | w | 1412 | vs | 1399 | 708.7 | 1416 | w | 1410 | vs | 1409 | w | 1380 | vs | 1377 | w | νs(COO−) | |
| 1345 | sh | 1324 | 19.83 | | | 1339 | m | 1321 | 22.08 | 1318 | m | 1339 | w | 1320 | 23.45 | 1342 | vw | 1344 | s | 1347 | vw | | | | | β(CH)C=C, β(CH)ar | 3 |
| | | 1360 | 36.02 | | | | | 1358 | 40.64 | | | | | 1359 | 35.57 | | | | | | | | | | | β(CH)C=C, β(CH)ar | 14 |
| 1298 | m | 1347 | 29.19 | 1290 | sh | 1298 | m | 1345 | 24.84 | | | 1298 | w | 1344 | 21.84 | | | 1303 | sh | 1308 | w | 1301 | m | | | β(CH)C=C | |
| 1254 | vs | 1276 | 16.74 | 1256 | s | 1242 | vs | 1275 | 18.2 | 1243 | s | 1241 | | 1271 | 20.04 | 1241 | s | 1244 | vs | 1245 | s | 1248 | s | 1263 | m | β(CH)C=C | |
| 1234 | sh | 1267 | 127.71 | | | 1213 | s | 1265 | 96.92 | 1215 | w | 1221 | | 1265 | 95.72 | | | 1223 | m | | | 1218 | w | | | ν(C-OH)ar | |
| | | 1220 | 6.36 | | | | | 1218 | 12.07 | | | | | 1217 | 8.34 | | | | | | | | | | | βCHar | |
| 1184 | w | 1191 | 57.63 | | | 1182 | m | 1193 | 56.94 | | | 1178 | | 1190 | 61.43 | 1181 | vw | 1180 | vw | | | | | | | β(CH)ar | 9a |
| 1155 | w | 1106 | 50.27 | 1162 | m | 1155 | m | 1106 | 45.17 | 1156 | m | 1163 | | 1105 | 48.44 | 1156 | w | 1163 | vw | 1165 | w | 1150 | m | 1158 | w | β(CH)ar | |
| 1094 | s | 1183 | 10 | 1097 | vw | 1090 | s | 1180 | 8.24 | 1094 | vw | 1090 | | 1181 | 7.46 | 1093 | vw | 1092 | m | 1094 | vw | 1093 | m | | | β(CH)ar | 18b |
| 1040 | m | 1066 | 2.65 | 1041 | m | 1040 | vw | 1062 | 3.7 | 1042 | m | 1035 | w | 1065 | 4.24 | 1038 | m | 1035 | vw | 1038 | m | 1040 | w | 1041 | w | β(CH)ar | 18a |
| | | | | | | 1007 | w | | | | | 1010 | w | | | 1011 | w | 1003 | m | 1002 | w | | | | | | |
| 980 | s | 973 | 0.16 | 984 | m | 974 | s | 971 | 0.05 | 976 | vw | 969 | s | 971 | 0.12 | 970 | w | 968 | m | 970 | vw | 970 | s | | | γ(CH)ar, | 5 |
| 937 | w | 1034 | 32.89 | | | 922 | vw | 1033 | 35.16 | | | | | 1032 | 35.34 | | | | | | | | | | | γ(CH)C=C | |
| | | 846 | 0.08 | | | | | 841 | 0.01 | | | | | 843 | 0.08 | | | | | | | | | | | γ(CH)ar | 17b |
| 883 | m | | | 887 | w | 878 | m | | | 879 | m | 879 | m | | | 881 | vw | 881 | m | 884 | vw | 881 | m | | | βs(COO−) | |
| 814 | s | | | 818 | w | 812 | m | | | 815 | w | 811 | m | | | 814 | w | 812 | m | 814 | w | 813 | m | | | γ(CH)C=C | |
| | | 826 | 27.14 | | | | | 820 | 18.8 | | | | | 819 | 22.26 | | | | | | | | | | | γ(CH)ar | 10b |
| | | | | | | 770 | w | 726 | 18.4 | 772 | vw | 763 | w | 726 | 38.13 | 764 | vw | | | | | | | | | γs(COO−) | |
| 748 | vs | | | 764 | w | 743 | s | | | 730 | w | 741 | s | | | | | 743 | vs | | | 751 | vs | | | φ(CCC) | 4 |
| 718 | s | 756 | 68.5 | 720 | w | | | 752 | 70.9 | | | | | 753 | 69.87 | | | | | | | 712 | w | | | γ(CH) | 11 |
| 669 | vw | 705 | 0.4 | | | 689 | vw | 703 | 0.07 | | | 695 | w | 703 | 0.03 | | | 669 | vw | | | | | | | α(CCC) | |
| 602 | s | | | 601 | vw | 600 | m | | | 603 | w | 597 | m | | | 600 | w | 598 | m | 599 | w | 596 | m | | | βas(COO−) | |
| 575 | m | | | | | 570 | s | | | 572 | vw | 569 | s | | | 572 | vw | 569 | m | 570 | vw | 565 | w | | | α(CCC) | 6a |
| 517 | w | 631 | 178.97 | 513 | vw | 515 | m | 610 | 21.82 | 516 | vw | 515 | w | 612 | 28.6 | 517 | w | 515 | m | 516 | vw | 515 | w | | | α(CCC) | 6b |
| | | 466 | 9.93 | | | 490 | w | 467 | 9.95 | | | 486 | w | 466 | 8.74 | 488 | w | 482 | vw | 484 | vw | | | | | φ(CC) | 16a |
| | | 517 | 0.36 | | | | | 512 | 5.44 | | | | | 514 | 7.96 | | | | | | | | | | | α(CCC) | |
| 460 | m | | | 458 | vw | 455 | m | | | | | 450 | m | | | 452 | w | 451 | w | 454 | w | 461 | w | | | φ (CC) | 16b |
These band are sensitive to the type of metal coordination and degree of complex hydration. βasCOO− and βsCOO− are in the range 883–596 cm−1 (IR), 887–596 cm−1(Raman) and γsCOO− modes of are at ~770 cm−1 (IR), (Raman). The difference between the wavenumbers of asymmetric and symmetric stretching of the carboxylic anion vibrations (ΔνCOO−) increased in the IR and Raman spectra from lithium to cesium o-coumarates. The increase in this value means the increase in the ionic character of metal-oxygen bond. The values of ∆βCOO− were also calculated and increased in the IR spectra along the series: Na→Li→K→Rb→Cs (278, 281, 282, 283, 285 cm−1) in Raman the order is: K→Na→Rb→Li (276, 281, 285, 286 cm−1).
Correlations between the chosen wavenumbers of vibrational bands in the IR spectra of o-coumarates and some alkali metal parameters, such as electronegativity, atomic and ionic radii, affinity, energy of ionization and ionic potential were analyzed. The best correlation was obtained for ionic radius (R = 0.9888) and ionization energy (R = 0.9687) for deformation band of carboxylate group (βasCOO−). The analysis of these data shows that the ionic radius and ionization energy are the most important metal parameters in view of influence of the metal on the vibration structure of o-coumarates.
In the spectra of trans o-coumaric acid and its metal salts bands from the hydroxyl group attached to the aromatic ring are present: (1) in the spectrum of the acid: νOHar 3356 cm−1 (IR), 3837 cm−1 (theoret.); νC-OHar 1219 cm−1 (IR), 1225 cm−1 (Raman), 1274 cm−1 (theoret..); (2) in the spectra of salts: νOHar 3564-3433 cm−1 (IR), 3840–3811 cm−1 (theoret..); νC-OHar 1234–1213 cm−1 (IR), 1215 cm−1 (Raman), 1267–1265 cm−1 (theoret.). Bands are located in the range 3154–2932 cm−1 (IR), 3074–2932 cm−1 (Raman) 3200–3143 cm−1 (theoret.), can be assigned to the CH stretching modes of aromatic ring and the double bond between C8 and C9 atoms. A peak around 1640 cm−1 (IR and Raman), 1680 cm−1 (theoret.) derives from the CC stretching vibrations of the double bond. The bands of the β(CH) vibrations are located in the range 1345–1035 cm−1 (IR), 1333–1038 cm−1 (Raman) and 1326–1062 cm−1 (theoret.). The bands at 980–712 cm−1 (IR), 984–720 cm−1 (Raman) and 1037–752 cm−1 (theoret.) are assigned to out-of-plane bending vibrations γCH. The wavenumbers of aromatic bands numbered as 20a, 20b, 7b, 19a, 9a, 5, 6b, 16a and 16b decrease in comparison to free acid, while the wavenumbers of 8b and 3 bands in the spectra increase.
2.4. UV Spectra
The UV spectra of trans o-coumaric acid and lithium, sodium, potassium, rubidium and cesium o-coumarates were recorded. The maxima of π→π* bands for trans o-coumaric acid were located at 210, 272, and 314 nm. In the UV spectra of o-coumarates the bands were shifted toward lower wavelengths, with the maxima at 210, 268, 312 nm respectively, similarly for all the salts. The noted hypsochromic shifts indicate a decrease in the delocalization energy and therefore, a perturbation effect of alkali metals on the electronic system of ligand.
2.5. Microbial Activity
Evaluation of the antimicrobial activity of
trans o-coumaric acid and lithium, sodium, potassium, rubidium and cesium
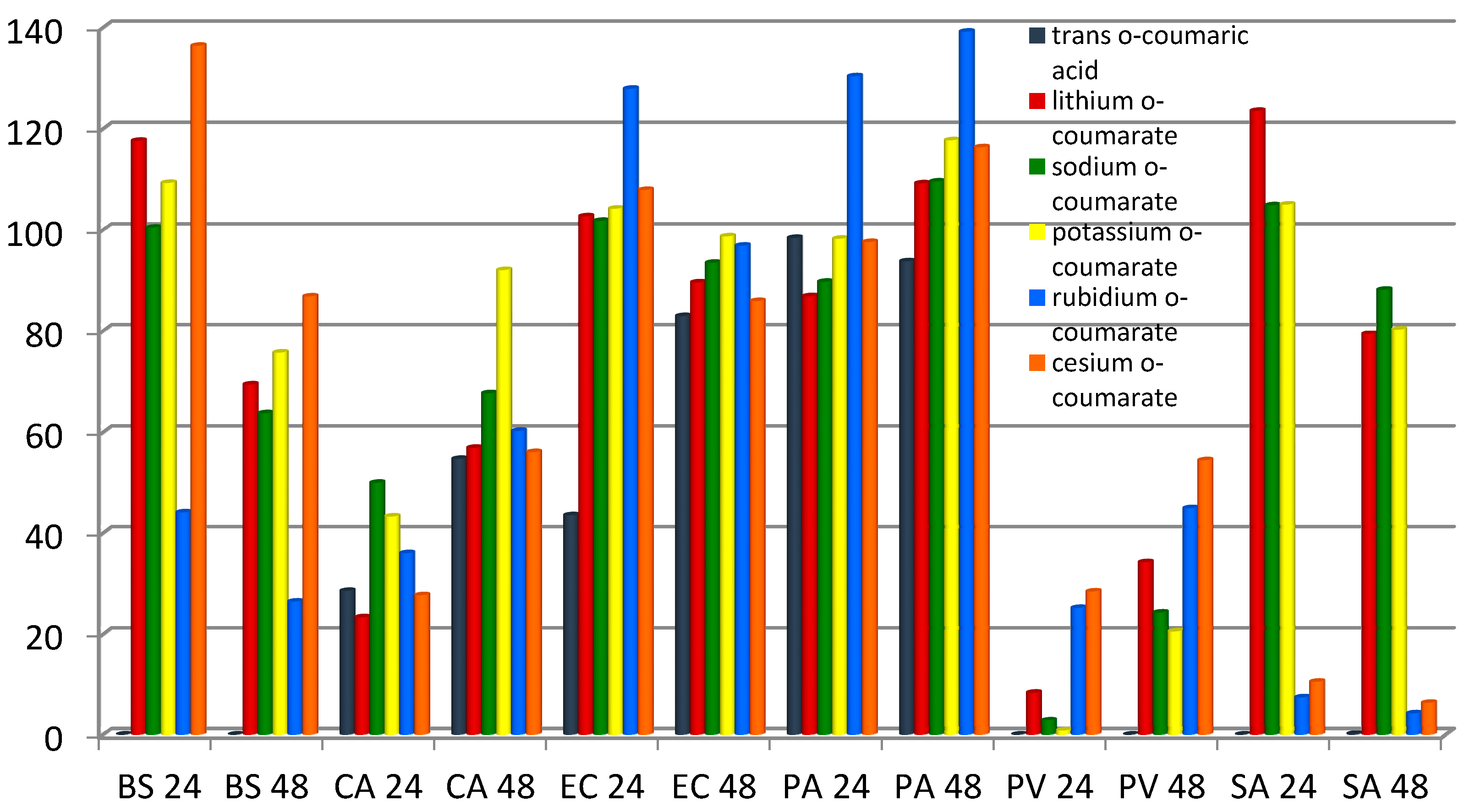
o-coumarates was measured as a degree of growth inhibition or stimulation in individual bacteria or fungi cultures, in relation to the control (non-treated) sample and depicted as a percent of control. The average values given in
Figure 9 were calculated from the data obtained in the four experiments taken for each preparation and strain. The results obtained after 24 and 48 h of incubation are shown in
Figure 9.
Figure 9.
The degree of growth inhibition induced by the studied compounds in Baccillus ssubtilis (BS), Candida albicans (CA), Escherichia coli (EC), Pseudomonas aeruginosa (PA), Proteus vulgaris (PV) and Staphylococus aureus (SA); evaluated after 24 and 48 h of incubation.
Figure 9.
The degree of growth inhibition induced by the studied compounds in Baccillus ssubtilis (BS), Candida albicans (CA), Escherichia coli (EC), Pseudomonas aeruginosa (PA), Proteus vulgaris (PV) and Staphylococus aureus (SA); evaluated after 24 and 48 h of incubation.
Trans o-coumaric acid induced a considerable growth inhibition in Bacillus subtilis, Proteus vulgaris and Staphylococcus aureus after 24 h and 48 h of treatment. The studied compounds strongly inhibited the growth of the bacterium Proteus vulgaris after 24 h of incubation. Lithium, sodium and potassium salts support the growth of Bacillus subtilis, Staphylococcus aureus and Escherichia coli. All salts showed a stimulating effect after 48 hours incubation in relation to bacteria Pseudomonas aeruginosa. Potassium and rubidium salts inhibited the growth of Escherichia coli to the lowest degree. Strong inhibitory effects of cesium and rubidium o-coumarates shown relative to Staphylococcus aureus (~95%).
The correlation between the physicochemical parameters and microbial properties of
trans o-coumaric acid and alkali metal
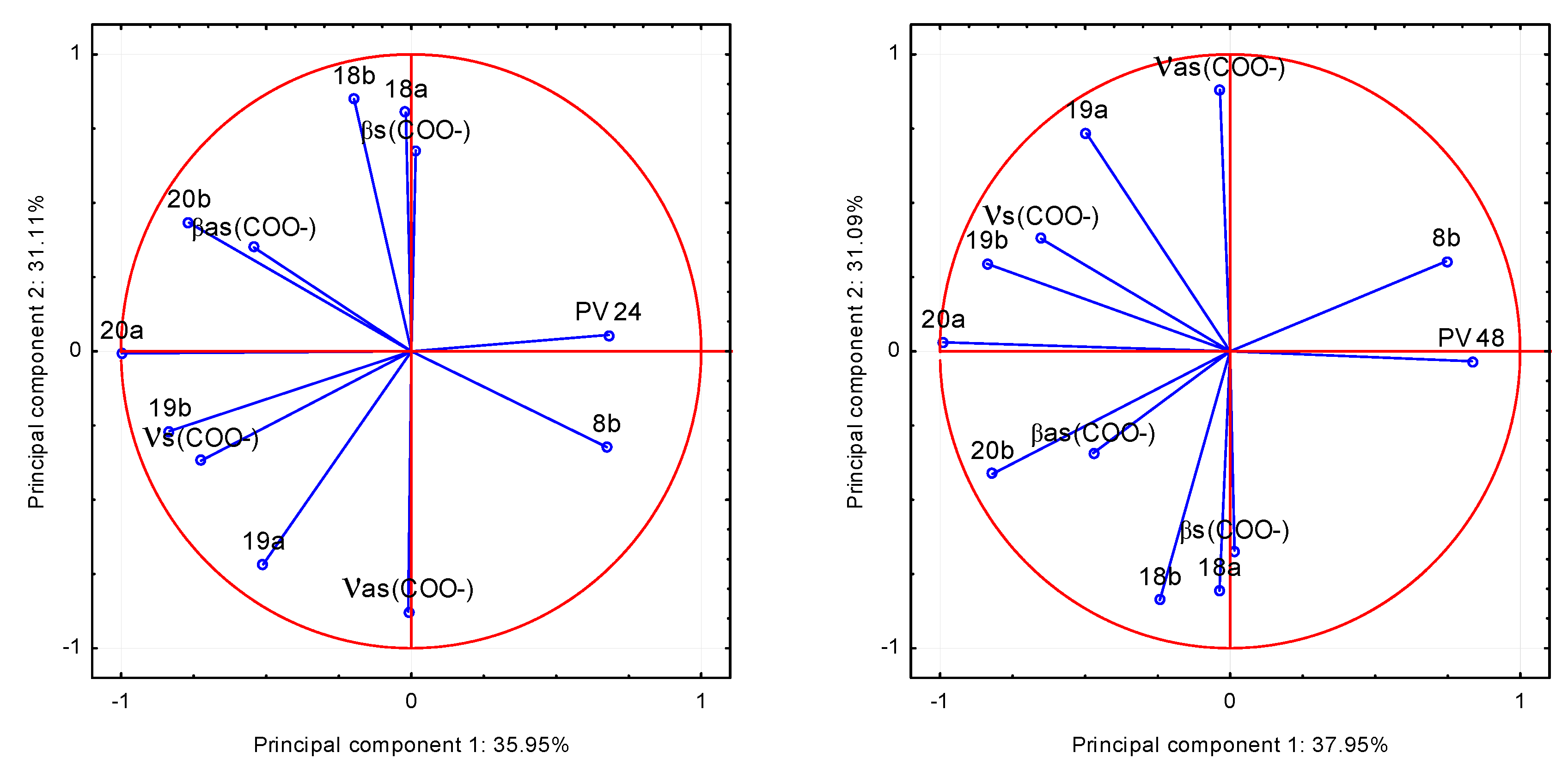
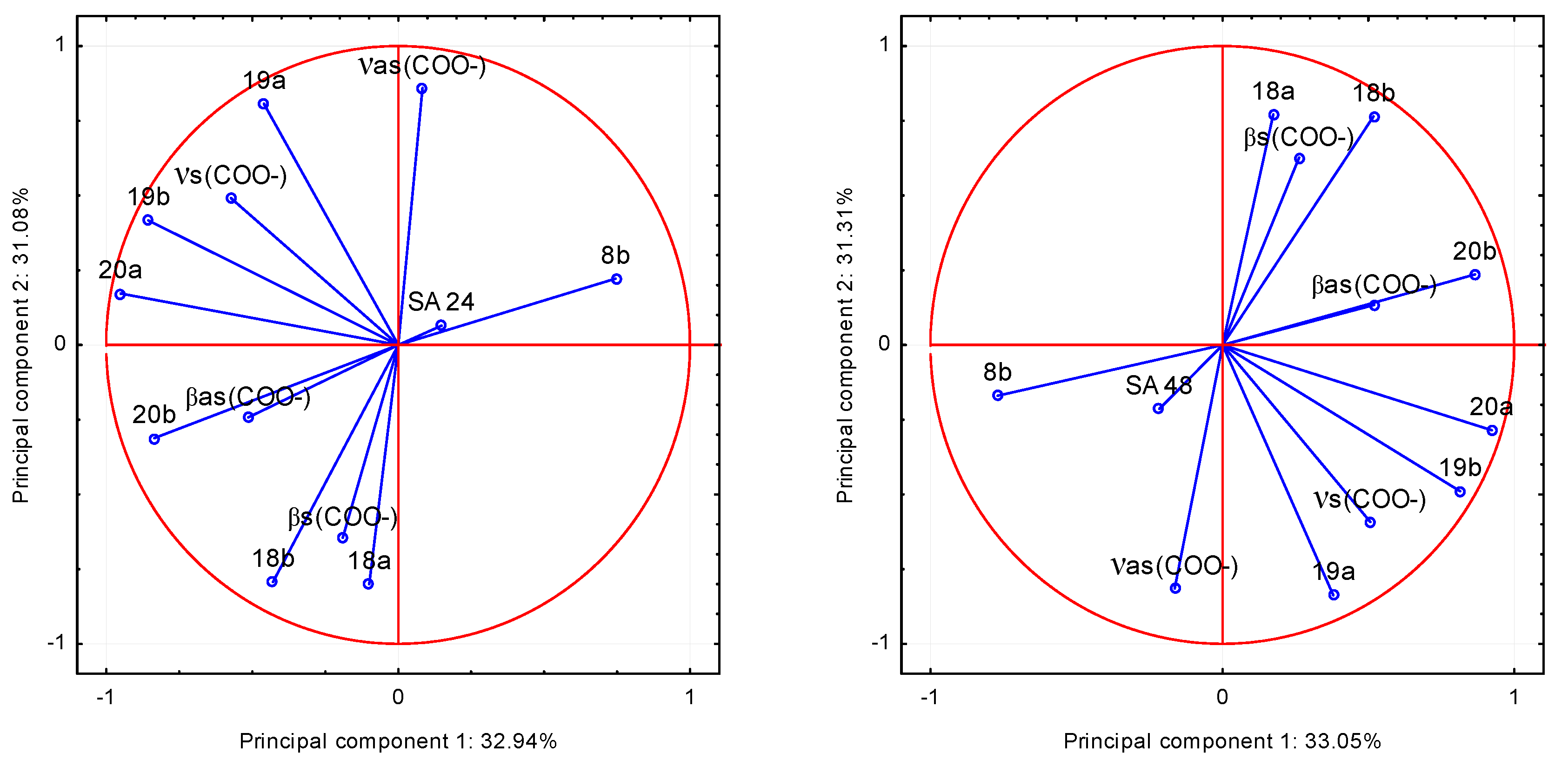
o-coumarates was examined. On the basis of the spectroscopic data: the values of wavenumbers of infrared spectra of the compounds and the parameters describing their antimicrobial activity the principal component analysis (PCA) was performed. This method is most commonly used for multivariate exploratory analysis of experimental data. Analysis was made on the basis of the factor loadings values being correlation coefficients describing the correlation between the antimicrobial properties of the compounds and the wavenumbers of selected bands in FT-IR spectra. Based on the screen chart, two main components were selected to data analysis. To investigate which of the bands described by the wavenumbers, are strongly positively or negatively correlated with the microbiological data, the projection weights on the plane defined by pairs of main factors was made. Correlations are determined by the angle between the two weight vectors, which initial point and the ends are defined by respective weight values. The relationship between the input variables and the main components is presented as a graph of configuration of points representing the variables in the system of the first two principal components (
Figure 10 and
Figure 11). The coordinates of these points are corresponding coefficients of variables, in this case, the wavenumbers of selected bands in the FT-IR spectra. The more given variable is closer to the edge of the wheel, the better is its representation of the main components defining the coordinate system,
i.e., the greater part of the information contained in the variable is carried by the main components.
Figure 10.
The values of the band wavenumbers in the FT–IR spectra of trans o-coumaric acid and alkali metal o-coumarates in relation to the degree of growth inhibition of the bacteria Proteus vulgaris (PV) after 24 and 48 h of incubation in the two principal components system.
Figure 10.
The values of the band wavenumbers in the FT–IR spectra of trans o-coumaric acid and alkali metal o-coumarates in relation to the degree of growth inhibition of the bacteria Proteus vulgaris (PV) after 24 and 48 h of incubation in the two principal components system.
Figure 11.
The values of the band wavenumbers in the FT–IR spectra of trans o-coumaric acid and alkali metal o-coumarates in relation to the degree of inhibition of growth in Staphylococcus aureus (SA) after 24 and 48 h of incubation in the two principal components system.
Figure 11.
The values of the band wavenumbers in the FT–IR spectra of trans o-coumaric acid and alkali metal o-coumarates in relation to the degree of inhibition of growth in Staphylococcus aureus (SA) after 24 and 48 h of incubation in the two principal components system.
Analysis of the data leads to the conclusion that the most statistically significant correlation was demonstrated between the degree of inhibition of bacterial growth: Proteus vulgaris (PV) and Staphylococcus aureus (SA) and the wavenumbers of the following bands in the spectra of the studied compounds: positive: 20b, βas(COO−) and negative: 8b, both after 24 and 48 h of incubation. It shows that the FT-IR spectra can be a good source of data for the quantitative analysis of the relationship between the molecular structure of the compound and its biological activity. This finding strongly points to the fact, that the analysis of the physicochemical structures can bring significant prerequisites on the biological activity of compounds. The presented method and analysis can be found useful in design of the syntheses of microbial active analogues.
4. Conclusions
In this paper the influence of lithium, sodium, potassium, rubidium and cesium cations on the electronic system of trans o-coumaric acid was studied by means of FT-IR, FT-Raman, UV-VIS, 1H- and 13C-NMR, ab initio calculations and microbiological tests. Substitution of hydrogen with alkali metals ions in the carboxylic group brought about some characteristic changes in the molecular spectra as well as in the geometrical structures of the investigated molecules. The experimental parameters were compared to calculated characteristics of studied compounds and they indicate good linear correlations.
The calculated aromaticity indices (Aj, BAC and I6–Bird’s index) for trans o-coumaric acid were almost the same as those of all salts. The aromaticity increased in the series: trans o-coumaric acid→ p-coumaric acid→cinnamic acid, what suggests that the compounds studied here in possess less aromatic properties than p-coumaric acid and cinnamic acid. In the case of salts the same relationship is observed. Theoretical studies of the geometrical parameters have shown that the substitution of the metal in the carboxyl group causes a slight change in the geometry of the aromatic ring. Geometric parameters (almost the same values of bond lengths and angles in the ring for all the salts, independently on the metal ion also suggest that p-coumaric acid has a higher aromaticity. Perturbation of the electronic system of molecules increases along the series: acid→Li→Na→K.
Chemical shift changes of were also noted in the proton and carbon NMR spectra. The chemical shifts of all protons decreased in the series Li→Na→K→Rb→Cs and the chemical shifts of carbon no. 5 and 6 increased in the same series. The highest correlation coefficients were obtained between the experimental data and the theoretical ones calculated by the NBO and APT methods.
The intensity and wavenumbers in the case of bands No. 20a, 20b, 7b, 19a, 9a, 5, 6b, 16a and 16b decrease in comparison to the free acid, which points to a perturbation in the electron charge distribution of the ligand. Ionic radius and ionization energy are the most important metal parameters in view of the influence of the metal on the vibration structure of
o-coumarates. In the case of benzoates and
o-coumarates the Δν
COO− values increase in the series: lithium→sodium→potassium→rubidium→cesium (in FT-IR and FT-Raman spectra). In the case of salicylates and
p-coumarates these values scatter [
27]. The increase in the value of Δν
COO− means the increase in the ionic character of metal-oxygen bond. In addition, the value of Δν
COO− for
p-coumaric acid was higher than
o-coumaric acid (in FT-IR, FT-Raman and theoretical spectra). In the UV-VIS spectra of the studied compounds, the hipsochromic shifts indicate that the alkali metal cations perturb the electronic system of the molecule.
After 24 and 48 h of incubation, trans o-coumaric completely inhibited growth of the bacteria: Bacillus subtilis, Proteus vulgaris and Staphylococcus aureus. Based on the principal component analysis, it was found that the most statistically significant correlations exist between the degree of inhibition of bacterial growth Proteus vulgaris and Staphylococcus aureus and the wavenumbers of 20b, βas(COO−) and 8b bands in the spectra of studied compounds.
The electronic properties of substituent have a significant effect on the degree of ionization and the polarity of chemical compounds, which influences their ability to penetrate cell membranes and the strength of binding to receptors. Therefore it is very important to be able to measure the electronic parameters of molecules and connect them with the corresponding biological properties. In addition to the physicochemical parameters (dipole moments, hydrogen bonding, conformation and distances between the atoms) discussed above, there are others which also play a role in molecular modeling. The use of these parameters is limited, because of the difficulty in measuring them. However, the biological activity of most compounds is a result of many physicochemical parameters.