An Effective Synthesis Method for Tilorone Dihydrochloride with Obvious IFN-α Inducing Activity
Abstract
:1. Introduction
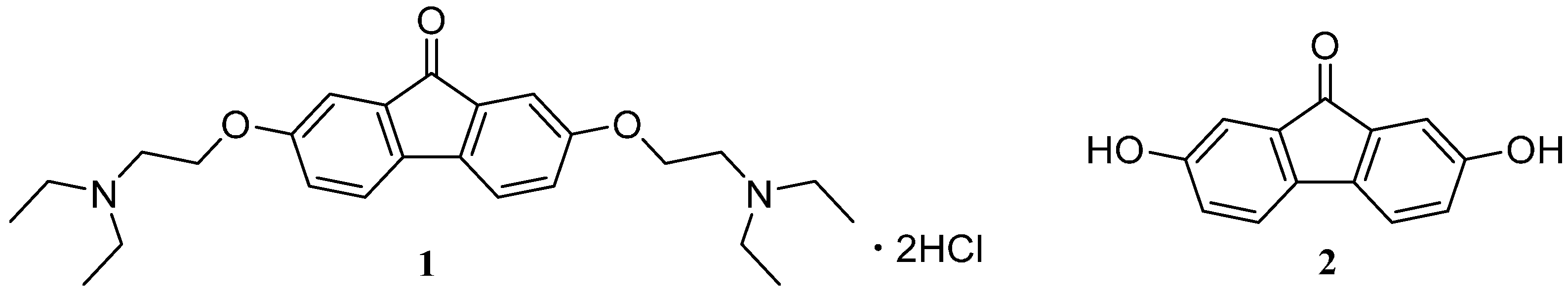
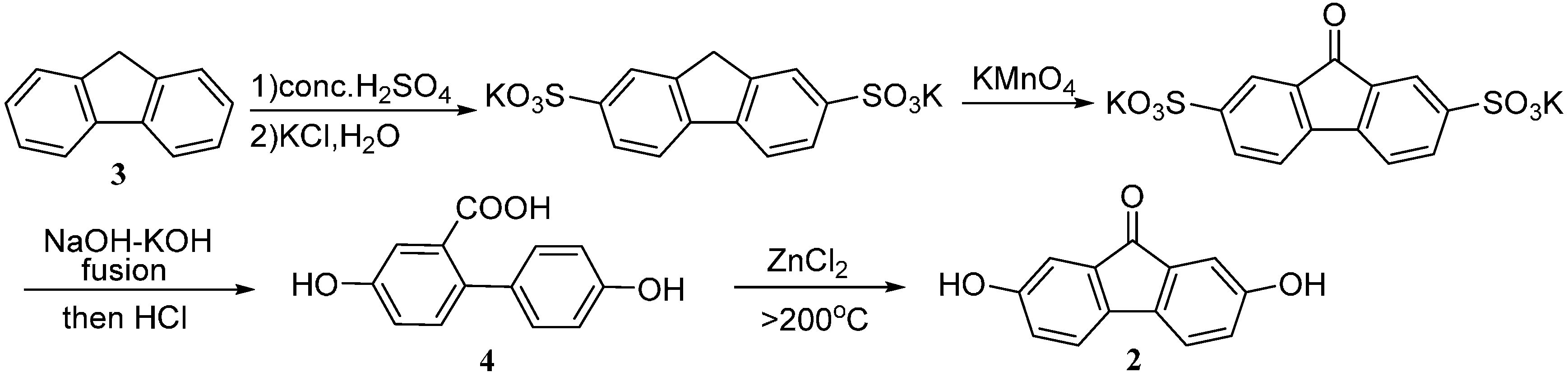
2. Results and Discussion
2.1 Synthesis of Tilorone Dihydrochloride
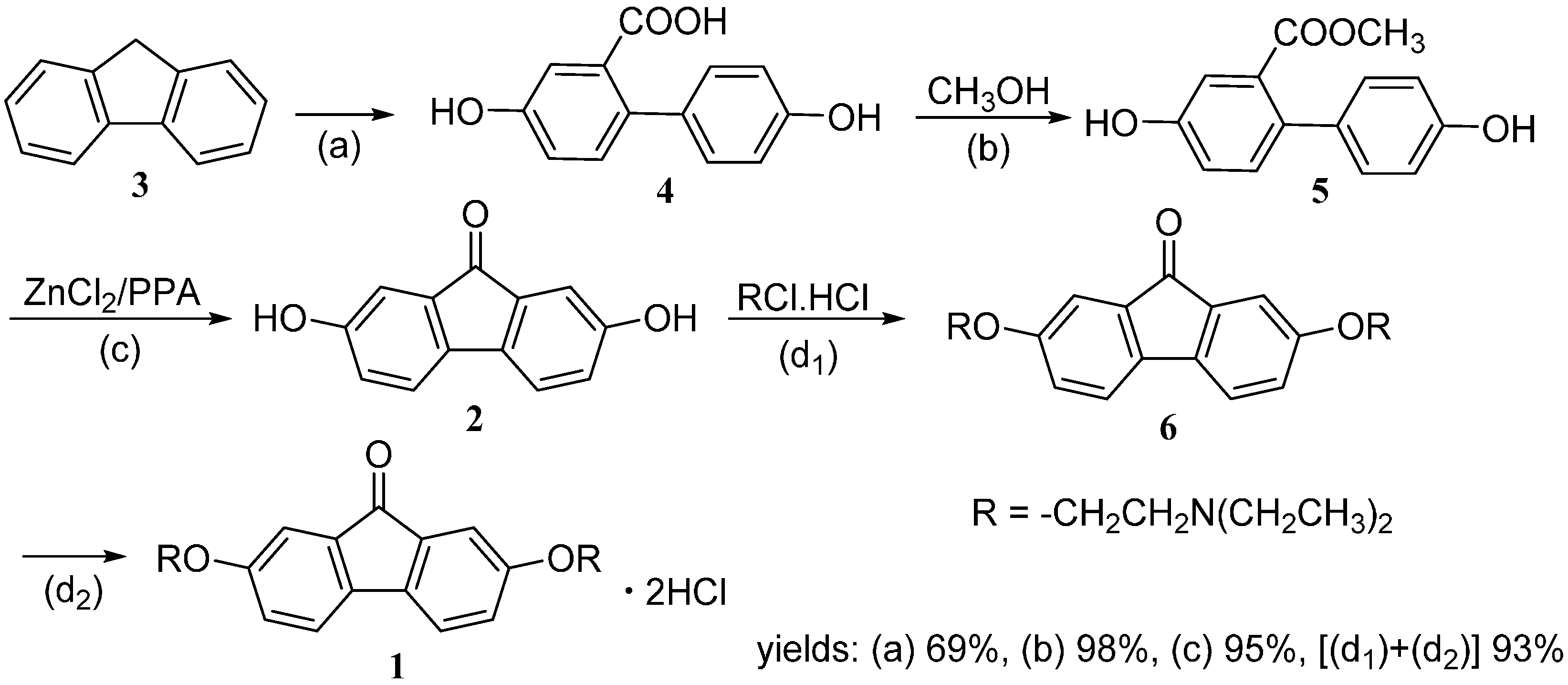
2.2 Evaluation on Induction Activity of IFN-α
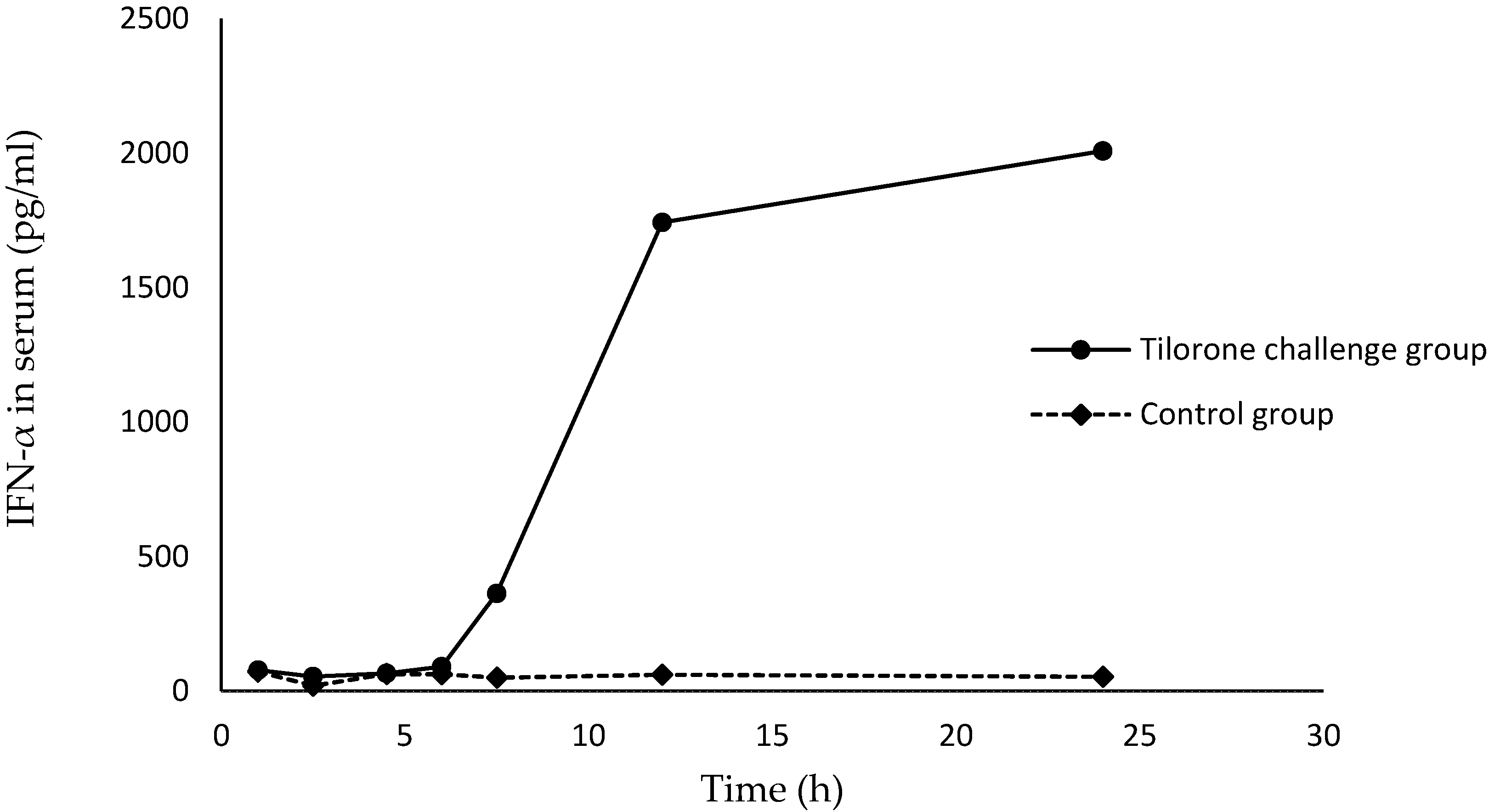
3. Experimental Section
3.1. Synthesis of Tilorone Dihydrochloride (1)
General
3.2. Determination of Serum IFN-α Induced by Tilorone Dihydrochloride by ELISA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krueger, R.E.; Mayer, G.D. Tilorone hydrochloride: An orally active antiviral agent. Science 1970, 169, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.F.; Mayer, G.D.; Yoshimura, S.; Ludwig, K.A. In vivo evaluations of tilorone hydrochloride against semliki forest virus. Antimicrob. Agents Chemother. 1970, 10, 486–490. [Google Scholar] [PubMed]
- Gaforio, J.J.; Ortega, E.; Algarra, I.; Serrano, M.J.; Alvarez de Cienfuegos, G. Nk cells mediate increase of phagocytic activity but not of proinflammatory cytokine (interleukin-6 [IL-6], tumor necrosis factor alpha, and IL-12) production elicited in splenic macrophages by tilorone treatment of mice during acute systemic candidiasis. Clin. Vaccine Immunol. 2002, 9, 1282–1294. [Google Scholar] [CrossRef]
- Clark, W.G.; Robins, J.A. The antipyretic effect of tilorone hydrochloride in the cat. Br. J. Pharmacol. 1978, 62, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Lepparanta, O.; Tikkanen, J.M.; Bespalov, M.M.; Koli, K.; Myllarniemi, M. Bone morphogenetic protein-inducer tilorone identified by high-throughput screening is antifibrotic in vivo. Am. J. Respir. Cell Mol. Biol. 2013, 48, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Megel, H.; Raychaudhuri, A.; Shemano, I.; Beaver, T.H.; Thomas, L.L. The anti-inflammatory actions of tilorone hydrochloride. Proc. Soc. Exp. Biol. Med. 1975, 149, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, P.; Sauer, I.; Schaljo, B. Molecular mechanisms of the anti-inflammatory functions of interferons. Immunobiology 2007, 212, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Skrots’ka, O.I.; Zholobak, N.M.; Antonenko, S.V.; Spivak, M.; Karpov, O.V. Antiherpetic effect of rna-tilorone molecular complex in cell culture. Mikrobiol. Z. 2007, 69, 62–68. [Google Scholar] [PubMed]
- Zholobak, N.M.; Kavok, N.S.; Bogorad-Kobelska, O.S.; Borovoy, I.A.; Malyukina, M.Y.; Spivak, M.Y. Effect of tilorone and its analogues on the change of mitochondrial potential of rat hepatocytes. Fiziolohichnyi Zhurnal 2012, 58, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.A.; Schrimpf, M.R.; Anderson, D.J.; Gubbins, E.J.; Gronlien, J.H.; Hakerud, M.; Ween, H.; Thorin-Hagene, K.; Malysz, J.; Li, J.; et al. Alpha7 nicotinic acetylcholine receptor agonist properties of tilorone and related tricyclic analogues. Br. J. Pharmacol. 2008, 153, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.V.; Zholobak, N.M.; Spivak, N.Y.; Rybalko, S.L.; Antonenko, S.V.; Krivokhatskaya, L.D. Virus-inhibitory effect of a yeast RNA-tilorone molecular complex in cell cultures. Acta Virol. 2001, 45, 181–184. [Google Scholar] [PubMed]
- Karpov, A.V.; Antonenko, S.V.; Barbasheva, E.V.; Spivak, N. Study of anti-HIV activity of the yeast RNA-tilorone molecular complex. Vopr. Virusol. 1997, 42, 17–19. [Google Scholar] [PubMed]
- Wissing, M.D.; Dadon, T.; Kim, E.; Piontek, K.B.; Shim, J.S.; Kaelber, N.S.; Liu, J.O.; Kachhap, S.K.; Nelkin, B.D. Small-molecule screening of pc3 prostate cancer cells identifies tilorone dihydrochloride to selectively inhibit cell growth based on cyclin-dependent kinase 5 expression. Oncol. Rep. 2014, 32, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Algarra, I.; Perez, M.; Hoglund, P.; Gaforio, J.J.; Ljunggren, H.G.; Garrido, F. Generation and control of metastasis in experimental tumor systems; inhibition of experimental metastases by a tilorone analogue. Int. J. Cancer 1993, 54, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tuo, W.; Hu, H.; Xu, J.; Chen, H.; Rao, Z.; Xiao, Y.; Hu, X.; Liu, P. Synthesis and activity evaluation of tilorone analogs as potential anticancer agents. Eur. J. Med. Chem. 2013, 64, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sherry, B. Ifn-alpha expression and antiviral effects are subtype and cell type specific in the cardiac response to viral infection. Virology 2010, 396, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, Y.; Kuriyama, K. Dissociation between antiinflammatory action of tilorone and its interferon inducing activity. Agents Actions 1991, 33, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Kunz, C. The protective effect of the interferon inducers tilorone hydrochloride and poly i:C on experimental tick-borne encephalitis in mice. Arch. Gesamte Virusforsch. 1972, 37, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chang, D.M.; Huang, K.F.; Chen, C.L.; Chen, T.C.; Lo, Y.; Guh, J.H.; Huang, H.S. Design, synthesis and antiproliferative evaluation of fluorenone analogs with DNA topoisomerase i inhibitory properties. Bioorg. Med. Chem. 2013, 21, 7125–7133. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Morrell, A.; Dexheimer, T.S.; Scher, E.S.; Pommier, Y.; Cushman, M. Design, synthesis, and biological evaluation of 14-substituted aromathecins as topoisomerase i inhibitors. J. Med. Chem. 2008, 51, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Waldo, J.P.; Zhang, X.; Shi, F.; Larock, R.C. Efficient synthesis of fluoren-9-ones by the palladium-catalyzed annulation of arynes by 2-haloarenecarboxaldehydes. J. Org. Chem. 2008, 73, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.R.; Fleming, R.W.; Grisar, J.M.; Kihm, J.C.; Wenstrup, D.L.; Mayer, G.D. Bis-basic-substituted polycyclic aromatic compounds. A new class of antiviral agents. 2. Tilorone and related bis-basic ethers of fluorenone, fluorenol, and fluorene. J. Med. Chem. 1974, 17, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.M.; Joullie, M.M. New synthetic routes to tilorone dihydrochloride and some of its analogues. J. Med. Chem. 1978, 21, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.C. Fluorene derivatives for antitumor activity. J. Med. Chem. 1967, 10, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Balezina, T.I.; Korneeva, L.E.; Zemskov, V.M.; Loidina, G.I.; Nikolaeva, O.V.; Fainshtein, S.L.; Fadeeva, L.L.; Eromolieva, Z.V. Comparison of interferon-inducing activities and antiviral effects of tobacco mosaic virus, tilorone and sodium nucleinate. Acta Virol. 1977, 21, 338–343. [Google Scholar] [PubMed]
- Hane, H.; Muro, Y.; Watanabe, K.; Ogawa, Y.; Sugiura, K.; Akiyama, M. Establishment of an ELISA to detect anti-glycyl-tRNA synthetase antibody (anti-EJ), a serological marker of dermatomyositis/polymyositis and interstitial lung disease. Clin. Chim. Acta 2014, 431, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Meroni, V.; Genco, F.; Tamarozzi, F.; Tinelli, C.; Filice, C.; Brunetti, E. Serum cytokine profile by ELISA in patients with echinococcal cysts of the liver: A stage-specific approach to assess their biological activity. Clin. Dev. Immunol. 2012, 2012, 483935. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, S.G.; Gruffydd-Jones, T.; Gunn-Moore, D.; Jahans, K. Adaptation of IFN-gamma ELISA and ELISPOT tests for feline tuberculosis. Vet. Immunol. Immunopathol. 2008, 124, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: All the samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yao, Q.; Liu, Z. An Effective Synthesis Method for Tilorone Dihydrochloride with Obvious IFN-α Inducing Activity. Molecules 2015, 20, 21458-21463. https://doi.org/10.3390/molecules201219781
Zhang J, Yao Q, Liu Z. An Effective Synthesis Method for Tilorone Dihydrochloride with Obvious IFN-α Inducing Activity. Molecules. 2015; 20(12):21458-21463. https://doi.org/10.3390/molecules201219781
Chicago/Turabian StyleZhang, Junren, Qizheng Yao, and Zuliang Liu. 2015. "An Effective Synthesis Method for Tilorone Dihydrochloride with Obvious IFN-α Inducing Activity" Molecules 20, no. 12: 21458-21463. https://doi.org/10.3390/molecules201219781
APA StyleZhang, J., Yao, Q., & Liu, Z. (2015). An Effective Synthesis Method for Tilorone Dihydrochloride with Obvious IFN-α Inducing Activity. Molecules, 20(12), 21458-21463. https://doi.org/10.3390/molecules201219781





