The Novel Multiple Inner Primers-Loop-Mediated Isothermal Amplification (MIP-LAMP) for Rapid Detection and Differentiation of Listeria monocytogenes
Abstract
:1. Introduction
2. Results
2.1. The Principle of the MIP-LAMP for Detecting Target DNA

2.2. Primers Design of MIP-LAMP
| Primers | Sequence (5′-3′) | Length |
|---|---|---|
| F3 | TCAAGTTGTGAATGCAATTTCGA | 23 nt |
| B3 | GCTCTTTAGTAACAGCTTTGCCG | 23 nt |
| FIP1(F1c-1 + F2-1) | CGTTTTACAGGGAGAACATCTGGTTGttttCTAACCTATCCAGGTGCTCTCG | 52 mer |
| FIP2(F1c-2 + F2-2) | GCGTTGTTAACGTTTGATTTAGTGGCttttCACTCAGCATTGATTTGCCAGGT | 53 mer |
| BIP2(B1c-1 + B2-1) | TGACGAAATGGCTTACAGTGAATCACttttGCGCCGAAGTTTACATTCAAGCT | 53 mer |
| BIP1(B1c-2 + B2-2) | AATCAGTGAAGGGAAAATGCAAGAAGttttCTGGAAGGTCTTGTAGGTTCAT | 52 mer |
| LF1 | TCTACTAATTCCGAATTCGCT | 21 nt |
| LF2 | CAACGATTTTATTGTCTTGATTAG | 24 nt |
| LB2 | TGCGAAATTTGGTACAGCAT | 20 nt |
| LB1 | GTCATTAGTTTTAAACAAATTTACTATAACG | 31 nt |
| P1 | CTAACCTATCCAGGTGCTCTCG | 22 nt |
| P2 | CACTCAGCATTGATTTGCCAGGT | 23 nt |
| B2 | GCGCCGAAGTTTACATTCAAGCT | 23 nt |
| B1 | CTGGAAGGTCTTGTAGGTTCAT | 22 nt |
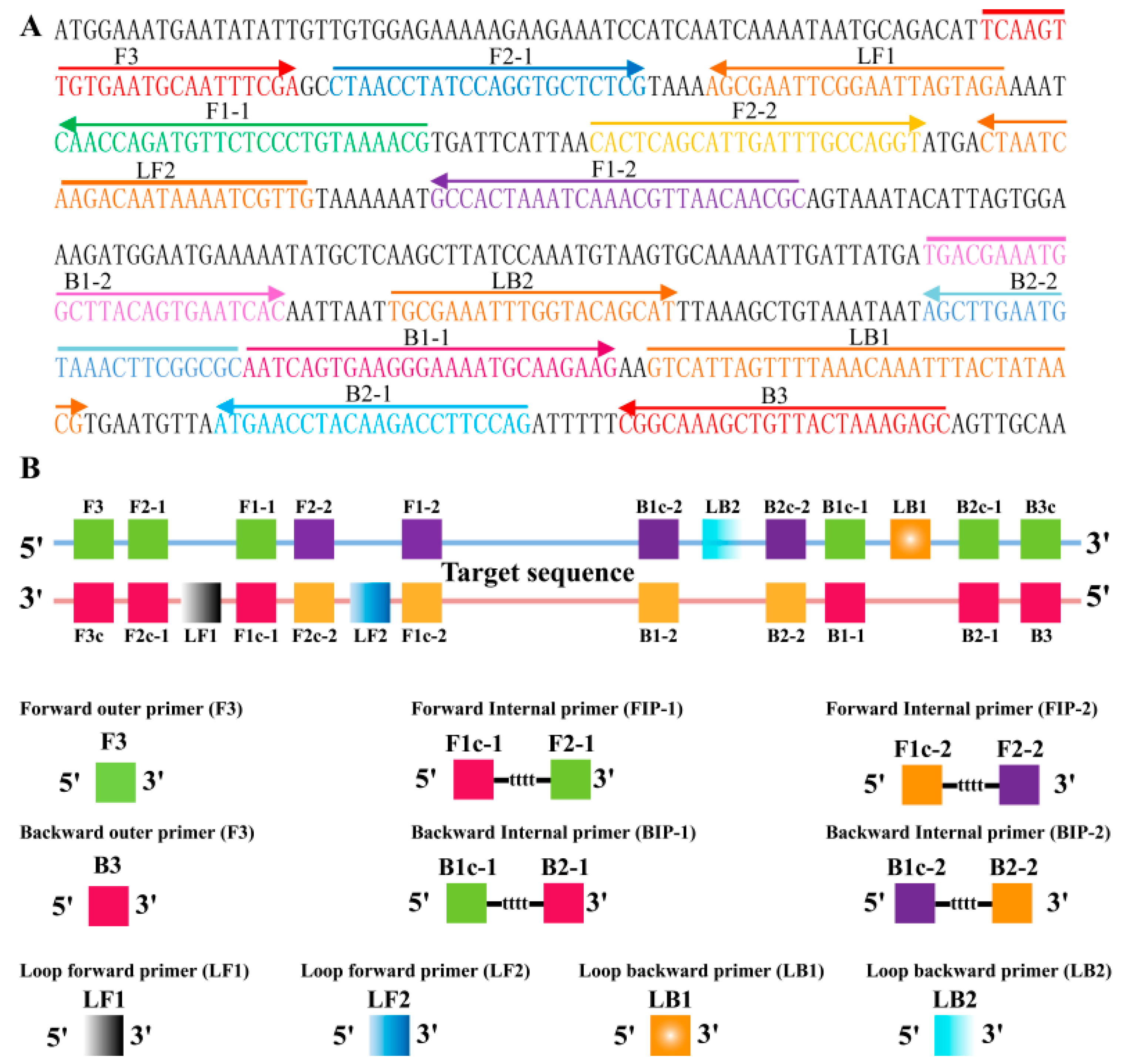
2.3. The Availability of MIP-LAMP Primers

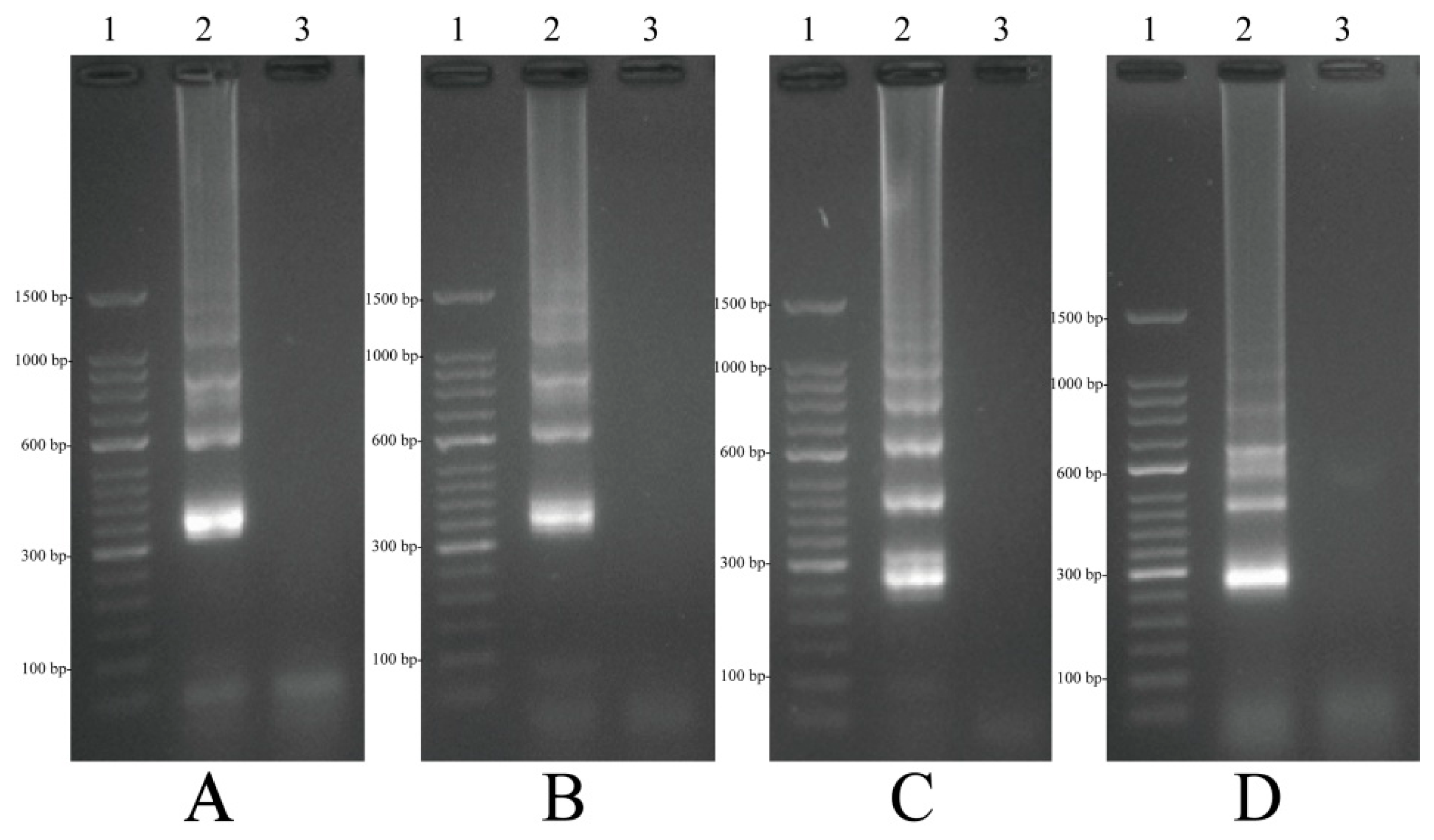
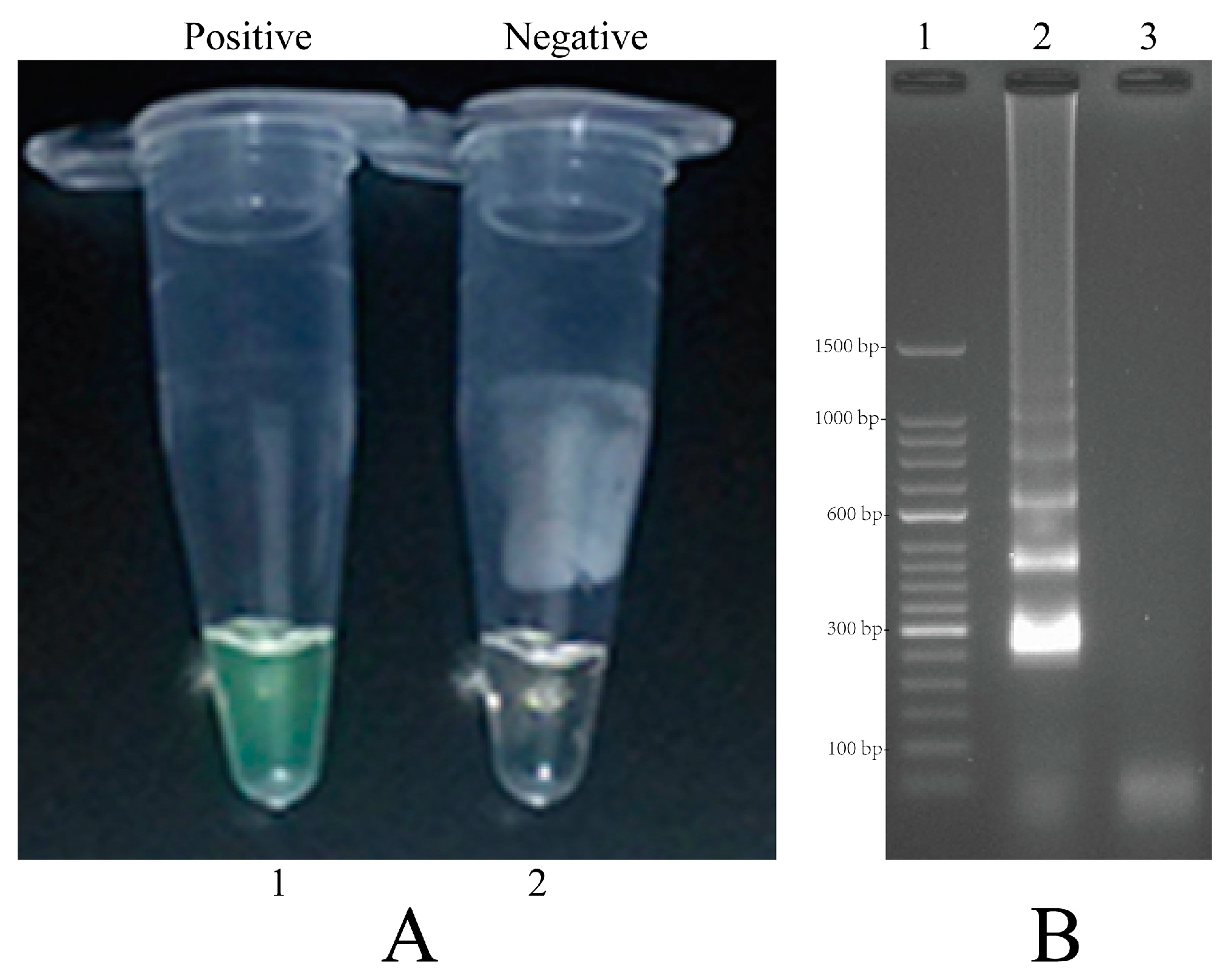
2.4. Confirmation and Detection of MIP-LAMP Products
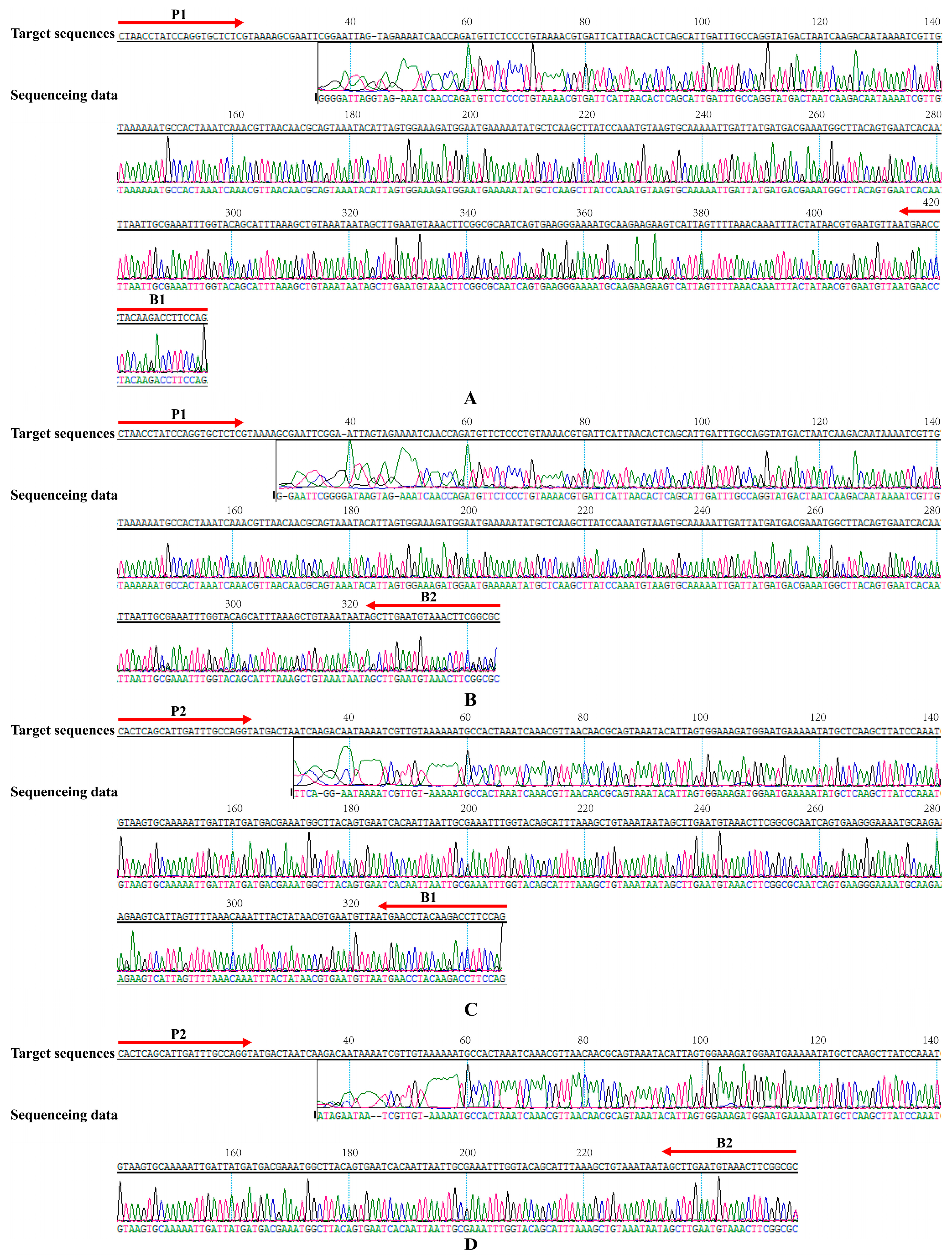
2.5. Validation of the Reliability of MIP-LAMP by Sequencing
2.6. The Optimal Temperature of MIP-LAMP Assay
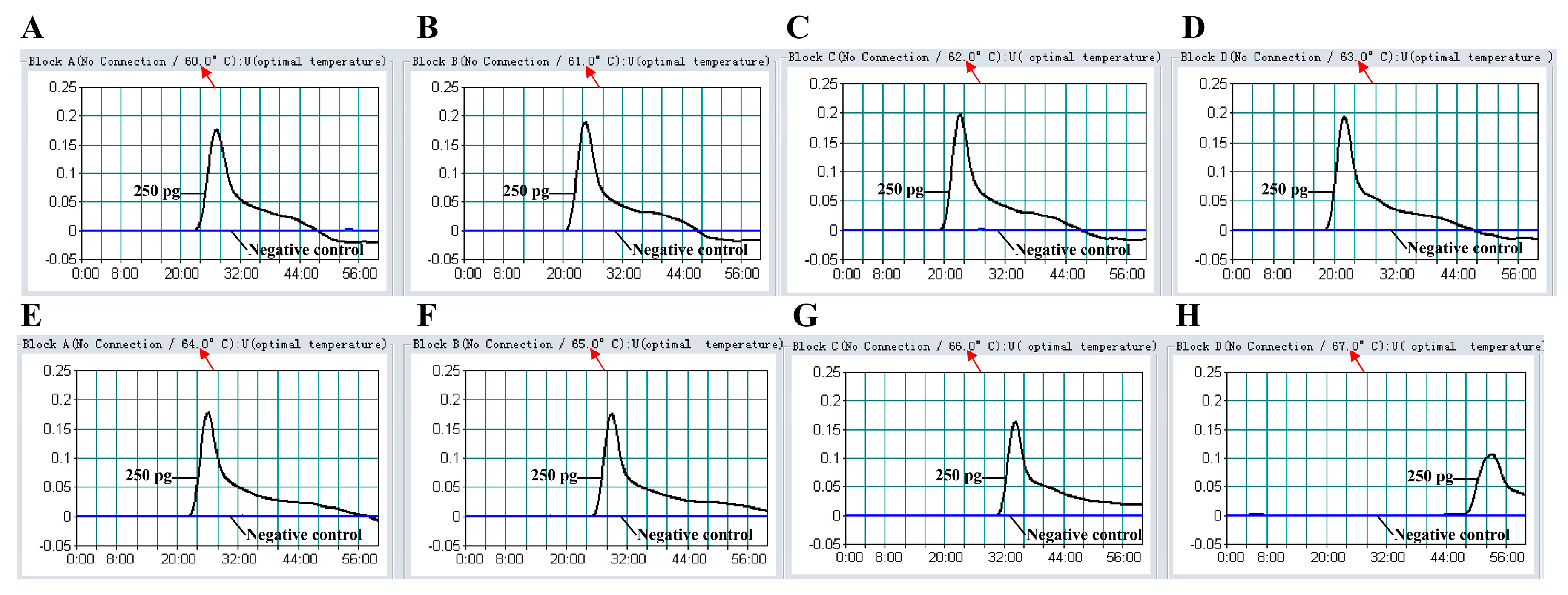
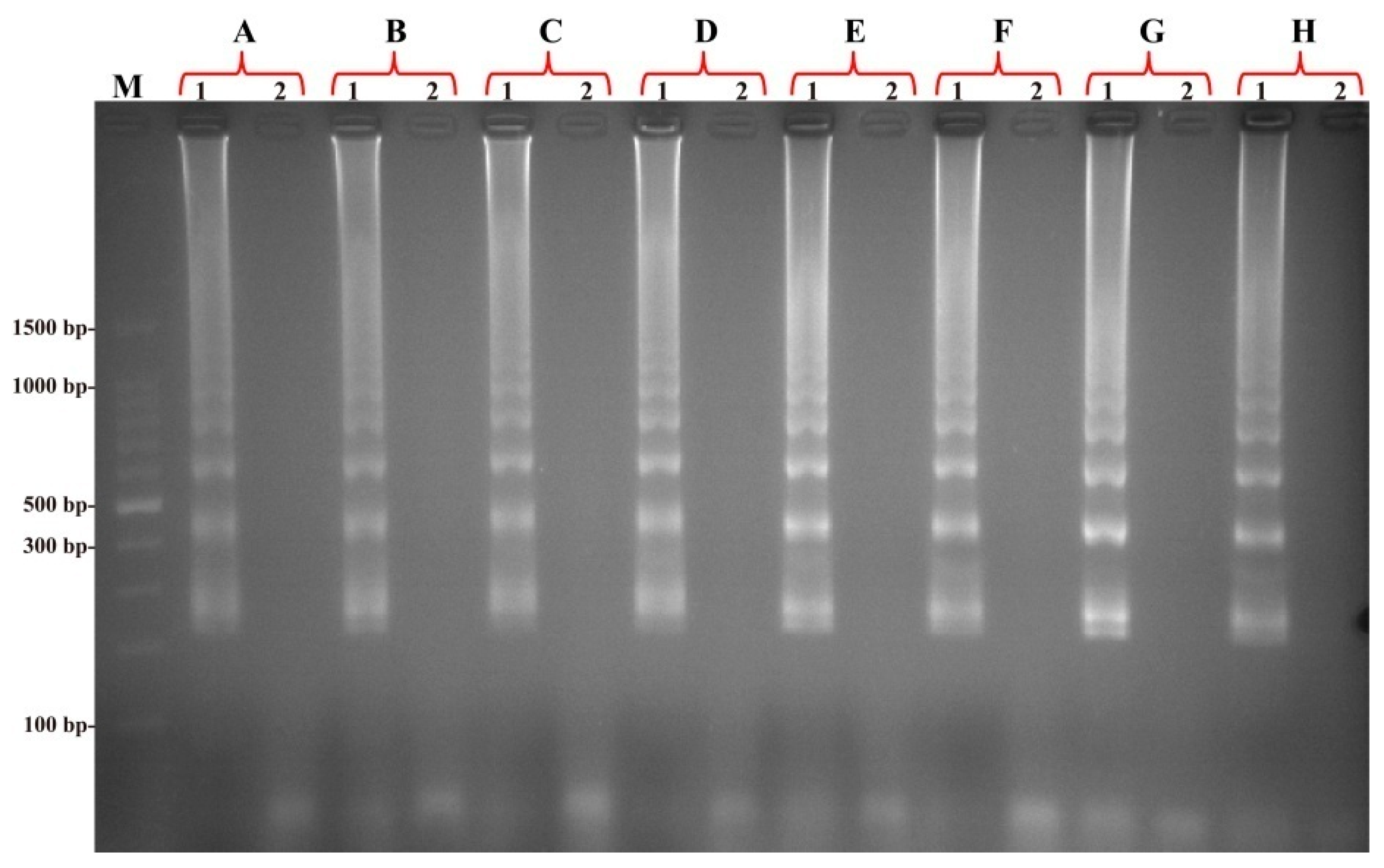
2.7. The Specificity of the MIP-LAMP Assay for Detection of L. monocytogenes
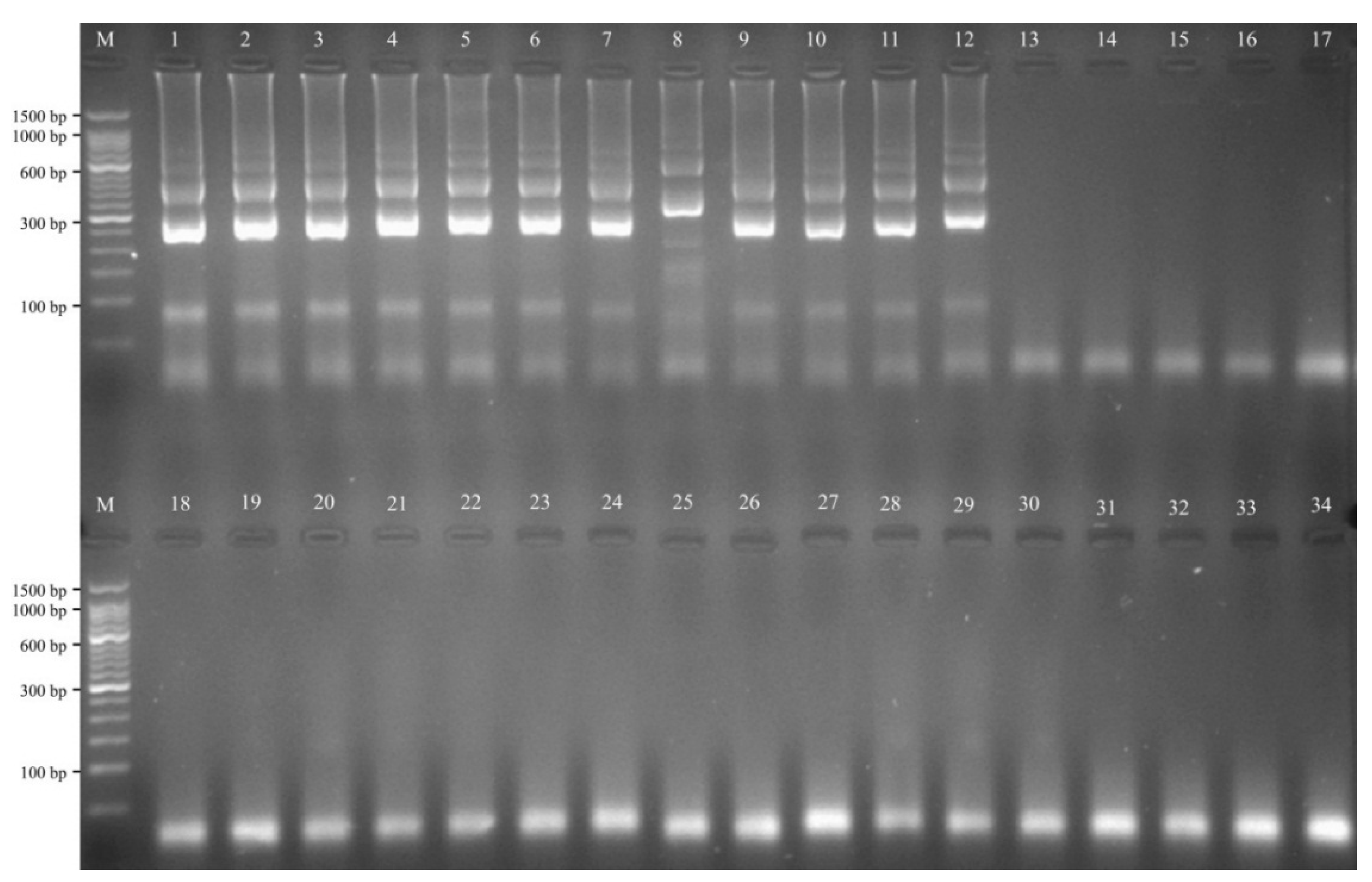
2.8. The Analytical Sensitivity of MIP-LAMP Assays
2.9. Sensitivity Comparison of nLAMP and MIP-LAMP in Test of Genomic DNA of L. monocytogenes
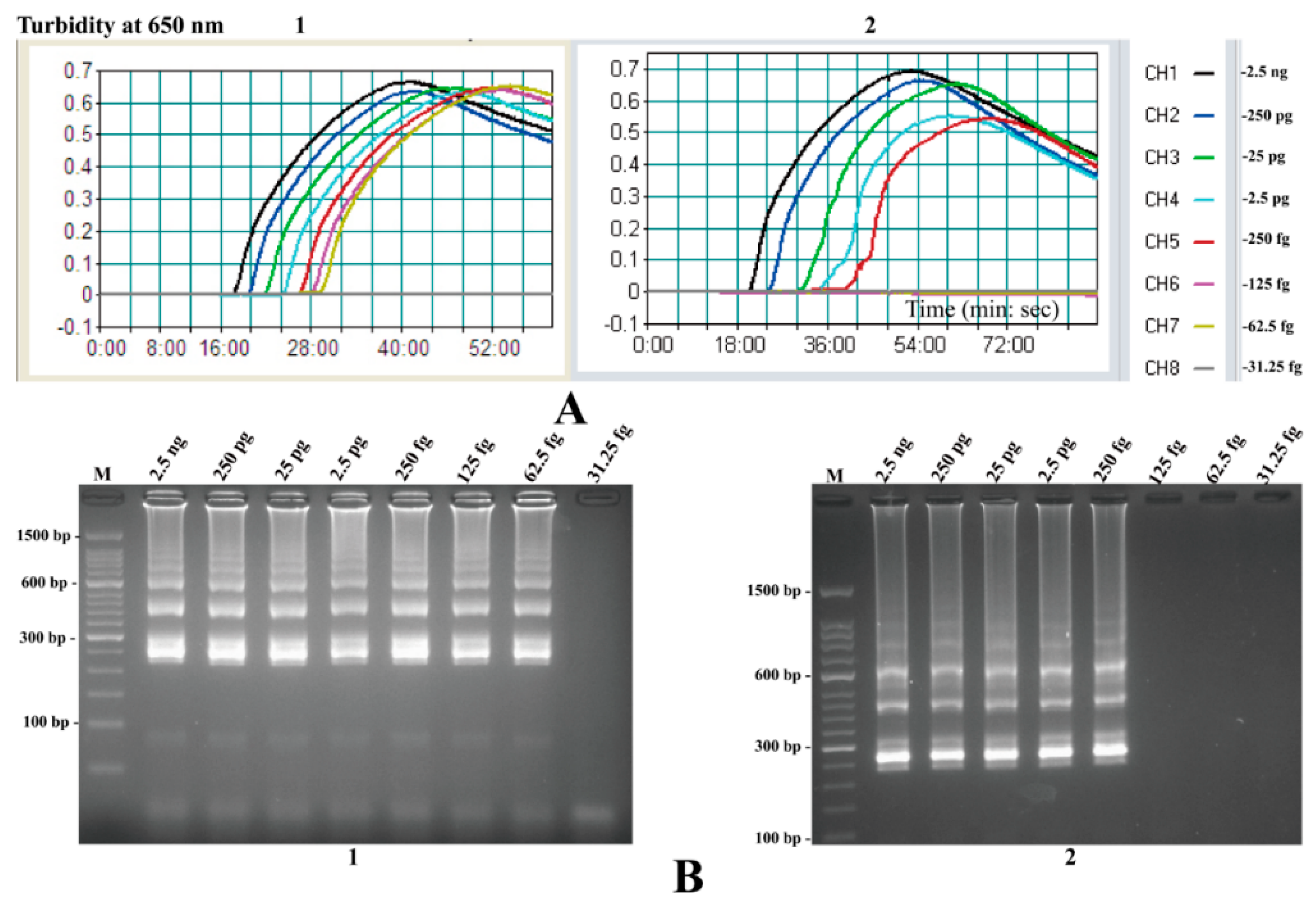
2.10. Evaluation of the MIP-LAMP Assay in Artificially Contaminated Milk
2.11. Evaluation of the MIP-LAMP Assay Using Pork Samples
| Samples | Cultures | nLAMP | MIP-LAMP | ||
|---|---|---|---|---|---|
| 24 h in FB | 48 h in FB | 24 h in FB | 48 h in FB | ||
| 1 | − | − | − | − | − |
| 2 | − | − | − | − | − |
| 3 | − | − | − | − | − |
| 4 | − | − | − | − | − |
| 5 | − | − | − | − | − |
| 6 | + | + | + | + | + |
| 7 | − | − | − | − | − |
| 8 | − | − | − | − | − |
| 9 | − | − | − | − | − |
| 10 | − | − | − | − | − |
| 11 | + | + | + | + | + |
| 12 | − | − | − | − | − |
| 13 | − | − | − | − | − |
| 14 | + | + | + | + | + |
| 15 | + | + | + | + | + |
| 16 | − | − | − | − | − |
| 17 | − | − | − | − | − |
| 18 | + | + | + | + | + |
| 19 | + | + | + | + | + |
| 20 | + | − | + | + | + |
| 21 | − | − | − | − | − |
| 22 | − | − | − | − | − |
| 23 | − | − | − | − | − |
| 24 | − | − | − | − | − |
| 25 | − | − | − | − | − |
| 26 | − | − | − | − | − |
| 27 | − | − | − | − | − |
| 28 | − | − | − | − | − |
| 29 | + | − | + | + | + |
| 30 | − | − | − | − | − |
| 31 | − | − | − | − | − |
| 32 | − | − | − | − | − |
| 33 | − | − | − | − | − |
| 34 | − | − | − | − | − |
| 35 | − | − | − | − | − |
| 36 | − | − | − | − | − |
| 37 | − | − | − | − | − |
| 38 | − | − | − | − | − |
| 39 | − | − | − | − | − |
| 40 | − | − | − | − | − |
| 41 | + | + | + | + | + |
| 42 | + | + | + | + | + |
| 43 | − | − | − | − | − |
| 44 | + | − | + | + | + |
| 45 | − | − | − | − | − |
| 46 | + | + | + | + | + |
| 47 | − | − | − | − | − |
| 48 | + | + | + | + | + |
| Total | 13 | 10 | 13 | 13 | 13 |
3. Discussion
4. Experimental Section
4.1. Primers for the MIP-LAMP Amplification
4.2. Bacterial Strains and Genomic DNA Extraction
| Bacteria | Serovar a | Strain No. (Source of Strain) b | No. of Strains |
|---|---|---|---|
| Listeria monocytogenes | 1/2a | EGD-e | 1 |
| Isolated strains (ICDC) | 4 | ||
| 3a | Isolated strains (ICDC) | 5 | |
| 1/2b | Isolated strains (ICDC) | 5 | |
| 3b | Isolated strains (ICDC) | 1 | |
| 1/2c | Isolated strains (ICDC) | 5 | |
| 3c | Isolated strains (ICDC) | 1 | |
| 7 | NCTC10890 | 1 | |
| 4a | ATCC19114 | 1 | |
| Isolated strains (ICDC) | 2 | ||
| 4c | ATCC19116 | 1 | |
| Isolated strains (ICDC) | 2 | ||
| 4b | Isolated strains (ICDC) | 5 | |
| 4d | ATCC19117 | 1 | |
| Isolated strains (ICDC) | 2 | ||
| 4e | ATCC19118 | 1 | |
| Isolated strains (ICDC) | 1 | ||
| Listeria ivanovii | U | ATCCBAA-678 | 1 |
| Isolated strains (ICDC) | 2 | ||
| Listeria innocua | U | ATCCBAA-680 | 1 |
| Isolated strains (ICDC) | 2 | ||
| Listeria grayi | U | ATCC25402 | 1 |
| Isolated strains (ICDC) | 2 | ||
| Listeria seeligeri | U | ATCC35967 | 1 |
| Isolated strains (ICDC) | 1 | ||
| Listeria welshimeri | U | ATCC35897 | 1 |
| Isolated strains (ICDC) | 2 | ||
| Bacillus cereus | U | Isolated strains (ICDC) | 1 |
| Enteropathogenic E. coli | U | Isolated strains (ICDC) | 1 |
| Enterotoxigenic E. coli | U | Isolated strains (ICDC) | 1 |
| Enteroaggregative E. coli | U | Isolated strains (ICDC) | 1 |
| Enteroinvasive E. coli | U | Isolated strains (ICDC) | 1 |
| Enterohemorrhagic E. coli | U | EDL933 | 1 |
| Plesiomonas shigelloides | U | Isolated strains (ICDC) | 1 |
| Shigella flexneri | U | Isolated strains (ICDC) | 1 |
| Shigella sonnei | U | Isolated strains (ICDC) | 1 |
| Enterococcus faecalis | U | ATCC35667 | 1 |
| Enterococcus faecium | U | Isolated strains (ICDC) | 1 |
| Yersinia enterocolitica | U | Isolated strains (ICDC) | 1 |
| Aeromona shydrophila | U | ATCC7966 | 1 |
| Citrobacter freumdii | U | Isolated strains (ICDC) | 1 |
| Proteus vulgaris | U | Isolated strains (ICDC) | 1 |
| Vibrio fluvialis | U | Isolated strains (ICDC) | 1 |
| Streptococcus bovis | U | Isolated strains (ICDC) | 1 |
| Vibrio parahaemolyticus | U | Isolated strains (ICDC) | 1 |
| Klebsiella pneumoniae | U | Isolated strains (ICDC) | 1 |
| Salmonella enterica | U | Isolated strains (ICDC) | 1 |
4.3. The MIP-LAMP Assay
4.4. Validation by Sequencing
4.5. The MIP-LAMP Specificity Test
4.6. The MIP-LAMP Sensitivity Test
4.7. MIP-LAMP Application in Artificially Contaminated Milk
4.8. Practical Application of MIP-LAMP Detection to L. monocytogenes in Food Samples
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclosures
References
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Sauders, B.D.; Overdevest, J.; Fortes, E.; Windham, K.; Schukken, Y.; Lembo, A.; Wiedmann, M. Diversity of listeria species in urban and natural environments. Appl. Environ. Microbiol. 2012, 78, 4420–4433. [Google Scholar] [CrossRef] [PubMed]
- Maertens de Noordhout, C.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- Awofisayo, A.; Amar, C.; Ruggles, R.; Elson, R.; Adak, G.K.; Mook, P.; Grant, K.A. Pregnancy-associated listeriosis in england and wales. Epidemiol. Infect. 2015, 143, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kathariou, S. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 2002, 65, 1811–1829. [Google Scholar] [PubMed]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P.; et al. Multistate outbreak of listeriosis associated with cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Mateus, T.; Silva, J.; Maia, R.L.; Teixeira, P. Listeriosis during pregnancy: A public health concern. ISRN Obstet. Gynecol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Goulet, V.; Hebert, M.; Hedberg, C.; Laurent, E.; Vaillant, V.; de Valk, H.; Desenclos, J.C. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clin. Infect. Dis. 2012, 54, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.W. Detection and isolation of listeria monocytogenes from food samples: Implications of sublethal injury. J. AOAC Int. 2002, 85, 495–500. [Google Scholar] [PubMed]
- Somer, L.; Kashi, Y. A PCR method based on 16s rRNA sequence for simultaneous detection of the genus listeria and the species listeria monocytogenes in food products. J. Food Prot. 2003, 66, 1658–1665. [Google Scholar] [PubMed]
- Delibato, E.; Gattuso, A.; Minucci, A.; Auricchio, B.; de Medici, D.; Toti, L.; Castagnola, M.; Capoluongo, E.; Gianfranceschi, M.V. PCR experion automated electrophoresis system to detect listeria monocytogenes in foods. J. Sep. Sci. 2009, 32, 3817–3821. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; Ruttledge, M.; Sedano-Balbas, S.; Smith, T.J.; Barry, T.; Maher, M. Rapid detection of listeria monocytogenes in food using culture enrichment combined with real-time PCR. Food Microbiol. 2009, 26, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Molinos, A.C.; Abriouel, H.; Ben Omar, N.; Martinez-Canamero, M.; Galvez, A. A quantitative real-time pcr assay for quantification of viable listeria monocytogenes cells after bacteriocin injury in food-first insights. Curr. Microbiol. 2010, 61, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Chander, Y.; Koelbl, J.; Puckett, J.; Moser, M.J.; Klingele, A.J.; Liles, M.R.; Carrias, A.; Mead, D.A.; Schoenfeld, T.W. A novel thermostable polymerase for RNA and DNA loop-mediated isothermal amplification (LAMP). Front. Microbiol. 2014, 5, 395. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Niessen, L. Current state and future perspectives of loop-mediated isothermal amplification (LAMP)-based diagnosis of filamentous fungi and yeasts. Appl. Microbiol. Biotechnol. 2015, 99, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Watanabe, K.; Ohtsuka, K.; Hase, T.; Notomi, T. Loop-mediated isothermal amplification reaction using a nondenatured template. Clin. Chem. 2001, 47, 1742–1743. [Google Scholar] [PubMed]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Lan, R.; Xu, H.; Ma, A.; Li, D.; Dai, H.; Yuan, X.; Xu, J.; Ye, C. Multiple endonuclease restriction real-time loop-mediated isothermal amplification: A novel analytically rapid, sensitive, multiplex Loop-mediated isothermal amplification detection technique. J. Mol. Diagn. 2015, 17, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Churchill, R.L.; Lee, H.; Hall, J.C. Detection of listeria monocytogenes and the toxin listeriolysin O in food. J. Microbiol. Methods 2006, 64, 141–170. [Google Scholar] [CrossRef] [PubMed]
- De Boer, E.; Beumer, R.R. Methodology for detection and typing of foodborne microorganisms. Int. J. Food Microbiol. 1999, 50, 119–130. [Google Scholar] [CrossRef]
- Law, J.W.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2014, 5, 770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Yang, J.W.; Shao, J.B.; Liao, X.L.; Lu, Z.H.; Jiang, H. Real-time polymerase chain reaction for diagnosing infectious mononucleosis in pediatric patients: A systematic review and meta-analysis. J. Med. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Ghaemi, A. Nucleic acid isothermal amplification technologies: A review. Nucleosides Nucleotides Nucleic Acids 2008, 27, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Qu, G.; Guo, S.; Ma, L.; Zhang, N.; Zhang, S.; Gao, S.; Shen, Z. Applications of loop-mediated isothermal DNA amplification. Appl. Biochem. Biotechnol. 2011, 163, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.V.; Morita, K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Millet, V.; Lonvaud-Funel, A. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 2000, 30, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Chu, J.; Xu, Z.; Zhong, Q. Development and application of a simple loop-mediated isothermal amplification method on rapid detection of listeria monocytogenes strains. Mol. Biol. Rep. 2012, 39, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J. Advances in isothermal amplification: Novel strategies inspired by biological processes. Biosens. Bioelectron. 2014, 64C, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Xu, H.; Dai, H.; Meng, S.; Ye, C. Rapid and sensitive detection of listeria ivanovii by loop-mediated isothermal amplification of the smcl gene. PLoS ONE 2014, 9, e115868. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Barbuddhe, S.; Malik, S.V.; Singh, R.K. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.J.; Zhou, S.; Zhang, X.Y.; Pu, J.H.; Ge, Q.L.; Tang, X.J.; Gao, Y.S. Rapid and sensitive detection of listeria monocytogenes by loop-mediated isothermal amplification. Curr. Microbiol. 2011, 63, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Ma, A.; Li, D.; Ye, C. Rapid and sensitive detection of listeria monocytogenes by cross-priming amplification of lmo0733 gene. FEMS Microbiol. Lett. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Ma, A.; Li, D.; Luo, L.; Liu, D.; Hu, S.; Jin, D.; Liu, K.; Ye, C. The Novel Multiple Inner Primers-Loop-Mediated Isothermal Amplification (MIP-LAMP) for Rapid Detection and Differentiation of Listeria monocytogenes. Molecules 2015, 20, 21515-21531. https://doi.org/10.3390/molecules201219787
Wang Y, Wang Y, Ma A, Li D, Luo L, Liu D, Hu S, Jin D, Liu K, Ye C. The Novel Multiple Inner Primers-Loop-Mediated Isothermal Amplification (MIP-LAMP) for Rapid Detection and Differentiation of Listeria monocytogenes. Molecules. 2015; 20(12):21515-21531. https://doi.org/10.3390/molecules201219787
Chicago/Turabian StyleWang, Yi, Yan Wang, Aijing Ma, Dongxun Li, Lijuan Luo, Dongxin Liu, Shoukui Hu, Dong Jin, Kai Liu, and Changyun Ye. 2015. "The Novel Multiple Inner Primers-Loop-Mediated Isothermal Amplification (MIP-LAMP) for Rapid Detection and Differentiation of Listeria monocytogenes" Molecules 20, no. 12: 21515-21531. https://doi.org/10.3390/molecules201219787
APA StyleWang, Y., Wang, Y., Ma, A., Li, D., Luo, L., Liu, D., Hu, S., Jin, D., Liu, K., & Ye, C. (2015). The Novel Multiple Inner Primers-Loop-Mediated Isothermal Amplification (MIP-LAMP) for Rapid Detection and Differentiation of Listeria monocytogenes. Molecules, 20(12), 21515-21531. https://doi.org/10.3390/molecules201219787






