Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs
Abstract
:1. Introduction
2. Types of Hydrogels
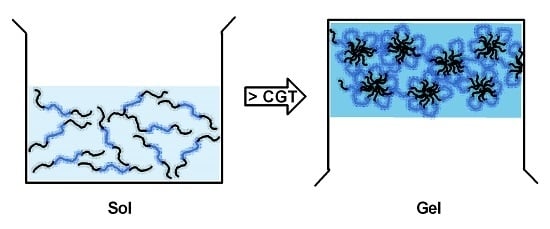
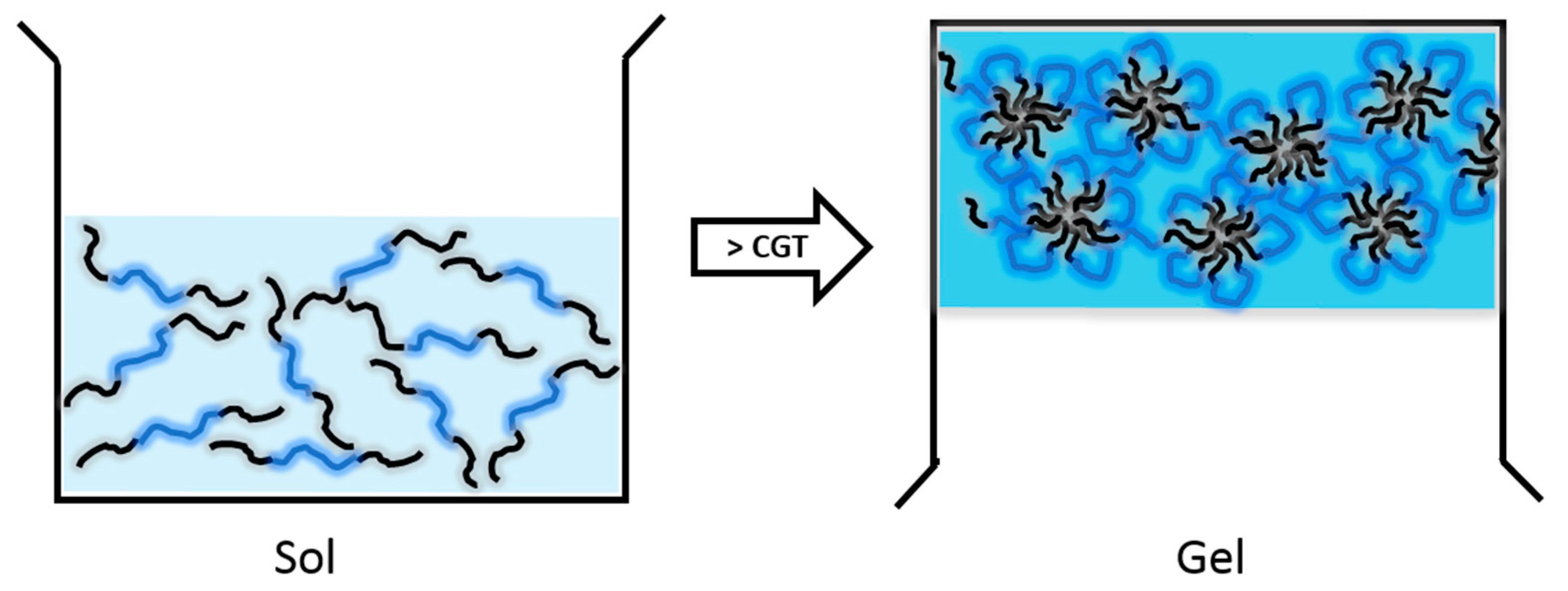
2.1. Thermosensitive Hydrogels
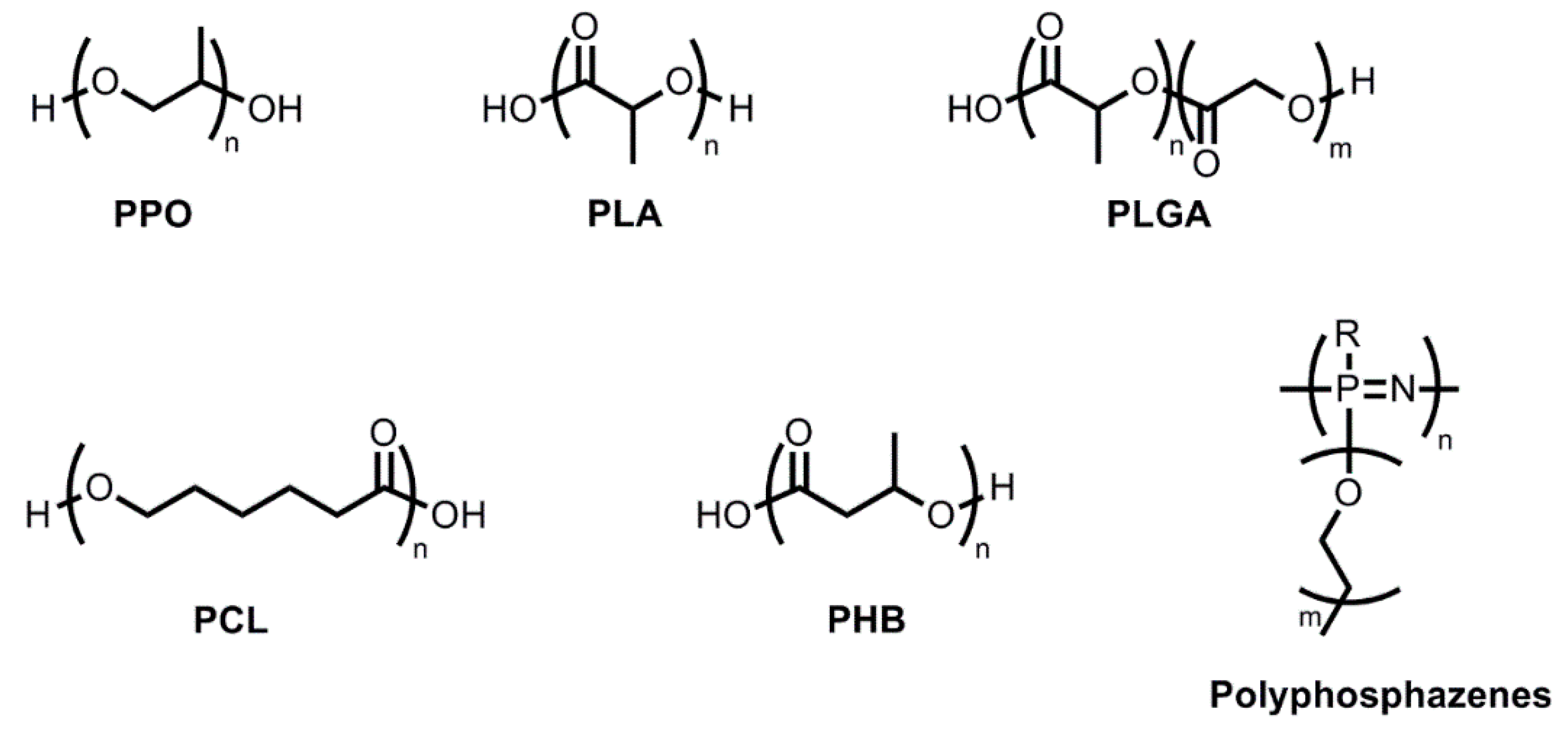
2.1.1. Hydrogels with Hydrophobic PPO Blocks
2.1.2. Hydrogels with Hydrophobic PLA Blocks
2.1.3. Hydrogels with Hydrophobic PLGA Blocks
2.1.4. Hydrogels with Hydrophobic PCL, PHB, or Polyphosphazene Blocks
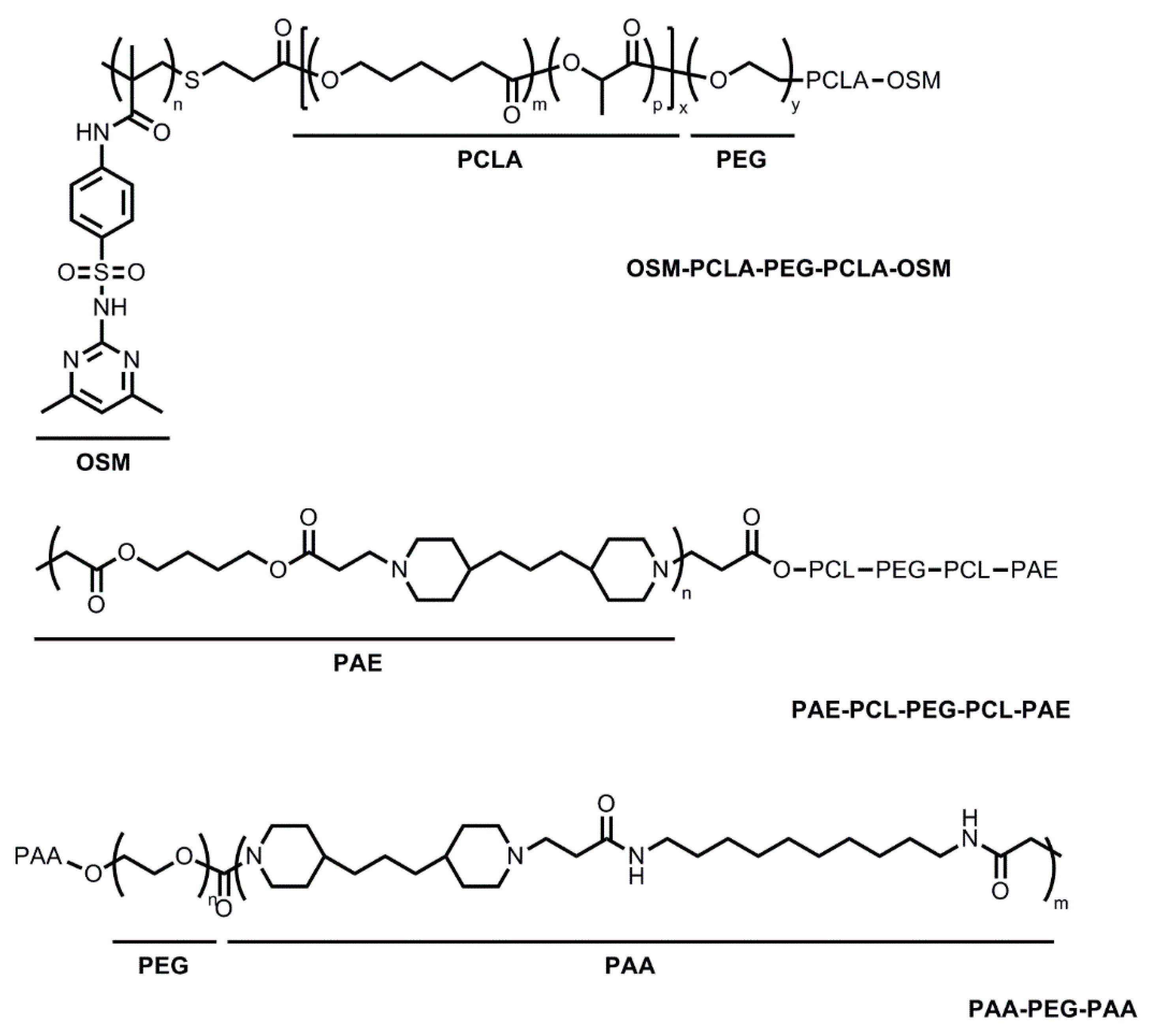
2.2. Thermo- and pH-Sensitive Hydrogels
3. Preparation of Hydrogels Carrying Hydrophobic Drugs
3.1. Simple Mixing below the CGT
3.2. Caging Drug-Loaded Nanoparticles or Complexes in Hydrogels
3.3. Solvent Evaporation Method
3.4. Modified Nanoprecipitation Method
4. Hydrogels in Cancer Therapy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogels from soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Lee, D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control Release 2014, 193, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Simoes, S.; Figueiras, A.; Francisco, V. Modular hydrogels for drug delivery. J. Biomater. Nanobiotechnol. 2012, 3, 185–199. [Google Scholar] [CrossRef]
- Sakai, S.; Ueda, K.; Taya, M. Peritoneal adhesion prevention by a biodegradable hyaluronic acid-based hydrogel formed in situ through a cascade enzyme reaction initiated by contact with body fluid on tissue surfaces. Acta Biomater. 2015, 24, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact. Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Maya, S.; Sarmento, B.; Nair, A.; Rejinold, N.S.; Nair, S.V.; Jayakumar, R. Smart stimuli sensitive nanogels in cancer drug delivery and imaging: A review. Curr. Pharm. Des. 2013, 19, 7203–7218. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kwon, G.S. Thermosensitive poly-(d,l-lactide-co-glycolide)-block-poly(ethylene glycol)-block-poly-(d,l-lactide-co-glycolide) hydrogels for multi-drug delivery. J. Drug Target 2014, 22, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Mencarelli, G.; Giorgioni, G.; Palmieri, G.F. Thermosensitive self-assembling block copolymers as drug delivery systems. Polymers 2011, 3, 779–811. [Google Scholar] [CrossRef]
- Raymond, E.G. On the theory of lower critical solution points in hydrogen-bonded mixtures. J. Chem. Phys. 1984, 80, 5340–5341. [Google Scholar] [CrossRef]
- Al-Saden, A.A.; Florence, A.T.; Morrison, H.; Whateley, T.L. Association of poloxamer block copolymers in aqueous solution [proceedings]. J. Pharm. Pharmacol. 1979, 31, 81. [Google Scholar] [CrossRef]
- Wang, P.; Johnson, T.P. Kinetics of sol-to-gel transition for poloxamer polyols. J. App. Polym. Sci. 1991, 43, 283–292. [Google Scholar] [CrossRef]
- Brown, W.; Schillen, K.; Almgren, M.; Hvidt, S.; Bahadur, P. Micelle and gel formation in a poly(ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) triblock copolymer in water solution: Dynamic and static light scattering and oscillatory shear measurements. J. Phys. Chem. 1991, 95, 1850–1858. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. The aggregation behavior of poly-(oxyethylene)-poly-(oxypropylene)-poly-(oxyethylene)-block-copolymers in aqueous solution. Colloid Polym. Sci. 1990, 268, 101–117. [Google Scholar] [CrossRef]
- Rassing, J.; Attwood, D. Ultrasonic velocity and light-scattering studies on the polyoxyethylene-polyoxypropylene copolymer pluronic F127 in aqueous solution. Int. J. Pharm. 1982, 13, 47–55. [Google Scholar] [CrossRef]
- Vadnere, M.; Amidon, G.; Lindenbaum, S.; Haslam, J.L. Thermodynamic studies on the gel-sol transition of some pluronic polyols. Int. J. Pharm. 1984, 22, 207–218. [Google Scholar] [CrossRef]
- Pandit, N.K.; Wang, D. Salt effects on the diffusion and release rate of propranolol from poloxamer 407 gels. Int. J. Pharm. 1998, 167, 183–189. [Google Scholar] [CrossRef]
- Devi, D.R.; Sandhya, P.; Hari, B.N.V. Poloxamer: A novel functional molecule for drug delivery and gene therapy. J. Pharm. Sci. Res. 2013, 5, 159–165. [Google Scholar]
- Lee, W.C.; Li, Y.C.; Chu, I.M. Amphiphilic poly(d,l-lactic acid)/poly(ethylene glycol)/poly(d,l-lactic acid) nanogels for controlled release of hydrophobic drugs. Macromol. Biosci. 2006, 6, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Rostamizadeh, K.; Salari, D.; Hamidi, M. Preparation and characterization of tri-block poly(lactide)-poly(ethylene glycol)-poly(lactide) nanogels for controlled release of naltrexone. Int. J. Pharm. 2011, 416, 356–364. [Google Scholar] [PubMed]
- Loh, X.J.; Li, J. Biodegradable thermosensitive copolymer hydrogels for drug delivery. Expert Opin. Ther. Pat. 2007, 17, 965–977. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric micelles for multi-drug delivery in cancer. AAPS Pharm. Sci. Technol. 2015, 16, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Chen, D.; Ma, X.; Liu, Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: Synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int. J. Pharm. 2005, 294, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of peg-plga-peg triblock copolymers. J. Control Release 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Gong, C.; Shi, S.; Dong, P.; Kan, B.; Gou, M.; Wang, X.; Li, X.; Luo, F.; Zhao, X.; Wei, Y.; et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, Y.M.; Sohn, Y.S.; Song, S.C. A thermosensitive poly(organophosphazene) gel. Macromolecules 2002, 35, 3876–3879. [Google Scholar] [CrossRef]
- Seong, J.Y.; Juna, Y.J.; Jeong, B.; Sohn, Y.S. New thermogelling poly(organophosphazenes) with methoxypoly(ethylene glycol) and oligopeptide as side groups. Polymer 2005, 46, 5075–5081. [Google Scholar] [CrossRef]
- Shim, W.S.; Yoo, J.S.; Bae, Y.H.; Lee, D.S. Novel injectable ph and temperature sensitive block copolymer hydrogel. Biomacromolecules 2005, 6, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Sohn, Y.S.; Jeong, B. Closed-loop sol-gel transition of peg-pec aqueous solution. J. Phys. Chem. B 2007, 111, 7715–7718. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Kim, M.S.; Lee, D.S.; Kim, B.S.; Kim, J.H. Novel pH and temperature-sensitive block copolymers: Poly(ethylene glycol)-b-poly(ε-caprolactone)-b-poly(β-amino ester). Macromol. Res. 2006, 14, 117–120. [Google Scholar] [CrossRef]
- Huynh, D.P.; Nguyen, M.K.; Pi, B.S.; Kim, M.S.; Chae, S.Y.; Lee, K.C.; Kim, B.S.; Kim, S.W.; Lee, D.S. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials 2008, 29, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.P.; Nguyen, M.K.; Kim, B.S.; Lee, D.S. Molecular design of novel pH/temperature-sensitive hydrogels. Polymer 2009, 50, 2565–2571. [Google Scholar] [CrossRef]
- Almomen, A.; Cho, S.; Yang, C.H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janat-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cho, S.; Kwon, I.C.; Janát-Amsbury, M.M.; Huh, K.M. Preparation and characterization of glycol chitin as a new thermogelling polymer for biomedical applications. Carbohydr. Polym. 2013, 92, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- De Alvarenga, E.S. Characterization and properties of chitosan. In Biotechnology of Biopolymers; Elnashar, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 91–108. [Google Scholar]
- Xuan, J.J.; Yan, Y.D.; Oh, D.H.; Choi, Y.K.; Yong, C.S.; Choi, H.G. Development of thermo-sensitive injectable hydrogel with sustained release of doxorubicin: Rheological characterization and in vivo evaluation in rats. Drug Deliv. 2011, 18, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Moinuddin, Z.; Patil-Sen, Y.; Littlefield, R.; Hood, M. Lipid-hydrogel films for sustained drug release. Int. J. Pharm. 2015, 479, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.K.S.; Rai, A.K.; Singh, S. Formulation of thermosensitive hydrogel containing cyclodextrin for controlled drug delivery of camptothecin. Trop. J. Pharm. Res. 2014, 13, 1007–1012. [Google Scholar] [CrossRef]
- Ju, C.; Sun, J.; Zi, P.; Jin, X.; Zhang, C. Thermosensitive micelles-hydrogel hybrid system based on poloxamer 407 for localized delivery of paclitaxel. J. Pharm. Sci. 2013, 102, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, F.; Ding, B.; Sun, N.; Liu, X.; Ding, X.; Gao, S. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J. Drug Target 2011, 19, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.J.; Thulasidasan, A.K.; Anto, R.J.; Chithralekha, D.N.; Narayanan, A.; Kumar, G.S. Folic acid conjugated cross-linked acrylic polymer (fa-clap) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J. Nanobiotechnol. 2014, 12, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Elstad, N.L.; Fowers, K.D. Oncogel (regel/paclitaxel)-clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Pados, G.; Makedos, A.; Tarlatzis, B. Adhesion prevention strategies in laparoscopic surgery. In Endoscopy; Amornyotin, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 49–72. [Google Scholar]
- Yang, B.; Gong, C.; Qian, Z.; Zhao, X.; Li, Z.; Qi, X.; Zhou, S.; Zhong, Q.; Luo, F.; Wei, Y. Prevention of post-surgical abdominal adhesions by a novel biodegradable thermosensitive pece hydrogel. BMC Biotechnol. 2010, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Di Zerega, G.S. Biochemical events in peritoneal tissue repair. Eur. J. Surg. Suppl. 1997, 577, 10–16. [Google Scholar] [PubMed]
- Gao, X.; Deng, X.; Wei, X.; Shi, H.; Wang, F.; Ye, T.; Shao, B.; Nie, W.; Li, Y.; Luo, M.; et al. Novel thermosensitive hydrogel for preventing formation of abdominal adhesions. Int. J. Nanomed. 2013, 8, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397-20408. https://doi.org/10.3390/molecules201119705
McKenzie M, Betts D, Suh A, Bui K, Kim LD, Cho H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules. 2015; 20(11):20397-20408. https://doi.org/10.3390/molecules201119705
Chicago/Turabian StyleMcKenzie, Matthew, David Betts, Amy Suh, Kathryn Bui, London Doyoung Kim, and Hyunah Cho. 2015. "Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs" Molecules 20, no. 11: 20397-20408. https://doi.org/10.3390/molecules201119705
APA StyleMcKenzie, M., Betts, D., Suh, A., Bui, K., Kim, L. D., & Cho, H. (2015). Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules, 20(11), 20397-20408. https://doi.org/10.3390/molecules201119705