Supramolecular Complexation of Carbohydrates for the Bioavailability Enhancement of Poorly Soluble Drugs
Abstract
:1. Introduction
2. Monosaccharides
2.1. Prodrug System
2.2. Glycosylated Carrier
3. Oligosaccharides
3.1. Binary Systems
3.1.1. Cyclic Oligosaccharides and the Derivatives
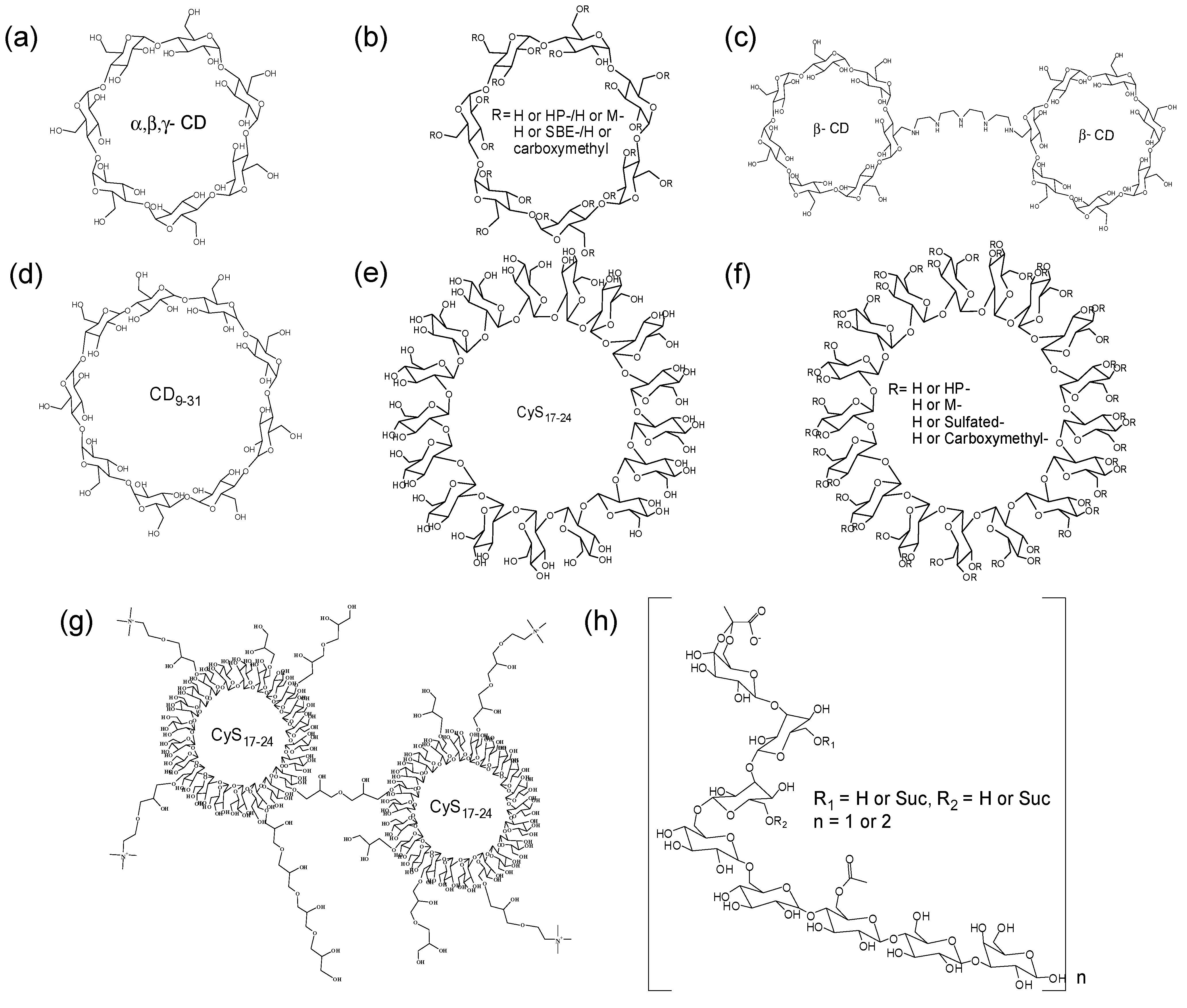
3.1.2. Linear Oligosaccharides
3.1.3. Preparation of Inclusion Complexes
3.1.4. Analysis of Inclusion Complex
- UV-Vis spectroscopy—Measurement of drug solubility enhancement by complexation.
- Nuclear Magnetic Resonance (NMR) spectroscopy—1H-NMR is a suitable method for the evaluation of noncovalent interactions at the molecular level [79]. To elucidate the intermolecular interaction of inclusion complexes, two-dimensional NMR spectroscopy (Nuclear Overhauser enhancement spectroscopy (NOESY) or Rotating frame nuclear Overhauser effect spectroscopy (ROESY)) has also been frequently used, because two protons located within 5 Å induce an NOE crosspeak [80].
- Thermogravimetric Analysis (TGA)/Differential Scanning Calorimetry (DSC)—TGA curves describe the weight losses of pure components and the complexes. DSC is performed to characterize the solid-state interactions for the inclusion complexes as compared to the melting points [81].
- Fourier Transform Infrared (FTIR) spectroscopy—The analysis of the vibrational changes upon the inclusion of drugs with a host.
- X-ray Powder Diffractometry (XRPD)—The powder diffraction patterns of drugs and complex are compared. In principle, drugs displayed sharp peaks, which are the characteristics of an organic molecule with crystallinity, and the complex shows different patterns with crystalline drugs.
- Electrospray mass spectrometry (ESI-MS)—Determination of molecular association of noncovalent bonding.
- Computational method (Molecular modeling)—The appropriate binding mode of complex between host and drug can be derived from molecular docking simulations [84].
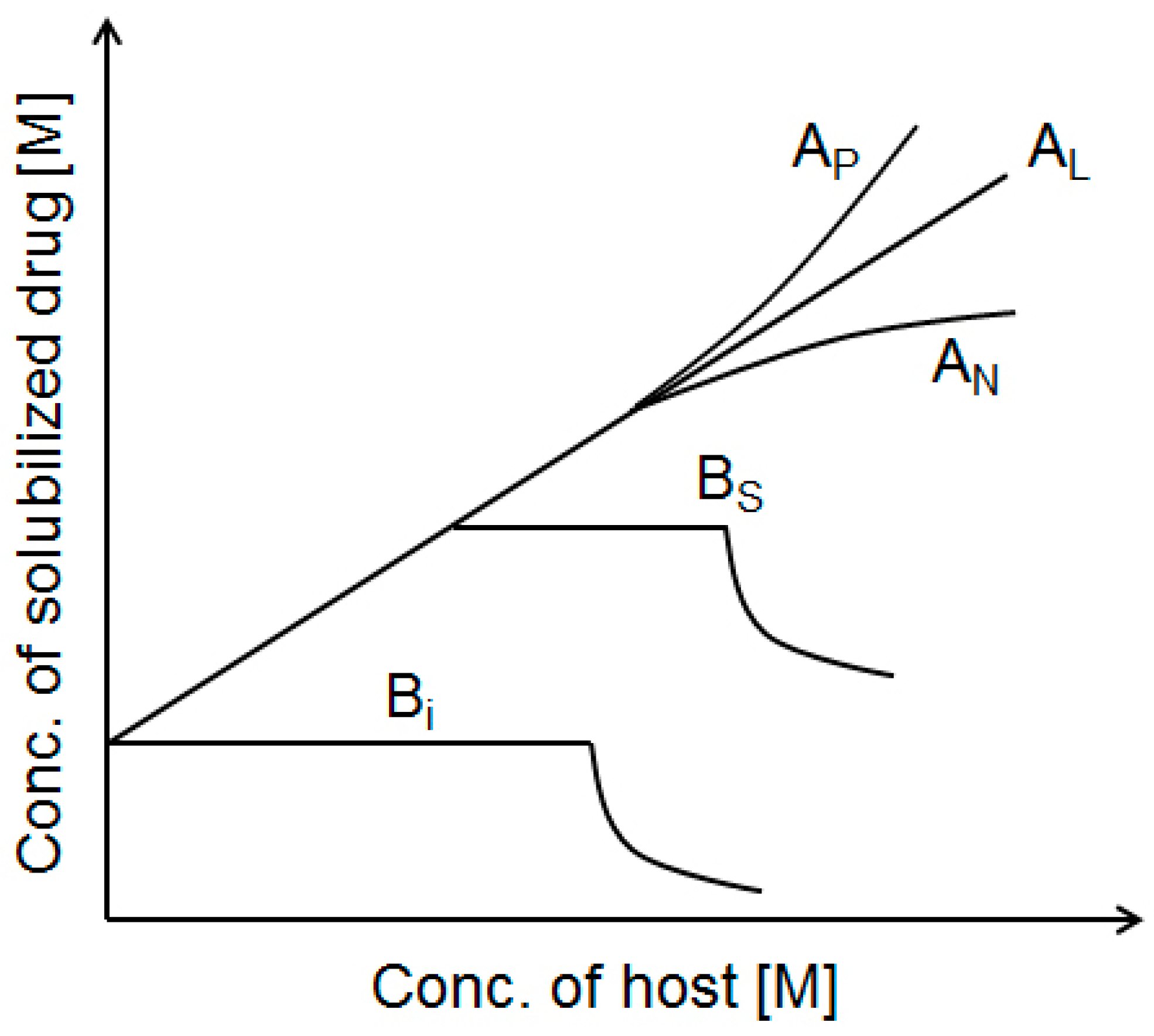
3.1.5. Phase Solubility Studies
3.1.6. Drug Delivery and CD Elimination from the Drug/CD Complexes
3.2. Ternary Systems
3.3. Multinary Systems
3.3.1. CD Amphiphiles
3.3.2. CD Pendent Polymers
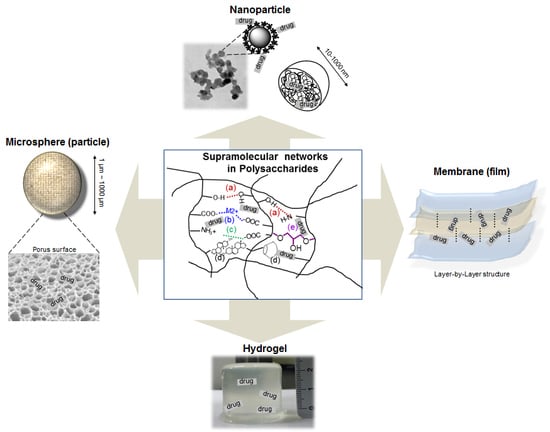
4. Polysaccharides
4.1. Polysaccharide Drug Conjugates
4.2. Supramolecular Architectures for Polysaccharide Drug Carriers

| Supramolecular Forces | Used Polysaccharides | Architecture Types | Drugs | References | |
|---|---|---|---|---|---|
| Non-covalent bond | Hydrogen bond | Hydrolyzed xyloglucan | Hydrogel | Ondansetron Indomethacin Mytomycin C | [133,134,135,136] |
| Hydroxypropyl Methylcellulose | Hydrogel | Indomethacin | [137] | ||
| Metal coordination | Alginate-calcium ion | Nanoparticle | Rifampicin, Doxorubicin | [138,139] | |
| N-succinyl chitosan-alginate | Hydrogel | Nifedipine | [140] | ||
| Alginate-calcium carbonate | Hydrogel | Ibuprofen | [141] | ||
| Ionic interaction | Chitosan-tripolyphosphate | Nanoparticle | Ciprofloxacin | [142] | |
| Chitosan-tripolyphosphate-hydroxypropylcyclodextrin | Nanoparticle | Furosemide, Triclosan | [143] | ||
| Chitosan-tripolyphosphate-dextran sulfate | Microsphere | Ibuprofen | [144] | ||
| Chitosan-dextran sulfate | Nanoparticle | Amphotericin B | [145] | ||
| Chitosan-glycyrrhetic acid | Nanoparticle | Glycyrrhetic acid | [146] | ||
| Chitosan-β-glycerophosphate | Hydrogel | Paclitaxel | [147] | ||
| Carrageenan Dextran sulfate | Nanosphere | Ciprofloxacin | [148] | ||
| Hydrophobic interaction | Ceramide modified hyaluronic acid | Nanoparticle | Docetaxel, Doxorubicin | [149,150] | |
| Deoxycholic acid modified hyaluronic acid | Nanoparticle | Paclitaxel | [151] | ||
| Histidine modified hyaluronic acid | Nanoparticle | Doxorubicin | [152] | ||
| Pullulan acetate | Nanoparticle | Silymarin | [153] | ||
| Cholesterol modified chitosan | Nanoparticle | Epirubicin | [154] | ||
| Deoxycholic acid-modified chitosan | Nanoparticle | Adriamycin, Doxorubicin | [155,156] | ||
| 5β-cholanic acid modified chitosan | Nanoparticle | Paclitaxel, Camptothecin | [157,158] | ||
| Stearic acid-g-chitosan | Nanosphere | Doxorubicin | [159,160] | ||
| N-acetyl histidine-conjugated glycol chitosan | Nanoparticle | Paclitaxel | [161] | ||
| Cholic acid modified dextran | Nanosphere | Indomethacin | [162] | ||
| CD polymer-dextran polymer | Nanogel | Benzophenone, Tamoxifen | [163] | ||
| Acetylated chondroitin sulfate | Nanogel | Doxorubicin | [164] | ||
| Covalent bond | Cross-linker or Copolymer | Chitosan (glutaraldehyde, sulphuric acid) | Microsphere | Diclofenac, Docetaxol Clozapine | [165,166,167] |
| Polyacrylamide-g-chitosan copolymer | Microsphere | Nifedipine | [168] | ||
| Chitosan-Pluronic copolymer | Nanoparticle | Indometacin, Doxorubicin | [169,170] | ||
| Pullulan-g-poly(l-lactide) copolymers | Hydrogel | Doxorubicin | [171] | ||
| Poly(dl-lactide-co-glycolide)-grafted pullulan | Nanosphere | Adriamycin | [172] | ||
| Cellulose-graft-poly(l-lactide) copolymers | Nanosphere | Paclitaxel | [173] | ||
| Dextran-b-poly(DL-lactide-coglycolide) copolymer | Nanosphere | Doxorubicin, Amphotericin B | [174,175] | ||
| Poly[lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer | Nanoparticle | Doxorubicin | [176] | ||
| Dextran-b-poly(ε-caprolactone) | Nanoparticle | Doxorubicin | [177] | ||
| Chondroitin sulfate-Pluronic copolymer | Nanoparticle | Doxorubicin | [178] | ||
| Starch (epichlorohydrin) | Microsphere | Ampicillin | [179] | ||
| Hyaluronic acid (1,3-diaminopropane) | Hydrogel | Ibuprofen | [180] | ||
4.2.1. Nano-Particles (Spheres)
4.2.2. Microspheres
4.2.3. Membrane (Film)
4.2.4. Hydrogels
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Okamoto, H. Novel Approaches for oral delivery of poorly soluble drugs. In Oral Delivery of Poorly Soluble Actives—From Drug Discovery to Marketed Products, Proceeding of Symposia on the Oral Delivery of Poorly Soluble Actives, Tokyo, Japan, 6 June 2003; Capsugel® Library: Morristown, NJ, USA.
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Blaabjerg, L.I.; Lenz, E.; Bohr, A.; Grohganz, H.; Kleinebudde, P.; Rades, T.; Löbmann, K. Preparation and characterization of spray-dried co-amorphous drug-amino acid salts. J. Pharm. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Supramolecular chemistry—Scope and perspectives: Molecules—Supermolecules—Molecular devices. J. Incl. Phenom. 1988, 6, 351–396. [Google Scholar] [CrossRef]
- Timsit, Y.; Moras, D. DNA self-fitting: The double helix directs the geometry of its supramolecular assembly. EMBO J. 1994, 13, 2737–2746. [Google Scholar] [PubMed]
- Uhlenheuer, D.A.; Petkau, K.; Brunsveld, L. Combining supramolecular chemistry with biology. Chem. Soc. Rev. 2010, 39, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sakai, F.; Su, L.; Liu, Y.; Wei, K.; Chen, G.; Jiang, M. Progressive macromolecular self-assembly: From biomimetic chemistry to bio-inspired materials. Adv. Mater. 2013, 25, 5215–5256. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Supramolecular Chemistry; Wiley-VCH: Weinheim, Germany, 1995; Volume 1. [Google Scholar]
- Lehn, J.M. Perspectives in supramolecular chemistry—From molecular recognition towards molecular information processing and self-organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Schneider, H.J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Kunitake, T. Molecular recognition at air-water and related interfaces: Complementary hydrogen bonding and multisite interaction. Acc. Chem. Res. 1998, 31, 371–378. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Carçabal, P.; Jockusch, R.A.; Hünig, I.; Snoek, L.C.; Kroemer, R.T.; Davis, B.G.; Gamblin, D.P.; Compagnon, I.; Oomens, J.; Simons, J.P. Hydrogen bonding and cooperativity in isolated and hydrated sugars: Mannose, galactose, glucose, and lactose. J. Am. Chem. Soc. 2005, 127, 11414–11425. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Kubota, N. Chemo-enzymatic synthesis of ester-linked docetaxel-monosaccharide conjugates as water-soluble prodrugs. Molecules 2011, 16, 6769–6777. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Rivault, F.; Tranoy-Opalinski, I.; Roche, J.; Gesson, J.-P.; Papot, S. Synthesis and biological evaluation of the suberoylanilide hydroxamic acid (SAHA) β-glucuronide and β-galactoside for application in selective prodrug chemotherapy. Bioorg. Med. Chem. Lett. 2007, 17, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Giorgioni, G.; Ruggieri, S.; di Stefano, A.; Sozio, P.; Cinque, B.; di Marzio, L.; Santoni, G.; Claudi, F. Glycosyl and polyalcoholic prodrugs of lonidamine. Bioorg. Med. Chem. Lett. 2008, 18, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Houba, P.; Boven, E.; van der Meulen-Muileman, I.; Leenders, R.; Scheeren, J.; Pinedo, H.; Haisma, H. A novel doxorubicin-glucuronide prodrug DOX-GA3 for tumour-selective chemotherapy: Distribution and efficacy in experimental human ovarian cancer. Br. J. Cancer 2001, 84, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Wahl, R.L. Overexpression of glut-1 glucose transporter in human breast cancer an immunohistochemical study. Cancer 1993, 72, 2979–2985. [Google Scholar] [CrossRef]
- Fernández, C.; Nieto, O.; Fontenla, J.A.; Rivas, E.; de Ceballos, M.L.; Fernández-Mayoralas, A. Synthesis of glycosyl derivatives as dopamine prodrugs: Interaction with glucose carrier GLUT-1. Org. Biomol. Chem. 2003, 1, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Nieto, O.; Rivas, E.; Montenegro, G.; Fontenla, J.A.; Fernández-Mayoralas, A. Synthesis and biological studies of glycosyl dopamine derivatives as potential antiparkinsonian agents. Carbohydr. Res. 2000, 327, 353–365. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Cudic, M. Optimization of physicochemical and pharmacological properties of Peptide drugs by glycosylation. In Peptide Modifications to Increase Metabolic Stability and Activity; Cudic, R., Ed.; Humana Press: New York City, NY, USA, 2013; pp. 107–136. [Google Scholar]
- Polt, R.; Porreca, F.; Szabo, L.Z.; Bilsky, E.J.; Davis, P.; Abbruscato, T.J.; Davis, T.P.; Harvath, R.; Yamamura, H.I.; Hruby, V.J. Glycopeptide enkephalin analogues produce analgesia in mice: Evidence for penetration of the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1994, 91, 7114–7118. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D.; Fahr, A. Drug Delivery Strategies for Poorly Water-Soluble Drugs; John Wiley & Sons: Weinheim, Germany, 2012. [Google Scholar]
- Sahoo, S.K.; Jain, T.K.; Reddy, M.K.; Labhasetwar, V. Nano-sized carriers for drug delivery. In NanoBioTechnology; Humana Press: New York City, NY, USA, 2008; pp. 329–348. [Google Scholar]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. A review of glycosylated carriers for drug delivery. Biomaterials 2012, 33, 4166–4186. [Google Scholar] [CrossRef] [PubMed]
- Monsigny, M.; Roche, A.-C.; Midoux, P.; Mayer, R. Glycoconjugates as carriers for specific delivery of therapeutic drugs and genes. Adv. Drug Deliv. Rev. 1994, 14, 1–24. [Google Scholar] [CrossRef]
- Agrawal, P.; Gupta, U.; Jain, N. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials 2007, 28, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Vyas, S. Mannosylated niosomes as adjuvant-carrier system for oral mucosal immunization. J. Liposome Res. 2006, 16, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Pichon, C.; Meunier, L.; Midoux, P.; Monsigny, M.; Roche, A. Enhanced biological activity of antisense oligonucleotides complexed with glycosylated poly-l-lysine. Mol. Pharmacol. 1996, 50, 1487–1494. [Google Scholar] [PubMed]
- Cramer, F. Einschlussverbindungen; Springer: Berlin, Germany, 1954. [Google Scholar]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schardinger, F. Bildung kristallisierter polysaccharide (dextrine) aus stärkekleister durch microben. Zentr. Bakt. Parasit. Abt. II 1911, 29, 188–197. [Google Scholar]
- Freudenberg, K.; Cramer, F.; Plieninger, H. Verfahren zur Herstellung von Einschlussverbindungen Physiologisch Wirksamer Organischer Verbindungen. German Patent DE895769C, 5 November 1953. [Google Scholar]
- Salústio, P.; Cabral-Marques, H.; Costa, P.; Pinto, J. Comparison of ibuprofen release from minitablets and capsules containing ibuprofen: β-Cyclodextrin complex. Eur. J. Pharm. Biopharm. 2011, 78, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cartledge, J.; Midgley, J.; Youle, M.; Gazzard, B. Itraconazole cyclodextrin solution—Effective treatment for HIV-related candidosis unresponsive to other azole therapy. J. Antimicrob. Chemother. 1994, 33, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, R.; Ficarra, P.; di Bella, M.; Raneri, D.; Tommasini, S.; Calabro, M.; Villari, A.; Coppolino, S. Study of the inclusion complex of atenolol with β-cyclodextrins. J. Pharm. Biomed. Anal. 2000, 23, 231–236. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Ennas, G.; Valenti, D.; Sinico, C.; Loy, G.; Fadda, A.M. Diclofenac-β-cyclodextrin binary systems: Physicochemical characterization and in vitro dissolution and diffusion studies. AAPS PharmSciTech 2005, 6, E464–E472. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.J.; Chen, J.Y.-J.; Jansson, J.L.; Widmalm, G.; Maliniak, A. Molecular properties related to the anomalous solubility of β-cyclodextrin. J. Phys. Chem. B 2004, 108, 4236–4238. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Cserháti, T. Interaction of some anticancer drugs with carboxymethyl-β-cyclodextrin. Int. J. Pharm. 1995, 124, 205–211. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y. Cooperative binding and multiple recognition by bridged bis (β-cyclodextrin)s with functional linkers. Acc. Chem. Res. 2006, 39, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lu, C.; Ren, X.; Meng, Q. New metallocene-bridged cyclodextrin dimer: A stable derivative of the antitumor drug titanocene dichloride and its potent cytotoxity against human breast cancer (MCF-7) cells. J. Organomet. Chem. 2006, 691, 5895–5899. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.-S.; Li, L.; Zhang, H.-Y.; Cao, D.-X.; Yuan, Y.-J. Inclusion complexation and solubilization of paclitaxel by bridged bis (β-cyclodextrin)s containing a tetraethylenepentaamino spacer. J. Med. Chem. 2003, 46, 4634–4637. [Google Scholar] [CrossRef] [PubMed]
- French, D. The schardinger dextrins. Adv. Carbohydr. Chem. 1957, 12, 189–260. [Google Scholar] [PubMed]
- Fujiwara, T.; Tanaka, N.; Kobayashi, S. Structure of. DELTA.-cyclodextrin 13.75 H2O. Chem. Lett. 1990, 739–742. [Google Scholar] [CrossRef]
- Miyazawa, I.; Ueda, H.; Nagase, H.; Endo, T.; Kobayashi, S.; Nagai, T. Physicochemical properties and inclusion complex formation of δ-cyclodextrin. Eur. J. Pharm. Sci. 1995, 3, 153–162. [Google Scholar] [CrossRef]
- Jacob, J.; Geβler, K.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T.; Saenger, W. Band-flip and kink as novel structural motifs in α-(1→4)-d-glucose oligosaccharides. Crystal structures of cyclodeca-and cyclotetradecaamylose. Carbohydr. Res. 1999, 322, 228–246. [Google Scholar] [CrossRef]
- Nimz, O.; Geßler, K.; Usón, I.; Saenger, W. An orthorhombic crystal form of cyclohexaicosaose, CA26···32.59 H2O: Comparison with the triclinic form. Carbohydr. Res. 2001, 336, 141–153. [Google Scholar] [CrossRef]
- Larsen, K.L. Large cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 1–13. [Google Scholar] [CrossRef]
- Koizumi, K.; Okada, Y.; Horiyama, S.; Utamura, T.; Higashiura, T.; Ikeda, M. Preparation of cyclosophoraose-A and its complex-forming ability. In Clathrate Compounds, Molecular Inclusion Phenomena, and Cyclodextrins; Springer: New York City, NY, USA, 1984; pp. 891–899. [Google Scholar]
- Lee, S.; Kwon, C.; Choi, Y.; Seo, D.-H.; Kim, H.-W.; Jung, S. Inclusion complexation of a family of cyclosophoraoses with indomethacin. J. Microbiol. Biotechnol. 2001, 11, 463–468. [Google Scholar]
- Lee, S.; Seo, D.-H.; Kim, H.-W.; Jung, S. Investigation of inclusion complexation of paclitaxel by cyclohenicosakis-(1→2)-(β-d-glucopyranosyl), by cyclic-(1→2)-β-d-glucans (cyclosophoraoses), and by cyclomaltoheptaoses (β-cyclodextrins). Carbohydr. Res. 2001, 334, 119–126. [Google Scholar] [CrossRef]
- Lee, S.; Seo, D.-H.; Park, H.-L.; Choi, Y.; Jung, S. Solubility enhancement of a hydrophobic flavonoid, luteolin by the complexation with cyclosophoraoses isolated from Rhizobium meliloti. Antonie Leeuwenhoek 2003, 84, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, K.; Lee, S.; Jung, S. Molecular dynamics simulation of cyclosophoroheptadecaose (Cys-A). J. Comput. Aided Mol. Des. 2002, 16, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Mimura, M.; Kitamura, S.; Gotoh, S.; Takeo, K.; Urakawa, H.; Kajiwara, K. Conformation of cyclic and linear (1→2)-β-d-glucans in aqueous solution. Carbohydr. Res. 1996, 289, 25–37. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.; Seo, D.; Choi, Y.; Jung, S. Synthesis and characterization of carboxymethylated cyclosophoraose, and its inclusion complexation behavior. Carbohydr. Res. 2004, 339, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jung, S. Separation of some chiral flavonoids by microbial cyclosophoraoses and their sulfated derivatives in micellar electrokinetic chromatography. Electrophoresis 2005, 26, 3833–3838. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.M.; Choi, Y.; Tahir, M.N.; Yang, Y.-H.; Cho, E.; Jung, S. Enhanced solubility of galangin based on the complexation with methylated microbial cyclosophoraoses. J. Incl. Phenom. Macrocycl. Chem. 2014, 79, 291–300. [Google Scholar] [CrossRef]
- Piao, J.; Jang, A.; Choi, Y.; Tahir, M.N.; Kim, Y.; Park, S.; Cho, E.; Jung, S. Solubility enhancement of α-naphthoflavone by synthesized hydroxypropyl cyclic-(1→2)-β-d-glucans (cyclosophoroases). Carbohydr. Polym. 2014, 101, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Choi, J.M.; Choi, Y.; Jeong, K.; Cho, E.; Jung, S. Complexation of fisetin with novel cyclosophoroase dimer to improve solubility and bioavailability. Carbohydr. Polym. 2013, 97, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Eggens, I.; Fenderson, B.; Toyokuni, T.; Dean, B.; Stroud, M.; Hakomori, S.-I. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J. Biol. Chem. 1989, 264, 9476–9484. [Google Scholar] [PubMed]
- Vyas, N.K. Atomic features of protein-carbohydrate interactions. Curr. Opin. Struct. Biol. 1991, 1, 732–740. [Google Scholar] [CrossRef]
- Aoyama, Y.; Otsuki, J.-I.; Nagai, Y.; Kobayashi, K.; Toi, H. Host-guest complexation of oligosaccharides: Interaction of maltodextrins with hydrophobic fluorescence probes in water. Tetrahedron Lett. 1992, 33, 3775–3778. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Choi, J.M.; Tahir, M.N.; Cho, E.; Choi, Y.; Lee, I.-S.; Jung, S. Solubilization of pyrimethamine, antibacterial drug, by low-molecular-weight succinoglycan dimers isolated from Shinorhizobium meliloti. Bull. Korean Chem. Soc. 2012, 33. [Google Scholar] [CrossRef]
- Choi, J.M.; Kim, H.; Cho, E.; Choi, Y.; Jung, S. Solubilization of haloperidol by acyclic succinoglycan oligosaccharides. Carbohydr. Polym. 2012, 89, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Choi, J.M.; Jung, S. Solubility enhancement of isoflavonoids by complexation with acyclic hexadecasaccharides, succinoglycan dimers isolated from Sinorhizobium meliloti. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 133–141. [Google Scholar] [CrossRef]
- Kim, K.; Cho, E.; Choi, J.M.; Kim, H.; Jang, A.; Choi, Y.; Yu, J.-H.; Jung, S. Intermolecular complexation of low-molecular-weight succinoglycans directs solubility enhancement of pindolol. Carbohydr. Polym. 2014, 106, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Hettiarachchi, G.; Nguyen, D.; Zhang, B.; Wittenberg, J.B.; Zavalij, P.Y.; Briken, V.; Isaacs, L. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat. Chem. 2012, 4, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Moyano, J.; Ginés, J.; Arias, M.; Rabasco, A. Study of the dissolution characteristics of oxazepam via complexation with β-cyclodextrin. Int. J. Pharm. 1995, 114, 95–102. [Google Scholar] [CrossRef]
- Pose-Vilarnovo, B.; Perdomo-López, I.; Echezarreta-López, M.; Schroth-Pardo, P.; Estrada, E.; Torres-Labandeira, J.J. Improvement of water solubility of sulfamethizole through its complexation with β-and hydroxypropyl-β-cyclodextrin: Characterization of the interaction in solution and in solid state. Eur. J. Pharm. Sci. 2001, 13, 325–331. [Google Scholar] [CrossRef]
- Yáñez, C.; Cañete-Rosales, P.; Castillo, J.P.; Catalán, N.; Undabeytia, T.; Morillo, E. Cyclodextrin inclusion complex to improve physicochemical properties of herbicide bentazon: Exploring better formulations. PLoS ONE 2012, 7, e41072. [Google Scholar] [CrossRef] [PubMed]
- Moyano, J.; Arias, M.; Gines, J.; Perez, J.; Rabasco, A. Dissolution Behavior of Oxazepam in Presence of Cyclodextrins: Evaluation of Oxazepam-Dimeb Binary Systemxs. Drug Dev. Ind. Pharm. 1997, 23, 379–385. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, Y.; Fujiwara, M.; Kanazawa, K.; Nemoto, T.; Fujita, K.-I.; Nakanishi, H. Three-dimensional structure of the inclusion complex between phloridzin and β-cyclodextrin. Carbohydr. Res. 2002, 337, 1737–1743. [Google Scholar] [CrossRef]
- Pînzaru, I.; Hadaruga, D.; Hadaruga, N.; Corpa, L.; Grozescu, I.; Peter, F. Hepatoprotective flavonoid bioconjugate/β-cyclodextrin nanoparticles: DSC-molecular modeling correlation. Dig. J. Nanomater. Biostruct. 2011, 6, 1605–1617. [Google Scholar]
- Bilensoy, E.; Doğan, L.; Şen, M.; Hıncal, A. Complexation behavior of antiestrogen drug tamoxifen citrate with natural and modified β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 651–655. [Google Scholar] [CrossRef]
- Sinha, V.; Anitha, R.; Ghosh, S.; Nanda, A.; Kumria, R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005, 94, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, M.M.; Zughul, M.B.; Davies, J.E.D.; Badwan, A.A. Sildenafil/cyclodextrin complexation: Stability constants, thermodynamics, and guest-host interactions probed by 1 H-NMR and molecular modeling studies. J. Pharm. Biomed. Anal. 2006, 41, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, A. Phase-solubility techniques. In Advances in Analytical Chemistry and Instrumentation; Jonh Wiley & Sons: Weinheim, Germany, 1965. [Google Scholar]
- Loftsson, T.; Magnúsdóttir, A.; Másson, M.; Sigurjónsdóttir, J.F. Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 2002, 91, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Zia, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Development of a myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Leong, W.W.Y.; Yang, J.A.; Lee, P.; Chan, S.Y.; Ho, P.C. Biopharmaceutics of 13-cis-retinoic acid (isotretinoin) formulated with modified β-cyclodextrins. Int. J. Pharm. 2007, 341, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, D.; Bombardiere, M.; Salomon, C. Effects of benznidazole: Cyclodextrin complexes on the drug bioavailability upon oral administration to rats. Int. J. Biol. Macromol. 2013, 62, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R. In vivo bioavailability and therapeutic assessment of host-guest inclusion phenomena for the hydrophobic molecule etodolac: Pharmacodynamic and pharmacokinetic evaluation. Sci. Pharm. 2010, 78, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.; Khanna, A.; Venkatpurwar, V.; Dhar, S.; Mandpe, L. Ternary complexation of carvedilol, β-cyclodextrin and citric acid for mouth-dissolving tablet formulation. Acta Pharm. 2009, 59, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Dua, K.; Ramana, M.; Singh Sara, U.; Himaja, M.; Agrawal, A.; Garg, V.; Pabreja, K. Investigation of enhancement of solubility of norfloxacin β-cyclodextrin in presence of acidic solubilizing additives. Curr. Drug Deliv. 2007, 4, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; Cirri, M. Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and aminoacids. Int. J. Pharm. 2003, 260, 293–302. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L.; Yalkowsky, S.H. Combined effect of cosolvent and cyclodextrin on solubilization of nonpolar drugs. J. Pharm. Sci. 1999, 88, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Másson, M. The effects of water-soluble polymers on cyclodextrins and cyclodextrin solubilization of drugs. J. Drug Deliv. Sci. Technol. 2004, 14, 35–43. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Gu, J.; Guo, T.; Yang, S.; Guo, Z.; Zhang, X.; Zhu, W.; Zhang, J. Ternary system of dihydroartemisinin with hydroxypropyl-β-cyclodextrin and lecithin: Simultaneous enhancement of drug solubility and stability in aqueous solutions. J. Pharm. Biomed. Anal. 2013, 83, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sallas, F.; Darcy, R. Amphiphilic cyclodextrins-advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008, 2008, 957–969. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Auzely-Velty, R.; Djedaini-Pilard, F.; Desert, S.; Perly, B.; Zemb, T. Micellization of hydrophobically modified cyclodextrins. 1. Micellar structure. Langmuir 2000, 16, 3727–3734. [Google Scholar] [CrossRef]
- Donohue, R.; Mazzaglia, A.; Ravoo, B.J.; Darcy, R. Cationic β-cyclodextrin bilayer vesicles. Chem. Commun. 2002, 2864–2865. [Google Scholar] [CrossRef]
- Kawabata, Y.; Matsumoto, M.; Tanaka, M.; Takahashi, H.; Irinatsu, Y.; Tamura, S.; Tagaki, W.; Nakahara, H.; Fukuda, K. Formation and deposition of monolayers of amphiphilic. β-cyclodextrin derivatives. Chem. Lett. 1986, 1933–1934. [Google Scholar] [CrossRef]
- Quaglia, F.; Ostacolo, L.; Mazzaglia, A.; Villari, V.; Zaccaria, D.; Sciortino, M.T. The intracellular effects of non-ionic amphiphilic cyclodextrin nanoparticles in the delivery of anticancer drugs. Biomaterials 2009, 30, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Guo, Q.; Zhang, C.; Hao, J.; Xing, P.; Su, J.; Li, S.; Hao, A.; Liu, G. Self-assembled vesicles prepared from amphiphilic cyclodextrins as drug carriers. Langmuir 2012, 28, 8625–8636. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Duffour, M.; Chevalier, Y.; Parrot-Lopez, H. Design, synthesis, and in vitro evaluation of new amphiphilic cyclodextrin-based nanoparticles for the incorporation and controlled release of acyclovir. Eur. J. Pharm. Biopharm. 2013, 83, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Szeman, J.; Fenyvesi, E.; Szejtli, J.; Ueda, H.; Machida, Y.; Nagai, T. Water soluble cyclodextrin polymers: Their interaction with drugs. In Proceedings of the Fourth International Symposium on Inclusion Phenomena and the Third International Symposium on Cyclodextrins, Lancaster, UK, 20–25 July 1986; Atwood, J.L., Davies, J.E., Eds.; Springer: New York City, NY, USA, 1987; pp. 319–323. [Google Scholar]
- Ma, M.; Li, D. Cyclodextrin Polymer Separation Materials. WO1998022197A9, 28 May 1998. [Google Scholar]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ritter, H. Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 2010, 1, 1552–1559. [Google Scholar] [CrossRef]
- Gil, E.S.; Li, J.; Xiao, H.; Lowe, T.L. Quaternary ammonium β-cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules 2009, 10, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.Y.; Wang, R.J.; Zheng, C.; Jin, Y.; Jin, L.Q. β-cyclodextrin-centered star-shaped amphiphilic polymers for doxorubicin delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 5, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Posocco, B.; Dreussi, E.; de Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G. Polysaccharides for the delivery of antitumor drugs. Materials 2015, 8, 2569–2615. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Bassi, P.; Volpe, A.; D’Agostino, D.; Palermo, G.; Renier, D.; Franchini, S.; Rosato, A.; Racioppi, M. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: Results of a phase I study. J. Urol. 2011, 185, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Yousefpour, P.; Atyabi, F.; Farahani, E.V.; Sakhtianchi, R.; Dinarvand, R. Polyanionic carbohydrate doxorubicin-dextran nanocomplex as a delivery system for anticancer drugs: In vitro analysis and evaluations. Int. J. Nanomed. 2011, 6, 1487–1496. [Google Scholar]
- Yousefpour, P.; Atyabi, F.; Vasheghani-Farahani, E.; Movahedi, A.-A.M.; Dinarvand, R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar]
- Wang, Y.; Xin, D.; Liu, K.; Zhu, M.; Xiang, J. Heparin-paclitaxel conjugates as drug delivery system: Synthesis, self-assembly property, drug release, and antitumor activity. Bioconjug. Chem. 2009, 20, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamkhani, A.; Duncan, R. Synthesis, controlled release properties and antitumour activity of alginate-cis-aconityl-daunomycin conjugates. Int. J. Pharm. 1995, 122, 107–119. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Yi, J.; Gu, C.; Fan, L.; Qiao, Y.; Tao, Y.; Cheng, C.; Wu, H. Folate-decorated maleilated pullulan-doxorubicin conjugate for active tumor-targeted drug delivery. Eur. J. Pharm. Sci. 2011, 42, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Elgart, A.; Farber, S.; Domb, A.J.; Polacheck, I.; Hoffman, A. Polysaccharide pharmacokinetics: Amphotericin B arabinogalactan conjugate—A drug delivery system or a new pharmaceutical entity? Biomacromolecules 2010, 11, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Pinhassi, R.I.; Assaraf, Y.G.; Farber, S.; Stark, M.; Ickowicz, D.; Drori, S.; Domb, A.J.; Livney, Y.D. Arabinogalactan-folic acid-drug conjugate for targeted delivery and target-activated release of anticancer drugs to folate receptor-overexpressing cells. Biomacromolecules 2009, 11, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr. Polym. 2013, 92, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, T.; Assmann, V.; Fieber, C.; Termeer, C.C.; Herrlich, P.; Hofmann, M.; Simon, J.C. CD44 is the principal mediator of hyaluronic-acid-induced melanoma cell proliferation. J. Investig. Dermatol. 2001, 116, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lamke, L.-O.; Liljedahl, S.-O. Plasma volume changes after infusion of various plasma expanders. Resuscitation 1976, 5, 93–102. [Google Scholar] [CrossRef]
- Soepenberg, O.; de Jonge, M.J.; Sparreboom, A.; de Bruin, P.; Eskens, F.A.; de Heus, G.; Wanders, J.; Cheverton, P.; Ducharme, M.P.; Verweij, J. Phase I and pharmacokinetic study of DE-310 in patients with advanced solid tumors. Clin. Cancer Res. 2005, 11, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Veltkamp, S.A.; Witteveen, E.O.; Capriati, A.; Crea, A.; Animati, F.; Voogel-Fuchs, M.; van den Heuvel, I.J.; Beijnen, J.H.; Voest, E.E.; Schellens, J.H. Clinical and pharmacologic study of the novel prodrug delimotecan (MEN 4901/T-0128) in patients with solid tumors. Clin. Cancer Res. 2008, 14, 7535–7544. [Google Scholar] [CrossRef] [PubMed]
- Danhauser-Riedl, S.; Hausmann, E.; Schick, H.-D.; Bender, R.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.-R. Phase I clinical and pharmacokinetic trial of dextran conjugated doxorubicin (AD-70, DOX-OXD). Investig. New Drugs 1993, 11, 187–195. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Tyagi, V.; Lohiya, G.; Nerkar, P. Thermally reversible xyloglucan gels as vehicles for nasal drug delivery. Drug Deliv. 2012, 19, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Suisha, F.; Kawasaki, N.; Shirakawa, M.; Yamatoya, K.; Attwood, D. Thermally reversible xyloglucan gels as vehicles for rectal drug delivery. J. Control. Release 1998, 56, 75–83. [Google Scholar] [CrossRef]
- Suisha, F.; Kawasaki, N.; Miyazaki, S.; Shirakawa, M.; Yamatoya, K.; Sasaki, M.; Attwood, D. Xyloglucan gels as sustained release vehicles for the intraperitoneal administration of mitomycin C. Int. J. Pharm. 1998, 172, 27–32. [Google Scholar] [CrossRef]
- Kawasaki, N.; Ohkura, R.; Miyazaki, S.; Uno, Y.; Sugimoto, S.; Attwood, D. Thermally reversible xyloglucan gels as vehicles for oral drug delivery. Int. J. Pharm. 1999, 181, 227–234. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-Gel behavior of hydroxypropyl methylcellulose (hpmc) in ionic media including drug release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Zahoor, A.; Sharma, S.; Khuller, G. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.N.; Li, P.; Zhang, J.P.; Wang, A.Q.; Wei, Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos. 2008, 29, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Gao, Q.; Liu, X.; Tong, Z. Alginate-calcium carbonate porous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr. Polym. 2008, 71, 476–480. [Google Scholar] [CrossRef]
- Jain, D.; Banerjee, R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Garcia-Fuentes, M.; Mura, P.; Alonso, M.J. A new drug nanocarrier consisting of chitosan and hydoxypropylcyclodextrin. Eur. J. Pharm. Biopharm. 2006, 63, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Yu, D.-G.; Yang, M.-C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Colloids Surf. B Biointerfaces 2005, 44, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int. J. Pharm. 2007, 329, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Y.; Yang, W.; Wang, C.; Fu, S.; Shen, X. Preparation, characterization, and drug release in vitro of chitosan-glycyrrhetic acid nanoparticles. J. Pharm. Sci. 2006, 95, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Shive, M.; Bichara, A.; Berrada, M.; le Garrec, D.; Chenite, A.; Leroux, J.-C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Amorphous nanodrugs prepared by complexation with polysaccharides: Carrageenan versus dextran sulfate. Carbohydr. Polym. 2015, 117, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Yoon, H.Y.; Koo, H.; Ko, S.-H.; Shim, J.-S.; Lee, J.-H.; Kim, K.; Kwon, I.C.; Kim, D.-D. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 2011, 32, 7181–7190. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Yoon, I.-S.; Yoon, H.Y.; Koo, H.; Jin, Y.-J.; Ko, S.-H.; Shim, J.-S.; Kim, K.; Kwon, I.C.; Kim, D.-D. Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials 2012, 33, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Liu, C.-G.; Wang, X.-L.; Huang, Z.-H. Preparation and characterization of nanoparticles based on histidine-hyaluronic acid conjugates as doxorubicin carriers. J. Mater. Sci. Mater. Med. 2012, 23, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.S.; Kumar, M.G.; Suguna, L.; Sastry, T.; Mandal, A. Pullulan acetate nanoparticles based delivery system for hydrophobic drug. Int. J. Pharma Biol. Sci. 2012, 3, 24–32. [Google Scholar]
- Wang, Y.-S.; Liu, L.-R.; Jiang, Q.; Zhang, Q.-Q. Self-aggregated nanoparticles of cholesterol-modified chitosan conjugate as a novel carrier of epirubicin. Eur. Polym. J. 2007, 43, 43–51. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.-H.; Kwon, I.; Jeong, S. Self-aggregates of deoxycholic acid-modified chitosan as a novel carrier of adriamycin. Colloid Polym. Sci. 2000, 278, 1216–1219. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Hu, H.-Y.; Qiao, M.-X.; Zhu, J.; Qi, J.-W.; Hu, C.-J.; Zhang, Q.; Chen, D.-W. pH-sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: Preparation and in vitro evaluation. Colloids Surf. B Biointerfaces 2012, 94, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, Y.-S.; Kim, S.; Park, J.H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.-W.; Kim, I.-S. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Park, K.; Kim, Y.-S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.-W.; Kim, I.-S.; Jeong, S.Y.; Kim, K. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, L.-J.; Du, Y.-Z.; Hu, F.-Q. Stearic acid-g-chitosan polymeric micelle for oral drug delivery: In vitro transport and in vivo absorption. Mol. Pharm. 2010, 8, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-T.; Du, Y.-Z.; Yuan, H.; Hu, F.-Q. Brain-targeting study of stearic acid-grafted chitosan micelle drug-delivery system. Int. J. Nanomed. 2012, 7, 3235–3244. [Google Scholar]
- Park, J.S.; Han, T.H.; Lee, K.Y.; Han, S.S.; Hwang, J.J.; Moon, D.H.; Kim, S.Y.; Cho, Y.W. N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: Endocytosis, exocytosis and drug release. J. Control. Release 2006, 115, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yuan, X.; Chang, J. Self-aggregates of cholic acid hydrazide-dextran conjugates as drug carriers. J. Appl. Polym. Sci. 2005, 95, 487–493. [Google Scholar] [CrossRef]
- Daoud-Mahammed, S.; Couvreur, P.; Bouchemal, K.; Chéron, M.; Lebas, G.; Amiel, C.; Gref, R. Cyclodextrin and polysaccharide-based nanogels: Entrapment of two hydrophobic molecules, benzophenone and tamoxifen. Biomacromolecules 2009, 10, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, S.-J.; Na, K. Potential of self-organizing nanogel with acetylated chondroitin sulfate as an anti-cancer drug carrier. Colloids Surf. B Biointerfaces 2010, 79, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.; Kulkarni, A.; Aminabhavi, T. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: Effect of crosslinking agent. J. Microencapsul. 2002, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.; Zhou, X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2014, 15, 3519–3532. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Aminabhavi, T.M. Controlled release of clozapine through chitosan microparticles prepared by a novel method. J. Control. Release 2004, 96, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; Aminabhavi, T.M. Synthesis and characterization of modified chitosan microspheres: Effect of the grafting ratio on the controlled release of nifedipine through microspheres. J. Appl. Polym. Sci. 2003, 89, 2940–2949. [Google Scholar] [CrossRef]
- Park, K.M.; Bae, J.W.; Joung, Y.K.; Shin, J.W.; Park, K.D. Nanoaggregate of thermosensitive chitosan-Pluronic for sustained release of hydrophobic drug. Colloids Surf. B Biointerfaces 2008, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manaspon, C.; Viravaidya-Pasuwat, K.; Pimpha, N. Preparation of folate-conjugated pluronic F127/chitosan core-shell nanoparticles encapsulating doxorubicin for breast cancer treatment. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.-S.; Jung, Y.-S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly (l-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Na, H.-S.; Oh, J.-S.; Choi, K.-C.; Song, C.-E.; Lee, H.-C. Adriamycin release from self-assembling nanospheres of poly (DL-lactide-co-glycolide)-grafted pullulan. Int. J. Pharm. 2006, 322, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Shu, X.; Shen, Z.; Sun, R.-C. Self-assembly and paclitaxel loading capacity of cellulose-graft-poly (lactide) nanomicelles. J. Agric. Food Chem. 2012, 60, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-I.; Kim, D.H.; Chung, C.-W.; Yoo, J.-J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly (DL-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011, 6, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.-Y.; Song, C.-E.; Kim, C.; Park, W.-D.; Cho, K.-R.; Kim, P.-I.; Lee, S.-R.; Chung, W.-T.; Choi, K.-C. Cytotoxicity of amphotericin B-incorporated polymeric micelles composed of poly (DL-lactide-co-glycolide)/dextran graft copolymer. Arch. Pharm. Res. 2008, 31, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, C.H.; Park, T.G. Poly [lactic-co-(glycolic acid)]-Grafted Hyaluronic Acid Copolymer Micelle Nanoparticles for Target-Specific Delivery of Doxorubicin. Macromol. Biosci. 2009, 9, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Q.; Wang, X.; Wang, C.; Jiang, X. Preparation, drug release and cellular uptake of doxorubicin-loaded dextran-b-poly(ε-caprolactone) nanoparticles. Carbohydr. Polym. 2013, 93, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-J.; Sun, S.-L.; Feng, T.-H.; Sung, K.-H.; Lui, W.-L.; Wang, L.-F. Folate-mediated chondroitin sulfate-Pluronic® 127 nanogels as a drug carrier. Eur. J. Pharm. Sci. 2009, 38, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Shelke, N.B.; Rokhade, A.P.; Patil, S.A.; Aminabhavi, T.M. Formulation and in vitro evaluation of novel starch-based tableted microspheres for controlled release of ampicillin. Carbohydr. Polym. 2008, 71, 42–53. [Google Scholar] [CrossRef]
- Barbucci, R.; Fini, M.; Giavaresi, G.; Torricelli, P.; Giardino, R.; Lamponi, S.; Leone, G. Hyaluronic acid hydrogel added with ibuprofen-lysine for the local treatment of chondral lesions in the knee: In vitro and in vivo investigations. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Tahir, M.N.; Choi, J.M.; Kim, H.; Yu, J.-H.; Jung, S. Novel magnetic nanoparticles coated by benzene-and β-cyclodextrin-bearing dextran, and the sorption of polycyclic aromatic hydrocarbon. Carbohydr. Polym. 2015, 133, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Jo, D.-G.; Park, J.H. Polysaccharide-based nanoparticles: A versatile platform for drug delivery and biomedical imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004, 4, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Baimark, Y.; Srisuwan, Y. Preparation of polysaccharide-based microspheres by a water-in-oil emulsion solvent diffusion method for drug carriers. Int. J. Polym. Sci. 2013, 2013, 761870. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Tostado, C.; Lan, W.; Luo, G. Preparation of monodispersed chitosan microspheres and in situ encapsulation of BSA in a co-axial microfluidic device. Biomed. Microdevices 2009, 11, 243–249. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar] [CrossRef]
- Li, B.-Z.; Wang, L.-J.; Li, D.; Bhandari, B.; Li, S.-J.; Lan, Y.; Chen, X.D.; Mao, Z.-H. Fabrication of starch-based microparticles by an emulsification-crosslinking method. J. Food Eng. 2009, 92, 250–254. [Google Scholar] [CrossRef]
- Mellors, R.; Benzeval, I.; Eisenthal, R.; Hubble, J. Preparation of self-assembled microspheres and their potential for drug delivery. Pharm. Dev. Technol. 2010, 15, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Illum, L. Sustained release chitosan microspheres prepared by novel spray drying methods. J. Microencapsul. 1999, 16, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Wood, K.C.; Chuang, H.F.; Batten, R.D.; Lynn, D.M.; Hammond, P.T. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc. Natl. Acad. Sci. USA 2006, 103, 10207–10212. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Du, Q.; Cao, D.-Y.; Xiang, B.; Fan, L.-F. In vitro and in vivo studies of pectin/ethylcellulose-film-coated pellets of 5-fluorouracil for colonic targeting. J. Pharm. Pharmacol. 2008, 60, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Macleod, G.S.; Collett, J.H.; Fell, J.T. The potential use of mixed films of pectin, chitosan and HPMC for bimodal drug release. J. Control. Release 1999, 58, 303–310. [Google Scholar] [CrossRef]
- Dhanikula, A.B.; Panchagnula, R. Development and characterization of biodegradable chitosan films for local delivery of paclitaxel. AAPS J. 2004, 6, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Eiselt, P.; Yeh, J.; Latvala, R.K.; Shea, L.D.; Mooney, D.J. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials 2000, 21, 1921–1927. [Google Scholar] [CrossRef]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F.; Lenourry, A.; Charrueau, C.; Chaumeil, J. The use of polysaccharides to target drugs to the colon. Carbohydr. Polym. 2002, 48, 219–231. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Jung, S. Supramolecular Complexation of Carbohydrates for the Bioavailability Enhancement of Poorly Soluble Drugs. Molecules 2015, 20, 19620-19646. https://doi.org/10.3390/molecules201019620
Cho E, Jung S. Supramolecular Complexation of Carbohydrates for the Bioavailability Enhancement of Poorly Soluble Drugs. Molecules. 2015; 20(10):19620-19646. https://doi.org/10.3390/molecules201019620
Chicago/Turabian StyleCho, Eunae, and Seunho Jung. 2015. "Supramolecular Complexation of Carbohydrates for the Bioavailability Enhancement of Poorly Soluble Drugs" Molecules 20, no. 10: 19620-19646. https://doi.org/10.3390/molecules201019620
APA StyleCho, E., & Jung, S. (2015). Supramolecular Complexation of Carbohydrates for the Bioavailability Enhancement of Poorly Soluble Drugs. Molecules, 20(10), 19620-19646. https://doi.org/10.3390/molecules201019620