Antioxidants of Edible Mushrooms
Abstract
:1. Introduction

2. ROS and Antioxidants in Cell Metabolism and Their Consequences in Human Cells and Health
2.1. Introduction to ROS
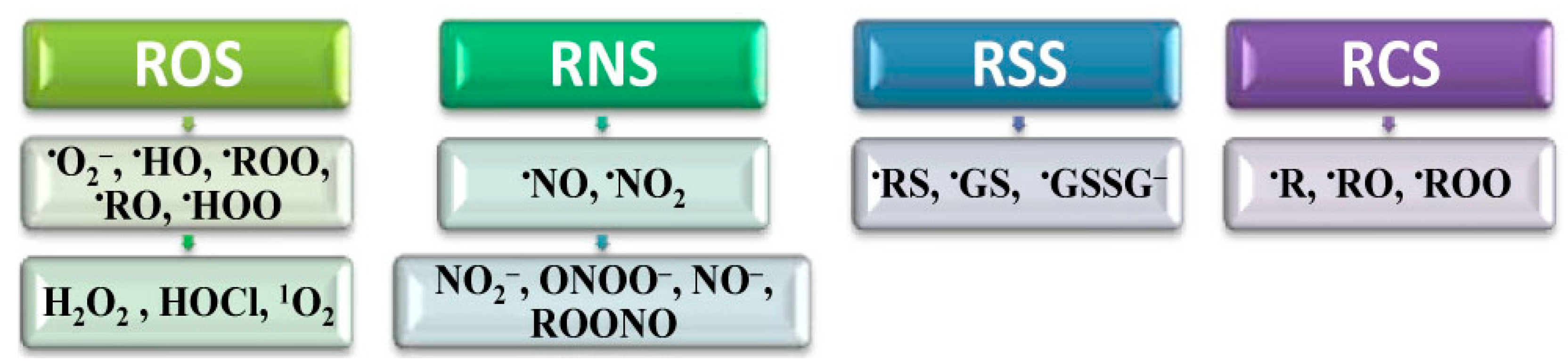
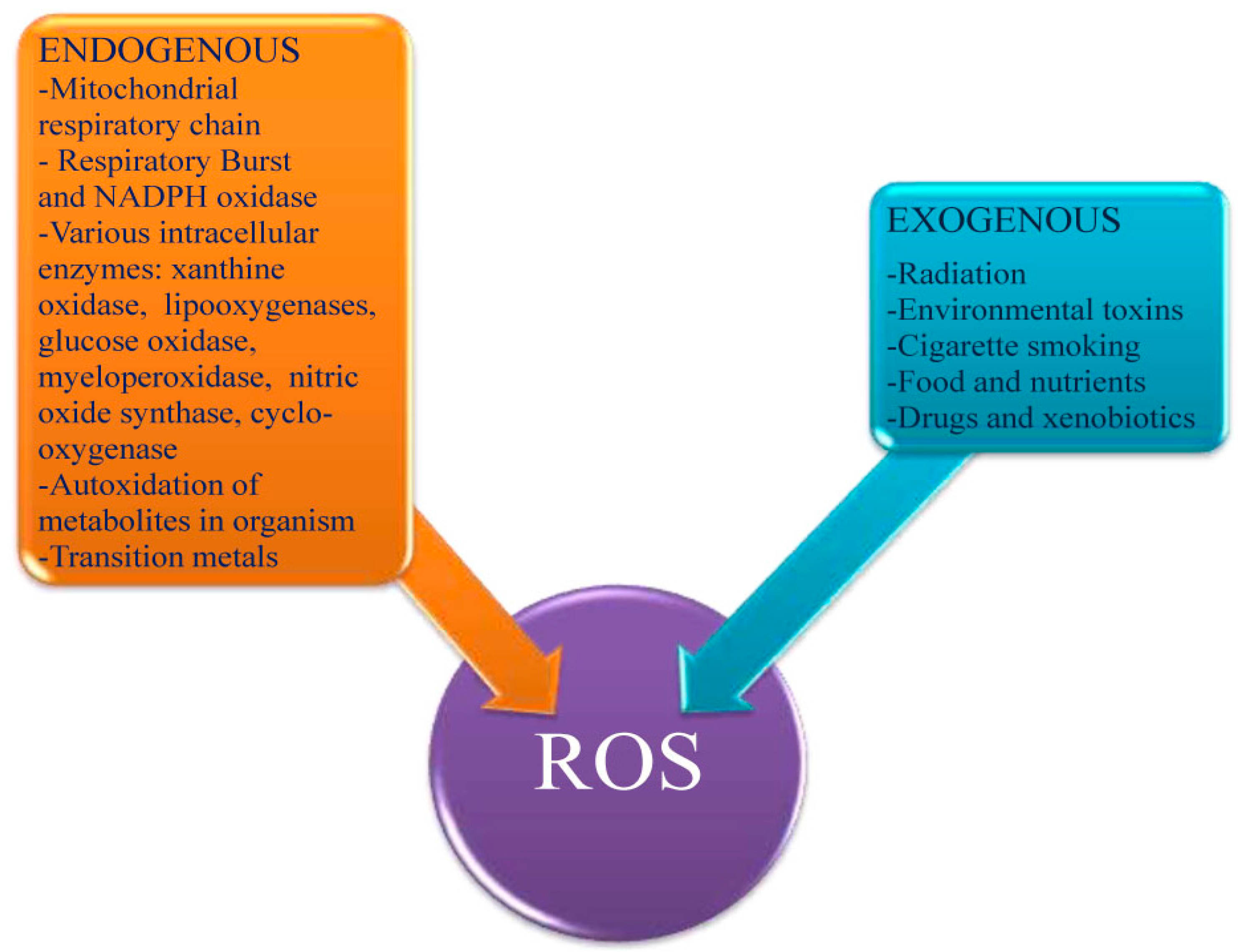
2.2. Positive Effects of ROS in Homeostasis
| No. | Cellular Pathways |
|---|---|
| 1 | proliferation and survival pathways through mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3), phosphatase and tensin homolog (PTEN), and protein tyrosine phosphatases |
| 2 | ROS homeostasis and antioxidant gene regulation through redox effector factor-1 (Ref-1), NF-E2-related factor (Nrf-2), thioredoxin |
| 3 | Aging through p66Shc, a member of the Src homologous-collagen homologue (ShcA) adaptor protein family |
| 4 | DNA damage response through ataxia-telangiectasia mutated kinase (ATM); this may lead to inhibition of the mammalian target of the rapamycin complex 1 (mTORC1) resulting in suppression of protein synthesis and activation of autophagy |
| 5 | Iron homeostasis through iron-regulatory proteins (IRP) and iron-responsive elements (IRE) |
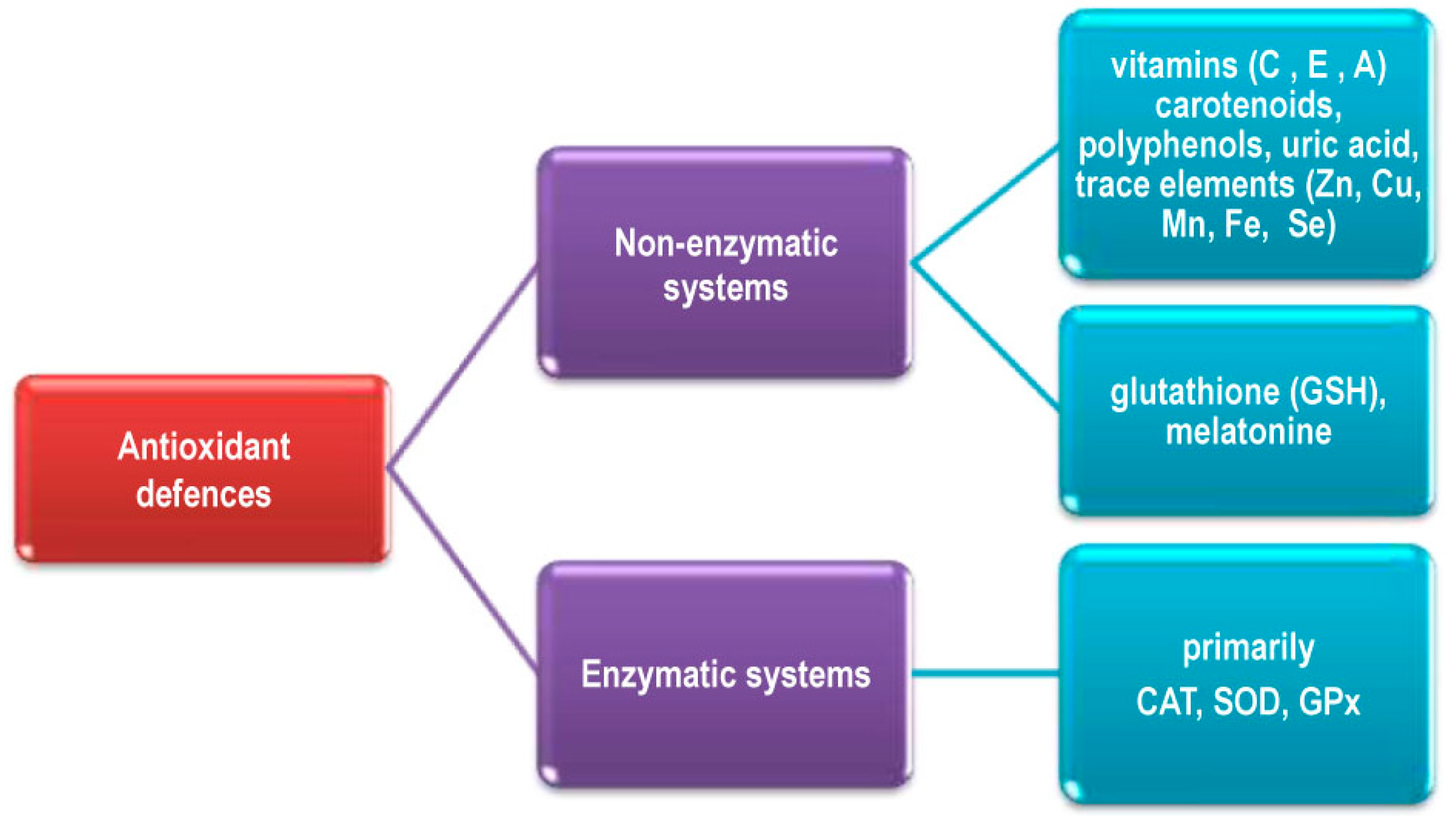
2.3. Elimination of ROS in Living Systems
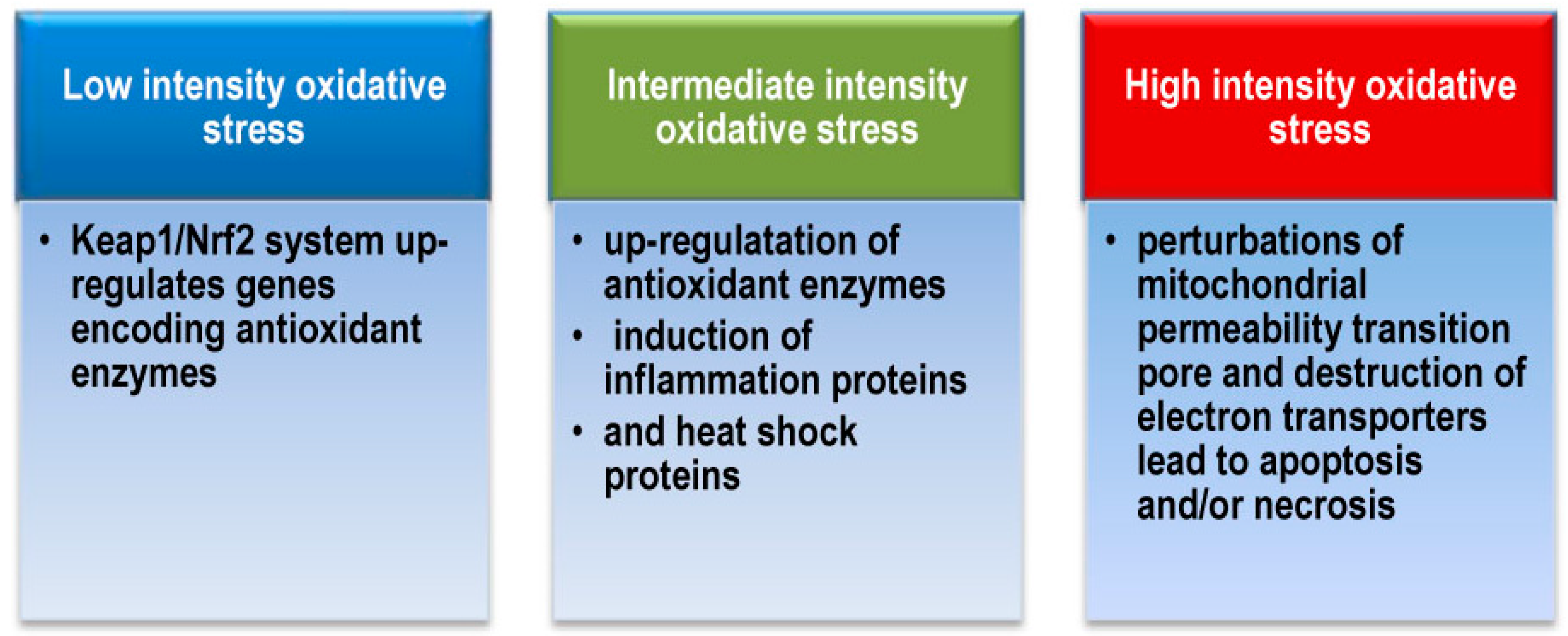
2.4. Regulation of Antioxidant Systems
3. Antioxidant Compounds in Mushrooms
| Mushroom Species | References |
|---|---|
| Agaricus bisporus, Agaricus brasiliensis (=Agaricus blazei ss. Heinem.), Agrocybe aegerita, Auricularia auricular, Auricularia cornea, Auricularia polytricha, Auricularia mesenterica, Auricularia fuscosuccinea, Agrocybe cylindracea, Amanita rubescens, Agaricus arvensis, Armillariella mellea, Agaricus silvicola, Agaricus silvaticus, Agaricus romagnesii, Antrodia camphorate | [75,77,81,82,83,90,91,93,97,103,104,112,115,117,121,123,125,129,130,131,132,133,135,139,140,142,144] |
| Boletus edulis, Boletus badius | [91,123,144] |
| Cantharellus lutescens, Cantharellus clavatus, Cantharellus cibarius, Cordyceps sinensis, Calvatia gigantea, Cerrena unicolor, Coprinus comatus | [16,91,93,97,104,115,134,140,142,144,147] |
| Dictophora indusiata | [114] |
| Flammulina velutipes (white), Flammulina velutipes (yellow) | [95,103,105,136,137,138] |
| Inonotus obliquus | [78,79,80] |
| Ganoderma lucidum, Ganoderma tsugae, Grifola frondosa, Ganoderma applanatum, Geastrum arenarius, Geastrum saccatum, Ganoderma atrum | [74,75,76,86,87,88,89,91,93,94,96,98,99,107,109,110,111,114,118,119,127,142,145,146] |
| Hericium erinaceus, Hericium coralloides, Hydnum repandum, Hygrophorus agathosmus, Hypsizigus marmoreus, Hypholoma fasciculare, Helvella crispa | [91,97,103,113,114,115,123,140,147] |
| Lepista nuda, Lentinus edodes, Lactarius sanguifluus, Lentinus squarrosulus, Lactarius deliciosus, Lentius sajor-caju, Leucopaxillus giganteus, Lactarius piperatus, Laetiporus sulphureus, Lycoperdon molle, Lycoperdon perlatum, Lactarius piperatus | [73,74,81,84,91,95,97,99,103,104,106,115,120,122,125,126,128,133,140,144,145,147] |
| Morchella esculenta, Morchella conica, Macrolepiota procera, Morchella angusticeps, Macrolepiota procera | [85,91,118,140] |
| Pleurotus ostreatus, Pleurotus eryngii, Pleurotus citrinopileatus, Pleurotus djamor, Pleurotus sajor-caju, Pleurotus cystidiosus, Pleurotus australis, Pleurotus tuber-regium, Phellinus linteus, Phellinus rimosus, Phellinus merrillii, Polyporus squamosus, Picoa juniperi, Pleurotus florida, Pleurotus pulmonarius, Paecilomyces japonica, Piptoporus betulinus | [50,75,81,88,91,92,93,95,97,102,103,104,108,115,116,124,133,141,142,144,148] |
| Russula brevipes, Russula cyanoxantha, Russula delica, Ramaria botrytis, Russula vinosa | [91,101,104,123,140] |
| Sparassis crispa, Suillus bellini, Suillus luteus, Suillus granulatus, Sarcodon imbricatus, Schizophyllum commune | [72,91,123,125,140,147] |
| Tricholoma acerbum, Tricholoma equestre, Tricholoma giganteum, Tricholomopsis rutilans, Termitomyces microcarpus, Termitomyces schimperi, Termitomyces mummiformis, Termitomyces tylerance, Termitomyces heimii, Termitomyces albuminosus, Termitomyces robustus, Terfezia claveryi, Tremella fuciformis, Trametes (Coriolus) versicolor, Trametes orientalis | [74,90,91,93,99,100,114,115,118,123,133,140,145,147] |
| Verpa conica, Volvariella volvacea | [103,104,106,120] |
3.1. Polyphenols Including Flavonoids
Flavonoids
3.2. Polysaccharides
3.3. Vitamins
3.3.1. Vitamin C
3.3.2. Vitamin E
3.3.3. Vitamin A Including Carotenoids
3.3.4. Vitamin D
3.3.5. Ergothioneine
3.4. Minerals
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Antioxidants Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2014–2020. Available online: http://www.prnewswire.com/news-releases/antioxidants-market-global-industry-analysis-size-share-growth-trends-and-forecast-2014-2020-300098101.html (accessed on 26 June 2015).
- Frost & Sullivan. Report, 2010. Available online: http://www.frost.com/prod/servlet/cio/236145272 (accessed on 30 June 2015).
- Knott, M. Natural Demand. Available online: http://www.foodmanufacture.co.uk/Ingredients/Natural-demand (accessed on 28 June 2015).
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanism of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Venkatesh, R.; Sood, D. Review of the Physiological Implications of Antioxidants in Food Interactive Qualifying; Project Report; Faculty of the Worcester Polytechnic Institute: Worcester, MA, USA, 2011; pp. 1–72. [Google Scholar]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.S.; Klaus, A.S.; Niksic, M.P.; van Griensven, L.J.L.D.; Vrvic, M.M.; Jakovljevic, D.M. Polysaccharides of higher fungi: Biological role, structure and antioxidative activity. Chem. Ind. 2014, 68, 305–320. [Google Scholar] [CrossRef]
- Yu, R.; Tan, T.H.; Kong, A.N. Butylated hydroxyanisole and its metabolite tert-butylhydroquinone differentially regulate mitogen-activated protein kinases. The role of oxidative stress in the activation of mitogen-activated protein kinases by phenolic antioxidants. J. Biol. Chem. 1997, 272, 28962–28970. [Google Scholar] [CrossRef] [PubMed]
- Lundebye, A.K.; Hove, H.; Mage, A.; Bohne, V.J.; Hamre, K. Levels of synthetic antioxidants (ethoxyquin, butylated hydroxytoluene and butylated hydroxyanisole) in fish feed and commercially farmed fish. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2010, 27, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Paul, S.; Acharya, K. Mushroom as the potential source of new generation of antioxidant: A review. Res. J. Pharm. Technol. 2013, 6, 496–505. [Google Scholar]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Valverde, M.E.; Hernandez-Perez, T.; Paredes-Lopez, O. Edible mushrooms: improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387:1–376387:14. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.K. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Konko, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Zizak, Z.; Niksic, M.; Jakovljevic, D.; Vrvic, M.M.; van Griensven, L.J.L.D. Nutraceutical properties of the methanolic extract of edible mushroom Cantharellus cibarius (Fries): Primary mechanisms. Food Funct. 2015, 6, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Ho, K.J.; Hsieh, Y.J.; Wang, L.T.; Mau, J.L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Van Griensven, L.J.L.D. Culinary-medicinal mushrooms: Must action be taken? Int. J. Med. Mushrooms 2009, 11, 281–286. [Google Scholar] [CrossRef]
- Loria-Kohen, V.; Lourenco-Nogueira, T.; Espinosa-Salinas, I.; Marin, F.R.; Soler-Rivas, C.; Ramirez de Molina, A. Nutritional and functional properties of edible mushrooms: A food with promising health claims. J. Pharm. Nutr. Sci. 2014, 4, 187–198. [Google Scholar] [CrossRef]
- Caglarirmak, N. Edible Mushrooms: An Alternative Food Item. In Economical and Societal Features, Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Convention Centre, Arcachon, France, 4–7 October 2011; p. 548.
- The Situation in Hungarian Mushroom Production and Possibilities of Development. Available online: http://phd.lib.uni-corvinus.hu/369/2/gyorfi_julia_ten.pdf (accessed on 29 June 2015).
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1957, 2, 298–300. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Chirico, E.N.; Pialoux, V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012, 64, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, J.; Vaddi, D.R.; Khan, S.A.; Wang, N.; Makerenko, V.; Prabhakar, N.R. Xanthine oxidase mediates hypoxia-inducible factor-2a degradation by intermittent hypoxia. PLoS ONE 2013, 8, e75838:1–e75838:9. [Google Scholar] [CrossRef] [PubMed]
- Saller, S.; Merz-Lange, J.; Raffael, S.; Hecht, S.; Pavlik, R.; Thaler, C.; Berg, D.; Berg, U.; Kunz, L.; Mayerhofer, A. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology 2012, 153, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792:1–956792:11. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxigen species (ROS) homeostasis and redox regulation in cellular signalling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Murrell, G.A.C.; Francis, M.J.O.; Bromley, L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem. J. 1990, 265, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, P.K.; Kwon, Y.G.; Bai, S.K.; Nam, W.D.; Kim, Y.M. Regulation of apoptosis by nitrosative stress. J. Biochem. Mol. Biol. 2002, 35, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Felty, Q.; Xiong, W.C.; Sun, D.; Sarkar, S.; Singh, K.P.; Parkash, J.; Roy, D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry 2005, 44, 6900–6909. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [PubMed]
- Kroncke, K.D. Nitrosative stress and transcription. J. Biol. Chem. 2003, 384, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Rivera-Del Valle, N.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cross, C.E. Oxygen-derived species: Their relation to human disease and environmental stress. Environ. Health Perspect. 1994, 102, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S.; Demirovic, D. Hormesis can and does work in humans. Dose-Response 2010, 8, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Woodman, R.C. Chronic granulomatous disease. Semin. Hematol. 1990, 27, 247–259. [Google Scholar] [PubMed]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Antimicrobial actions of reactive oxygen species. MBIO 2011. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.K.; Mukhopadhyay, M.; Chatterjee, I.B. NADPH-initiated P450-dependent free-iron-dependent microsomal lipid peroxidation: Specific prevention by ascorbic acid. Mol. Cell Biochem. 1997, 166, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001, 20, 464S–472S. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Zamzami, N.; Susin, S.A. Mitochondrial control of apoptosis. Immunol. Today 1997, 18, 44–51. [Google Scholar] [CrossRef]
- Meier, B.; Radeke, H.H.; Selle, S.; Younes, M.; Sies, H.; Resch, K.; Habermehl, G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989, 263, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Tiku, M.L.; Liesch, J.B.; Robertson, F.M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J. Immunol. 1990, 145, 690–696. [Google Scholar] [PubMed]
- Lo, Y.Y.C.; Cruz, T.F. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J. Biol. Chem. 1995, 270, 11727–11730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Harashima, N.; Moritani, T.; Huang, W.; Harada, M. The roles of ROS and caspases in TRAIL-induced apoptosis and necroptosis in human pancreatic cancer cells. PLoS ONE 2015, 10, e0127386:1–e0127386:21. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 1999, 11, 255–260. [Google Scholar] [CrossRef]
- Almasan, A.; Ashkenazi, A. Apo2L/TRAIL: Apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003, 14, 337–348. [Google Scholar] [CrossRef]
- Van Griensven, L.J.L.D.; Verhoeven, H.A. Phellinus linteus polysaccharide extracts increase the mitochondrial membrane potential and cause apoptotic death of THP-1 monocytes. Chin. Med. 2013, 8, 25:1–25:13. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Ni, J.; Wei, Y.F.; Yu, G.; Gentz, R.; Dixit, V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Jouan-Lanhouet, S.; Arshad, M.I.; Piquiet-Pellorce, C.; Martin-Chouly, C.; Moigne-Muller, G.L.; van Herreweghe, F.; Takahashi, N.; Sergent, O.; Lagadic-Gossmann, D.; Vandenabeele, P.; et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell. Death Differ. 2012, 19, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Meurette, O.; Rebillard, A.; Huc, L.; Moigne, G.L.; Merino, D.; Micheau, O.; Lagadic-Gossmann, D.; Dimanche-Boitrel, M.T. TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell dearth under acidic extracellular conditions. Cancer Res. 2007, 67, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Karl, I.; Jossberger-Werner, M.; Schmidt, N.; Horn, S.; Goebeler, M.; Leverkus, M.; Wajant, H.; Giner, T. TRAF2 inhibits TRAIL- and CD95L-induced apoptosis and necroptosis. Cell. Death Dis. 2014, 5, e1444:1–e1444:12. [Google Scholar] [CrossRef]
- Guzman, M.L.; Li, X.; Corbett, C.A.; Rossi, R.M.; Bushnell, T.; Liesveld, J.L.; Hebert, J.; Young, F.; Jordan, C.T. Rapid and selective death of leukemia stem and progenitor cells induced by the compound 4-benzyl, 2-methyl, 1,2,4-thiadiazolidine, 3,5 dione (TDZD-8). Blood 2007, 110, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, R.C.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. PNAS 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- James, D.E.; Kraegen, E.W.; Chisholm, D.J. Effect of exercise training on whole-body insulin sensitivity and responsiveness. J. Appl. Physiol. 1984, 56, 1217–1222. [Google Scholar] [PubMed]
- Simoneau, J.A.; Kelley, D.E. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J. Appl. Physiol. 1997, 83, 166–171. [Google Scholar] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Cristiana, F.; Zamosteanu, N.; Albu, E. Homocysteine in red blood cells metabolism—Pharmacological approaches. In Blood Cell—An Overview of Studies in Hematology; Moschandreou, T.E., Ed.; In Tech: Iasi, Romania, 2012; Chapter 3. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Biomarkers of oxidative stress in red blood cells. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2011, 155, 131–136. [Google Scholar] [CrossRef]
- Lindeman, J.H.; Lentjes, E.G.; Houdkamp, E.; van Zoeren-Grobben, D.; Schrijver, J.; Berger, H.M. Effect of an exchange transfusion on plasma antioxidants in the newborn. Pediatrics 1992, 90, 200–203. [Google Scholar] [PubMed]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajo, J.C. Antioxidant extraction by supercritical fluids. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; Martinez, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Chapter 9; pp. 275–305. [Google Scholar]
- Durackova, Z. Oxidants, antioxidants and oxidative stress. In Mitochondrial Medicine Mitochondrial Metabolism, Diseases, Diagnosis and Therapy; Gvozdjakova, A., Ed.; Springer: New York, NY, USA, 2008; Chapter 2; pp. 19–54. [Google Scholar]
- Lardone, P.; Alvarez-Sanchez, N.; Guerrero, J.; Carrillo-Vico, A. Melatonin and glucose metabolism: Clinical relevance. Curr. Pharm. Des. 2014, 20, 4841–4853. [Google Scholar] [PubMed]
- Chakrabarti, S.; Lekontseva, O.; Davidge, S.T. Estrogen is a modulator of vascular inflammation. IUBMB Life 2008, 60, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohe, C.; Laufs, K.; Bohm, M.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Joh, T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J. Clin. Biochem. Nutr. 2007, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837:1–736837:26. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [PubMed]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharides extracted from the basidiomycete Schizophyllum commune. LWT Food Sci. Technol. 2011, 44, 2005–2011. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; van Griensven, L.J.L.D.; Stefanoska, I. The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Vrvic, M.M.; Todorovic, N.; van Griensven, L.J.L.D.; Jakovljevic, D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; Jakovljevic, D.; Zizak, Z.; Pavlovic, V.; Levic, S.; Niksic, M; van Griensven, L.J.L.D. Biological potential of extracts of the wild edible Basidiomycete mushroom Grifola frondosa. Food Res. Int. 2015, 67, 272–283. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Niksic, M.; van Griensven, L.J.L.D.; Vrvic, M.M. Dietary polysaccharide extracts of Agaricus brasiliensis fruiting bodies: Chemical characterization and bioactivities at different levels of purification. Food Res. Int. 2014, 64, 53–64. [Google Scholar] [CrossRef]
- Glamoclija, J.; Ciric, A.; Nikolic, M.; Fernandes, A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sokovic, M.; van Griensven, L.J.L.D. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Park, D.K.; Lee, B.R.; Jin, H.L.; Lee, S.Y.; Samad, N.B.; Lim, B.O. Antioxidant activity of Inonotus obliquus grown on germinated brown rice extracts. J. Biochem. 2013, 37, 456–464. [Google Scholar]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant small phenolic ingredients in Inonotus obliquus (Persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolics profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, D.; Reis, F.S.; Glamoclija, J.; Ciric, A.; Barros, L.; van Griensven, L.J.L.D.; Sokovic, M.; Ferreira, I.C.F.R. Cultivated strains of Agaricus bisporus and A. brasiliensis: Chemical characterization and evaluation of antioxidant and antimicrobial properties for final healthy product—Natural preservatives in yoghurt. Food Funct. 2014, 5, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Ker, Y.B.; Chen, K.C.; Chyau, C.C.; Chen, C.C.; Guo, J.H.; Hsien, C.L.; Wang, H.E.; Peng, C.C.; Chang, C.H.; Peng, R.Y. Antioxidant capability of polysaccharides fractionated from submerge-cultured Agaricus blazei Mycelia. J. Agric. Food Chem. 2005, 53, 7052–7058. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.C.T.; Chang, C.A.; Chiuc, K.H.; Tsayd, P.K.; Jena, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Heleno, S.A.; Stojkovic, D.; Barros, L.; Glamoclija, J.; Sokovic, M.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Res. Int. 2013, 51, 236–243. [Google Scholar] [CrossRef]
- Li, W.J.; Nie, S.P.; Liu, X.Z.; Zhang, H.; Yang, Y.; Yu, Q.; Xie, M.Y. Antimicrobial properties, antioxidant activity and cytotoxicity of ethanol-soluble acidic components from Ganoderma atrum. Food Chem. Toxicol. 2012, 50, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.Y.; Hsieh, L.H.; Wu, K.T.; Tsai, C.F. Antioxidant properties and antioxidant compounds of various extracts from the edible Basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3321. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Janardhanan, K.K. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.L.; Chao, G.R.; Wu, K.T. Antioxidant properties of methanolic extracts from several ear mushrooms. J. Agric. Food Chem. 2001, 49, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; Lim, S.S.; Lee, S.H.; Lee, Y.S.; Cho, S.Y. Antioxidant and immunostimulating activities of the fruiting bodies of Paecilomyces japonica, a new type of Cordyceps sp. Ann. N. Y. Acad. Sci. 2001, 928, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; van Griensven, L.J.L.D. Pro- and antioxidative properties of medicinal mushroom extracts. Int. J. Med. Mushrooms 2008, 10, 315–324. [Google Scholar]
- Tseng, Y.H.; Yang, J.H.; Mau, J.L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008, 107, 732–738. [Google Scholar] [CrossRef]
- Yang, J.H.; Lin, H.C.; Mau, J.L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002, 77, 229–235. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Hemar, Y.; Perera, C.O.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre 2014, 3, 41–51. [Google Scholar] [CrossRef]
- Yu, Y.; Guzha, N.; Ying, T. Extraction of Polysaccharide from Ganoderma lucidum assisted ultrafiltration and optimization of free radical scavenging capacity. J. Chin. Inst. Food Sci. Technol. 2014, 34, 40–46. [Google Scholar]
- Siu, K.C.; Chen, X.; Wu, J.Y. Constituents actually responsible for the antioxidant activities of crude polysaccharides isolated from mushrooms. J. Funct. Foods 2014, 11, 548–556. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W.D. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 3215–3323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, G.; Yan, H.; Geng, X.; Cao, Q.; Wang, H.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom Russula vinosa lindblad. J. Agric. Food Chem. 2014, 62, 8858–8866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Pei, J.J.; Ma, H.L.; Cai, P.F.; Yan, J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr. Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shieh, D.; Ho, C. Antioxidant and free radical scavenging activities of edible mushrooms. J. Food Lipids 2002, 9, 35–46. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Comp. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Bao, H.N.D.; Osako, K.; Ohshima, T. Value-added use of mushroom ergothioneine as a colour stabilizer in processed fish meats. J. Sci. Food Agric. 2010, 90, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef]
- Chang, H.Y.; Ho, Y.L.; Sheu, M.J.; Lin, Y.H.; Tseng, M.C.; Wu, S.H.; Huang, G.J.; Chang, Y.S. Antioxidant and free radical scavenging activities of Phellinus merrillii extracts. Bot. Stud. 2007, 48, 407–417. [Google Scholar]
- Guo, C.Y.; Ji, S.Z.; Ping, C.X. Modulatory effect of Ganoderma lucidum polysaccharides on serum antioxidant enzymes activities in ovarian cancer rats. Carbohydr. Polym. 2009, 78, 258–262. [Google Scholar]
- Ping, C.X.; Yan, C.; Bing, L.S.; Guo, C.Y.; Yun, L.J.; Ping, L.L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar]
- Jia, J.; Zhang, X.; Hu, Y.S.; Wu, Y.; Wang, Q.Z.; Li, N.N.; Guo, Q.C.; Dong, X.C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, S.; Yu, L.; Ma, L. Evaluation of antioxidant property and quality of breads containing Auricularia auricula polysaccharide flour. Food Chem. 2007, 101, 1158–1163. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jian, S.Y.; Lian, P.Y.; Mau, J.L. Antioxidant properties of extracts from a white mutant of the mushroom Hypsizigus marmoreus. J. Food Comp. Anal. 2008, 21, 116–124. [Google Scholar] [CrossRef]
- Mau, J.L.; Lin, H.C.; Song, S.F. Antioxidant properties of several specialty mushrooms. Food Res. Int. 2002, 35, 519–526. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martinez-Tome, M.; Jimenez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P.J. Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. Food Prot. 2002, 65, 1614–1622. [Google Scholar]
- Song, Y.S.; Kim, S.H.; Sa, J.H.; Jin, C.; Lim, C.J.; Park, E.H. Antiangiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J. Ethnopharmacol. 2003, 88, 113–116. [Google Scholar] [CrossRef]
- Acharya, K.; Samui, K.; Rai, M.; Dutta, B.B.; Acharya, R. Antioxidant and nitric oxide synthase activation properties of Auricularia auricula. Indian J. Exp. Biol. 2004, 42, 538–540. [Google Scholar] [PubMed]
- Mau, J.L.; Chang, C.N.; Huang, S.J.; Chen, C.C. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004, 87, 111–118. [Google Scholar] [CrossRef]
- Acharya, K.; Yonzone, P.; Rai, M.; Rupa, A. Antioxidant and nitric oxide synthase activation properties of Ganoderma applanatum. Indian J. Exp. Biol. 2005, 43, 926–929. [Google Scholar] [PubMed]
- Cheung, L.M.; Cheung, P.C.K. Mushroom extracts with antioxidant activity against lipid peroxidation. Food Chem. 2005, 89, 403–409. [Google Scholar] [CrossRef]
- Lo, K.M.; Cheung, P.C.K. Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. alba. Food Chem. 2005, 89, 533–539. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Ribeiro, B.; Rangel, J.; Valentao, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Contents of carboxylic acids and two phenolics and antioxidant activity of dried portuguese wild edible mushrooms. J. Agric. Food Chem. 2006, 54, 8530–8537. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Liang, Z.C.; Chia, Y.C.; Lien, J.L.; Chen, K.S.; Lee, M.Y.; Wang, J.C. Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 2006, 54, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Dore, C.M.P.G.; Azevedo, T.C.G.; de Souza, M.C.R.; Rego, L.A.; de Dantas, J.C.M.; Silva, F.R.F.; Rocha, H.A.O.; Basela, I.G.; Leite, E.L. Antiinflammatory, antioxidant and cytotoxic actions of beta-glucan-rich extract from Geastrum saecatum mushroom. Int. Immunopharmacol. 2007, 7, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Kitzberger, C.S.G.; Smania, A.; Pedrosa, R.C.; Ferreira, S.R.S. Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J. Food Eng. 2007, 80, 631–638. [Google Scholar] [CrossRef]
- Ng, L.T.; Wu, S.J.; Tsai, J.Y.; Lai, M.N. Antioxidant activities of cultured Armillariella mellea. Prikl. Biokhim. Mikrobiol. 2007, 43, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.M.; Vellosa, J.C.; Fernandes, A.S.; Buffa-Filho, W.; Hakime-Silva, R.A.; Furlan, M.; Brunetti, I.L. Antioxidant activity of Agaricus blazei. Fitoterapia 2007, 78, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Falcao, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant activity of Agaricus sp. Mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- Soares, A.A.; de Souza, C.G.M.; Daniel, F.M.; Ferrari, G.P.; da Costa, S.M.G.; Peralta, R.M. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem. 2009, 112, 775–781. [Google Scholar] [CrossRef]
- Obodai, M.; Ferreira, I.C.F.R.; Fernandes, A.; Barros, L.; Narh Mensah, D.L.; Dzomeku, M.; Urben, A.F.; Prempeh, J.; Takli, R.K. Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules 2014, 19, 19532–19548. [Google Scholar] [CrossRef] [PubMed]
- Jaszek, M.; Osinska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkolazka, A. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed. Res. Int. 2013, 2013, 497492:1–497492:11. [Google Scholar] [CrossRef] [PubMed]
- Weigand-Heller, J.; Kris-Etherton, P.M.; Beelman, R.B. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, A.B.; Fagutao, F.; Jintasataporn, O.; Worawattanamateekul, W.; Hirono, I.; Ohshima, T. Application of ergothioneine-rich extract from an edible mushroom Flammulina velutipes for melanosis prevention in shrimp, Penaeus monodon and Litopenaeus vannamei. Food Res. Int. 2012, 45, 232–237. [Google Scholar] [CrossRef]
- Encarnacion, A.B.; Fagutao, F.; Hirono, I.; Ushio, H.; Ohshima, T. Effects of ergothioneine from mushrooms (Flammulina velutipes) on melanosis and lipid oxidation of kuruma shrimp (Marsupenaeus japonicus). J. Agric. Food Chem. 2010, 58, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.N.D.; Ushio, H.; Ohshima, T. Antioxidative activity and antidiscoloration efficacy of ergothioneine in mushroom (Flammulina velutipes) extract added to beef and fish meats. J. Agric. Food Chem. 2008, 56, 10032–10040. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Correia, D.M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem. 2008, 110, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Duenas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2008, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Vunduk, J.; Klaus, A.; Kozarski, M.; Petrovic, P.; Zizak, Z.; Niksic, M.; van Griensven, L.J.L.D. Did the “Iceman” know better: Screening of the medicinal properties of Piptoporus betulinus. Int. J. Med. Mushrooms 2015, in press. [Google Scholar]
- Wei, S.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Phenolic compounds present in medicinal mushroom extracts generate reactive oxygen species in human cells in vitro. Int. J. Med. Mushrooms 2008, 10, 1–13. [Google Scholar] [CrossRef]
- Dubost, N.J.; Beelman, R.B.; Peterson, D.; Royse, D.J. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int. J. Med. Mushrooms 2006, 8, 215–222. [Google Scholar] [CrossRef]
- Muszynska, B.; Sulkowska-Ziaja, K.; Ekiert, H. Phenolic acids in selected edible basidiomycota species: Armillaria mellea, Boletus badius, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus and Pleurotus ostreatus. Acta Sci. Pol. Hortorum Cultus 2013, 12, 107–116. [Google Scholar]
- Cheung, Y.C.; Siu, K.C.; Liu, Y.S.; Wu, J.Y. Molecular properties and antioxidant activities of polysaccharideprotein complexes from selected mushrooms by ultrasound assisted extraction. Process. Biochem. 2012, 47, 892–895. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Chemical composition and biological properties of Portuguese wild mushrooms: a comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Suabjakyong, P.; Saiki, R.; van Griensven, L.; Higashi, K.; Nishimura, K.; Igarashi, K.; Toida, T. Polyphenol extract from Phellinus igniarius protects against acrolein toxicity in vitro and provides protection in a mouse stroke model. PLoS ONE 2015, 10, e0122733:1–e0122733:14. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in foods: State of the science important to the food industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [PubMed]
- Vujovic, D.; Pejin, B.; Popovic-Djordjevic, J.; Velickovic, M.; Tesevic, V. Phenolic natural products of the wines obtained from three new Merlot clone candidates. Nat. Prod. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [PubMed]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.W.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [PubMed]
- Forman, H.J.; Torres, M.; Fukuto, J. Redox signaling. Mol. Cell Biochem. 2002, 234–235, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Sirtuins in aging and disease. Cold Spring Harbor Symp. Quant. Biol. 2007, 72, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.M.; Huber, F.M.; Hoelz, A. Structural and functional analysis of human SIRT1. J. Mol. Biol. 2014, 426, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox. Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Lagunes, I.; Trigos, A. Photo-oxidation of ergosterol: Indirect detection of antioxidants photosensitizers or quenchers of singlet oxygen. J. Photochem. Photobiol. B 2015, 145, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Das, C.K. Curcumin (diferuloylmethane), a singlet oxygen (1O2) quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef]
- Celaje, J.A.; Zhang, D.; Guerrero, A.M.; Selke, M. Chemistry of trans-resveratrol with singlet oxygen: [2 + 2] addition, [4 + 2] addition, and formation of the phytoalexin moracin M. Org. Lett. 2011, 13, 4846–4849. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, S.; Fotiou, D.; Alamanou, A.; Deliconstantinos, G. Resveratrol activation of nitric oxide synthase in rabbit brain synaptosomes: singlet oxygen (1O2) formation as a causative factor of neurotoxicity. In Vivo 2010, 24, 49–53. [Google Scholar] [PubMed]
- Buss, S.; Dobra, J.; Goerg, K.; Hoffmann, S.; Kippenberger, S.; Kaufmann, R.; Hofmann, M.; Bernd, A. Visible light is a better co-inducer of apoptosis for curcumin-treated human melanoma cells than UVA. PLoS ONE 2013, 8, e79748:1–e79748:8. [Google Scholar]
- Timmers, S.; Auwerx, J.; Schrauwen, P. The journey of resveratrol from yeast to human. Aging 2012, 4, 146–158. [Google Scholar] [PubMed]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef]
- Renaud, S.C.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Farkas, O.; Jakus, J.; Heberger, K. Quantitative structure–antioxidant activity relationships of flavonoid compounds. Molecules 2004, 9, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, F.B.A.; Perello, L.; Real, J.A.; Gonzalez-Alvarez, M.; Alzuet, G.; Garcia-Granda, S.; Borras, J.; Montejo-Bernardo, J. Oxidative DNA cleavage induced by an iron(III) flavonoid complex: Synthesis, crystal structure and characterization of chlorobis(flavonolato)(methanol) iron(III) complex. J. Inorg. Biochem. 2006, 100, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Liu, L.; Guo, W.; Meydani, M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J. Nutr. Biochem. 2006, 17, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Peng, C.C.; Cheng, Y.M.; Lin, L.Y.; Ker, Y.B.; Chang, C.H.; Chen, K.C.; Peng, R.Y. Quercetin and ferulic acid aggravate renal carcinoma in long-term diabetic victims. J. Agric. Food Chem. 2010, 58, 9273–9280. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev. 2010, 68, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Ooi, V.E.C.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermayer, T.H.J.; Julich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Alternat. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Alzorqi, I.; Manickam, S. Effects of axial circulation and dispersion geometry on the scale-up of ultrasonic extraction of polysaccharides. AlChE J. 2015, 61, 1483–1491. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Baggio, C.H.; Borato, D.G.; Santana-Filho, A.P.; Sassaki, G.L.; Iacomini, M.; van Griensven, L.J.L.D. Anti-inflammatory properties of the medicinal mushroom Cordyceps militaris might be related to its linear (1–3)-β-d-Glucan. PLoS ONE 2014, 9, e110266:1–e110266:26. [Google Scholar]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.P.; M’Rabet, L.; Stahl, B.; Boehm, G.; Garssen, J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit. Rev. Immunol. 2007, 27, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.W.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med. 2001, 30, 393–402. [Google Scholar] [CrossRef]
- Kishk, Y.F.M.; Al-Sayed, H.M.A. Free-radical scavenging and antioxidative activities of some polysaccharides in emulsions. LWT Food Sci. Technol. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Cipriani, T.R.; Mellinger, C.G.; de Souza, L.M.; Baggio, C.H.; Freitas, C.S.; Marques, M.C.; Gorin, P.A.; Sassaki, G.L.; Iacomini, M. A polysaccharide from a tea (infusion) of Maytenus ilicifolia leaves with anti-ulcer protective effects. J. Nat. Prod. 2006, 69, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.Z.; Ding, G.C.; Gu, C.M.; Huang, T.; Rui, C.; Wang, Y.X.; Lu, Q. CDX2 enhances HTR-8/SVneo trophoblast cell invasion by altering the expression of matrix metalloproteinases. Cell Physiol. Biochem. 2014, 34, 628–636. [Google Scholar]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins—vitamin-E and vitamin-C, beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant l-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Yamamoto, K.; Niki, E. Interaction of alpha-tocopherol with iron: antioxidant and prooxidant effects of alpha-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochim. Biophys. Acta 1998, 958, 19–23. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef]
- Tong, L.; Chuang, C.C.; Wu, S.; Zuo, L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Savas, E.; Aksoy, N.; Pehlivan, Y.; Sayiner, Z.A.; Ozturk, Z.A.; Tabur, S.; Orkmez, M.; Onat, A.M. Evaluation of oxidant and antioxidant status and relation with prolidase in systemic sclerosis. Wien. Klin. Wochenschr. 2014, 126, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Top 10 Foods Highest in Vitamin E You Can’t Miss. Available online: http://healthaliciousness.com/artcles/vitamin-E.php (accessed on 1 July 2015).
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.J.; Passmore, A.P.; Vahidassr, M.D.; Young, I.S.; Lawson, J.T. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM 1999, 92, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pilz, D.; Norvell, L.; Danell, E.; Molina, R. General Technical Report PNW-GTR-576; United States Department of Agriculture (USDA): Portland, OR, USA, 2003. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [PubMed]
- Paiva, S.A.; Russell, R.M. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P., Jr.; Caan, B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the life after cancer epidemiology (LACE) cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.W.; Khalafi, R.; Cassidy, M.M.; Vahouny, G.V. Alterations in calcium, magnesium, iron, and zinc metabolism by dietary cholestyramine. Dig. Dis. Sci. 1985, 30, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, A.; Denis, I.; Colin, C. Effects of dietary vitamin D on magnesium absorption and bone mineral contents in pigs on normal magnesium intakes. Magnes Res. 1995, 8, 19–26. [Google Scholar] [PubMed]
- Wiseman, H. Vitamin D is a membrane antioxidant Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702:1–e40702:20. [Google Scholar] [CrossRef] [PubMed]
- Outila, T.A.; Mattila, P.H.; Piironen, V.I.; Lamberg-Allardt, C.J.E. Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human. Am. J. Clin. Nutr. 1999, 69, 95–98. [Google Scholar] [PubMed]
- Mattila, P.H.; Piironen, V.I.; Uusi-Rauva, E.J.; Koivistoinent, P.E. Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 1994, 42, 2449–2453. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.G.; Rinker, D.L.; Tsao, R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue and developmental stage. J Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef] [PubMed]
- Souci, S.W.; Fachman, W.; Kraut, H. Food Composition and Nutrition Tables, 3rd ed.; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 1986. [Google Scholar]
- Holland, B.; Welch, A.A.; Unwin, I.D.; Buss, D.H.; Paul, A.A.; Southgate, D.A.T. McCance and Widdowson’s The Composition of Foods; The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food, Richard Clay Ltd.: Bungay, Suffolk, England, UK, 1991. [Google Scholar]
- Moller, A. Levnedsmiddeltabeller; Statens Levnedmiddelinstitute: Frederikshavn, Germany, 1985. [Google Scholar]
- Takeuchi, A.; Okano, T.; Teraoka, S.; Murakami, Y.; Kobayashi, T. High-performance liquid chromatographic determination of vitamin D in foods, feeds and pharmaceuticals by successive use of reversed-phase and straight-phasecolumns. J. Nutr. Sei. Vitaminol. 1984, 30, 11–25. [Google Scholar] [CrossRef]
- Takamura, K.; Hoshino, H.; Sugahara, T.; Amano, H. Determination of vitamin D2 in shiitake mushroom by highperformance liquid chromatography. J. Chromatogr. 1991, 545, 201–204. [Google Scholar] [CrossRef]
- Koyyalamudi, S.R.; Jeong, S.C.; Song, C.H.; Cho, K.Y.; Pang, G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- Koyyalamudi, S.R.; Jeong, S.C.; Pang, G.; Teal, A.; Biggs, T. Concentration of vitamin D2 in white button mushrooms (Agaricus bisporus) exposed to pulsed UV light. J. Food Comp. Anal. 2011, 24, 976–979. [Google Scholar] [CrossRef]
- Roberts, J.S.; Teichert, A.; McHugh, T.H. Vitamin D2 formation from postharvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agric. Food Chem. 2008, 56, 4541–4544. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jagerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effect of UV radiation. LWT Food Sci Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Taraboletti, G.; Perin, L.; Bottazzi, B.; Mantovani, A.; Giavazzi, R.; Salmona, M. Membrane fluidity affects tumor-cell motility, invasion and lung-colonizing potential. Int. J. Cancer 1989, 44, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Baldassari, F.; Bononi, A.; Bonora, M.; de Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; et al. Mitochondrial Ca2+ and apoptosis. Cell Calcium 2012, 52, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, B.J.; Stockwell, B.R.; Ingram, V.M. Eliminating membrane depolarization caused by the Alzheimer peptide Aβ(1–42, aggr.). Biochem. Biophys. Res. Commun. 2002, 293, 1204–1208. [Google Scholar] [CrossRef]
- Ho, P.W.L.; Ho, J.W.M.; Liu, H.F.; So, D.H.F.; Tse, Z.H.M.; Chan, K.H.; Ramsden, D.B.; Ho, S.L. Mitochondrial neuronal uncoupling proteins: A target for potential disease-modification in Parkinson’s disease. Transl. Neurodegener. 2012, 1, 3:1–3:9. [Google Scholar] [CrossRef] [PubMed]
- Grundemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schomig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [PubMed]
- Grundemann, D. The ergothioneine transporter controls and indicates ergothioneine activity. Prev. Med. 2012, 54, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, C.; Stihi, C.; Busuioc, G.; Gheboianu, A.I.; Popescu, I.V. Studies concerning heavy metals bioaccumulation of wild edible mushrooms from industrial area by using spectrometric techniques. Bull. Environ. Contam. Toxicol. 2010, 84, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Borovicka, B. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Turlo, J.; Gutkowska, B.; Klimaszewska, M.; Kapusta, C.; Schneider, K.; Sikora, M.; Cieslak, M.; Kazmierczak-Baranska, J.; Gorski, A.; Purchla, S.; et al. Selenium-Enriched Polysaccharide Fraction Isolated from Mycelial Culture of Lentinula edodes (Berk.)—Preliminary Analysis of the Structure and Biological Activity. In Mycosourced Molecules and Nutritional Quality, Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Convention Centre, Arcachon, France, 4–7 October 2011; pp. 242–246.
- Milovanovic, I.; Brceski, I.; Stajic, M.; Knezevic, A.; Vukojevic, J. Potential enrichment of medicinal mushrooms with selenium to obtain new dietary supplements. Int. J. Med. Mushrooms 2013, 15, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, I.; Stajic, M.; Stanojkovic, T.; Knezevic, A.; Vukojevic, J. Effects of selenium presence in mycelia of Ganoderma species (higher basidiomycetes) on their medicinal properties. Int. J. Med. Mushrooms 2015, 17, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Vunduk, J.; Klaus, A.; Kozarski, M.; Dordevic, R.; Jovanovic, L.; Niksic, M. Zeolites as possible biofortifiers in Maitake cultivation. Arch. Biol. Sci. 2014, 66, 123–129. [Google Scholar] [CrossRef]
- Lipinski, B. Rationale for the treatment of cancer with sodium selenite. Med. Hypotheses 2005, 64, 806–810. [Google Scholar] [CrossRef]
- Tobe, T.; Ueda, K.; Ando, M.; Okamoto, Y.; Kojima, N. Thiol-mediated multiple mechanisms centered on selenodiglutathione determine selenium cytotoxicity against MCF-7 cancer cells. J. Biol. Inorg. Chem. 2015, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Stihi, C.; Radulescu, C.; Busuioc, G.; Popescu, I.V.; Gheboianu, A.; Ene, A. Studies on accumulation of heavy metals from substrate to edible wild mushrooms. Rom. J. Phys. 2011, 56, 257–264. [Google Scholar]
- Das, N. Heavy metals biosorption by mushrooms. Nat. Prod. Radiance 2005, 4, 454–459. [Google Scholar]
- Kalac, P.; Ninanska, M.; Bevilaqua, D.; Staskova, I. Concentartion of mercury, copper, cadmium and lead in fruiting bodies of edible mushrooms in the vicinity of a mercury smelter and a copper smelter. Sci. Total Environ. 1996, 177, 251–258. [Google Scholar] [CrossRef]
- Mironczuk-Chodakowska, I.; Socha, K.; Witkowska, A.M.; Zujko, M.E.; Borawska, M.H. Cadmium and lead in wild edible mushrooms from the eastern region of Poland s “Green Lungs”. Pol. J. Environ. Stud. 2013, 22, 1759–1765. [Google Scholar]
- Hagemeyer, J. Heavy Metal Stress in Plants: From Molecules to Ecosystems; Prasad, M.N.V., Ed.; Springer: New York, NY, USA, 1999; p. 401. [Google Scholar]
- Sharma, B.; Singh, S.; Siddiqi, N.J. Biomedical implications of heavy metals induced imbalances in redox systems. Biomed. Res. Int. 2014, 2014, 640754:1–640754:26. [Google Scholar]
- Casalino, E.; Sblano, C.; Landriscina, C. Enzyme activity alteration by cadmium administration to rats: The possibility of iron involvement in lipid peroxidation. Arch. Biochem. Biophys. 1997, 346, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M.K. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Arce, C.; Oset-Gasque, M.J.; Canadas, S.; Gonzalez, M.P. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Biol. Med. 2006, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, H.; Sato, M.; Kezuka, K.; Sato, I.; Feng, X.; Okumura, S.; Fujita, T.; Yokoyama, U.; Eguchi, H.; Ishikawa, Y.; et al. Effect of ascorbic acid on reactive oxygen species production in chemotherapy and hyperthermia in prostate cancer cells. J. Physiol. Sci. 2012, 62, 251–257. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489-19525. https://doi.org/10.3390/molecules201019489
Kozarski M, Klaus A, Jakovljevic D, Todorovic N, Vunduk J, Petrović P, Niksic M, Vrvic MM, Van Griensven L. Antioxidants of Edible Mushrooms. Molecules. 2015; 20(10):19489-19525. https://doi.org/10.3390/molecules201019489
Chicago/Turabian StyleKozarski, Maja, Anita Klaus, Dragica Jakovljevic, Nina Todorovic, Jovana Vunduk, Predrag Petrović, Miomir Niksic, Miroslav M. Vrvic, and Leo Van Griensven. 2015. "Antioxidants of Edible Mushrooms" Molecules 20, no. 10: 19489-19525. https://doi.org/10.3390/molecules201019489
APA StyleKozarski, M., Klaus, A., Jakovljevic, D., Todorovic, N., Vunduk, J., Petrović, P., Niksic, M., Vrvic, M. M., & Van Griensven, L. (2015). Antioxidants of Edible Mushrooms. Molecules, 20(10), 19489-19525. https://doi.org/10.3390/molecules201019489








