γ-Alumina Nanoparticle Catalyzed Efficient Synthesis of Highly Substituted Imidazoles
Abstract
:1. Introduction
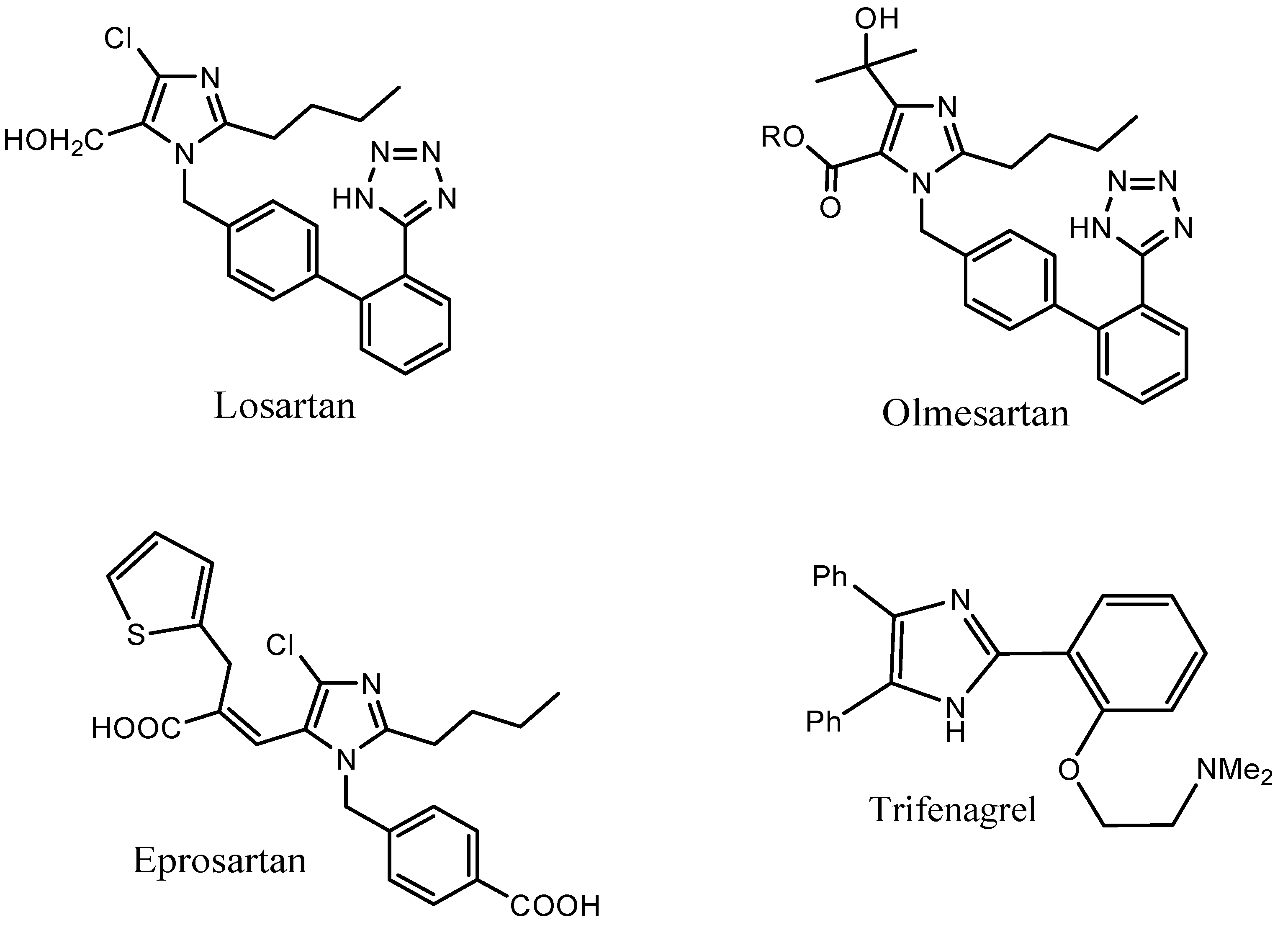
2. Results and Discussion
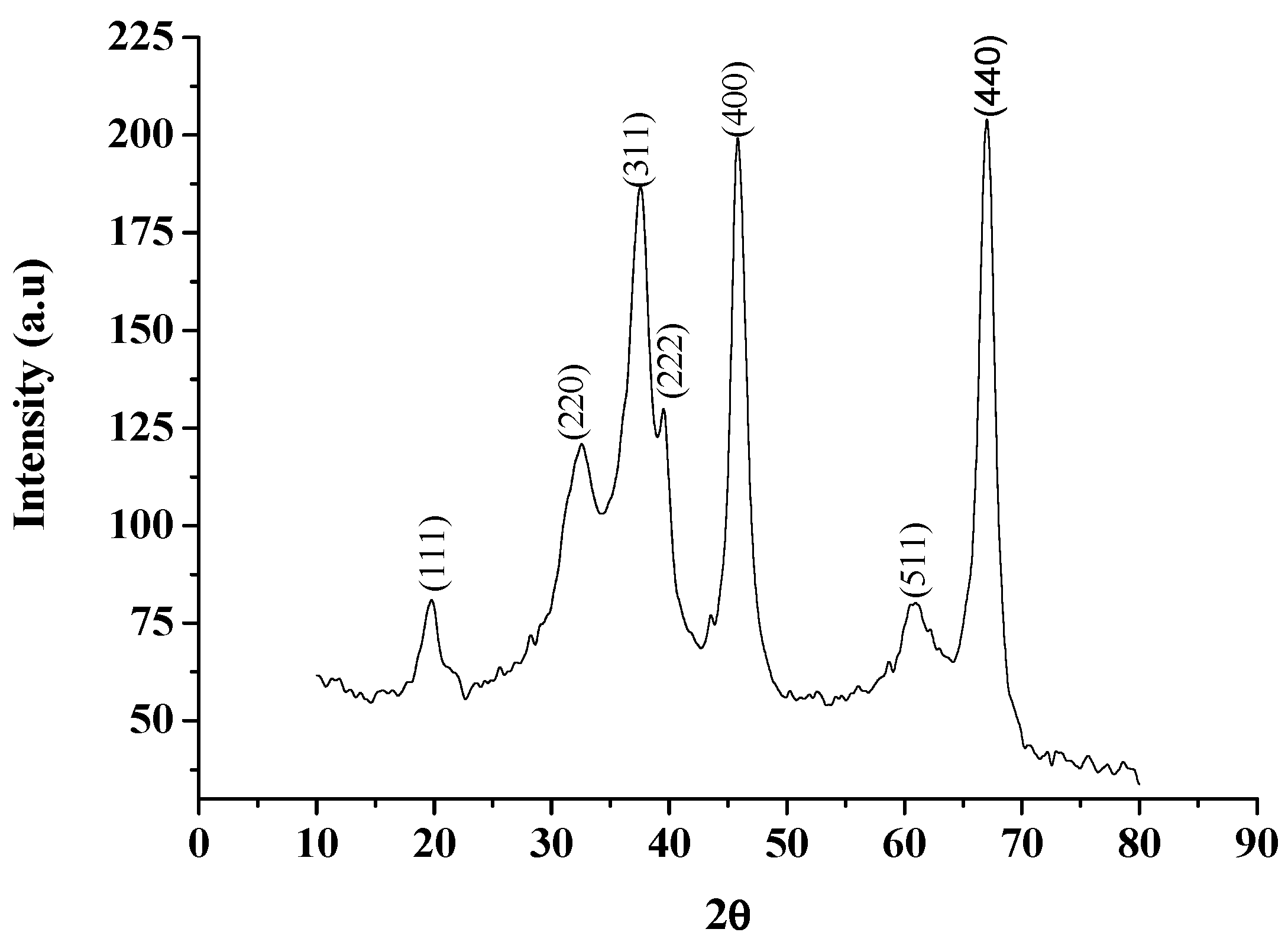
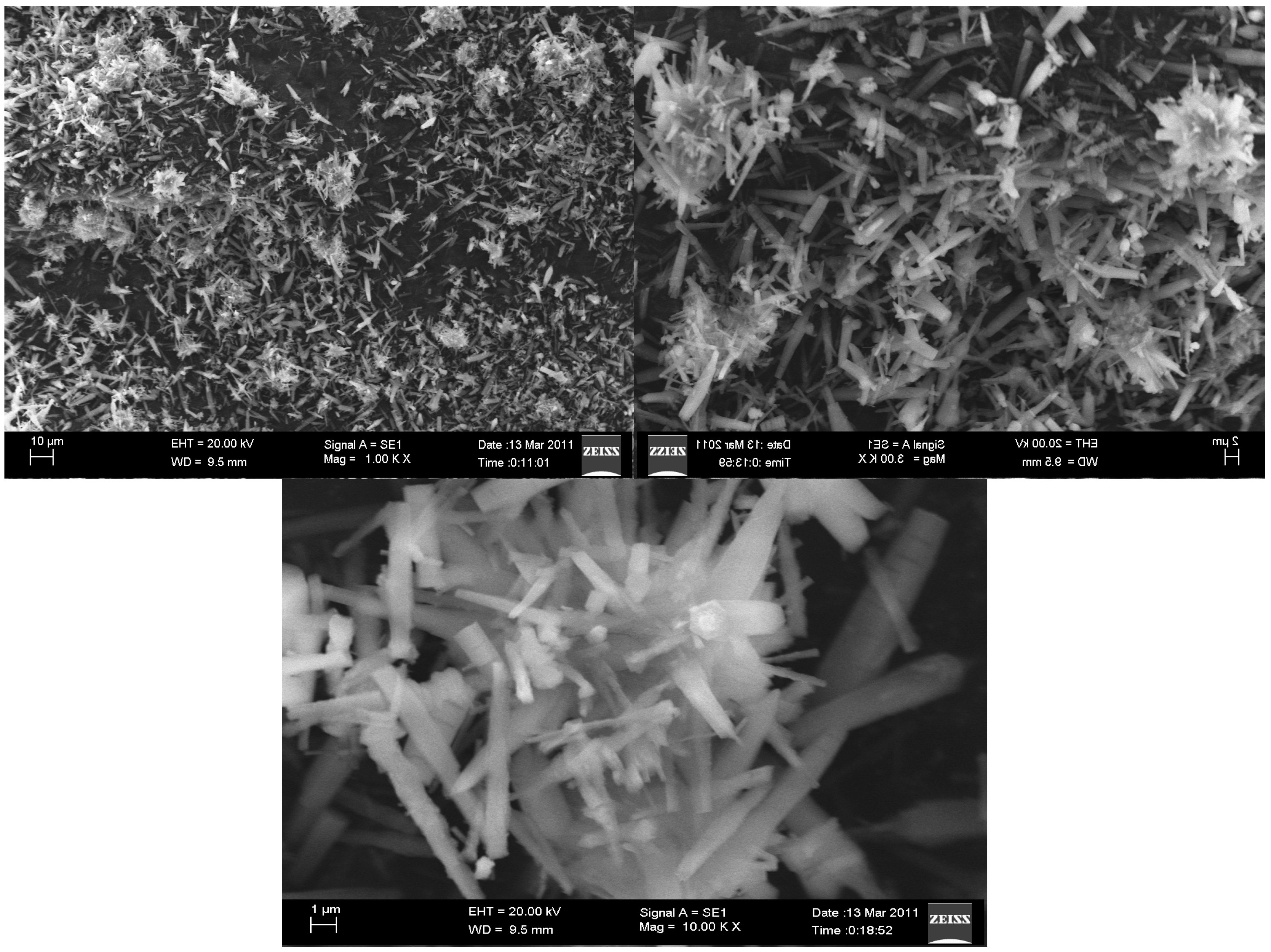
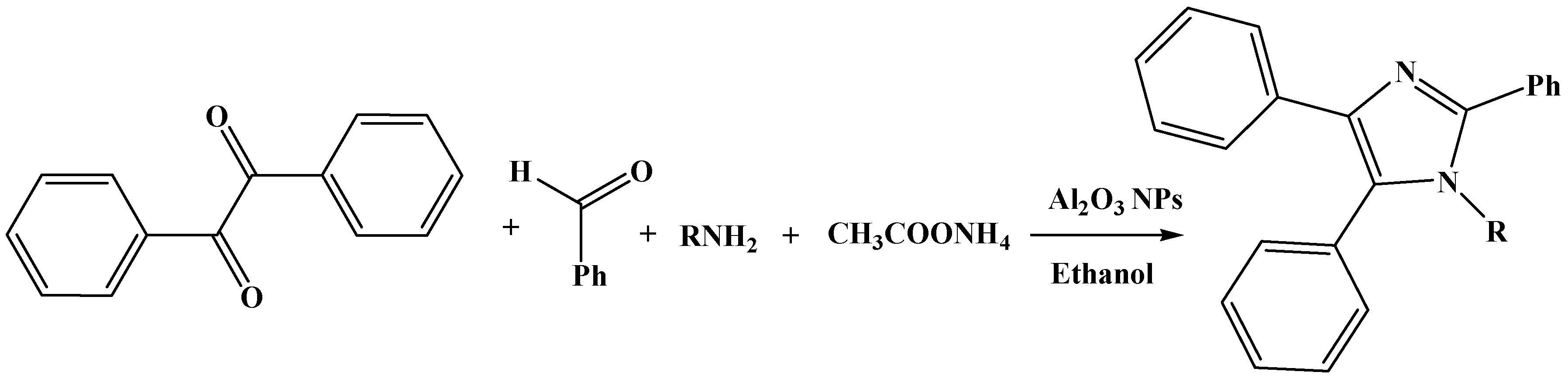
| In Conventional Method | Under Ultrasonication | ||||||
|---|---|---|---|---|---|---|---|
| Entry | Al2O3 (mol %) | Time (min) | Yield (%) b | Entry | Al2O3 (mol %) | Time (min) | Yield (%) b |
| 1 | 20 | 40 | 93 | 1 | 20 | 25 | 95 |
| 2 | 15 | 40 | 93 | 2 | 15 | 25 | 94 |
| 3 | 10 | 40 | 93 | 3 | 10 | 25 | 94 |
| 4 | 05 | 60 | 82 | 4 | 05 | 45 | 80 |
| 5 | 00 | 240 | 33 | 5 | 00 | 120 | 35 |


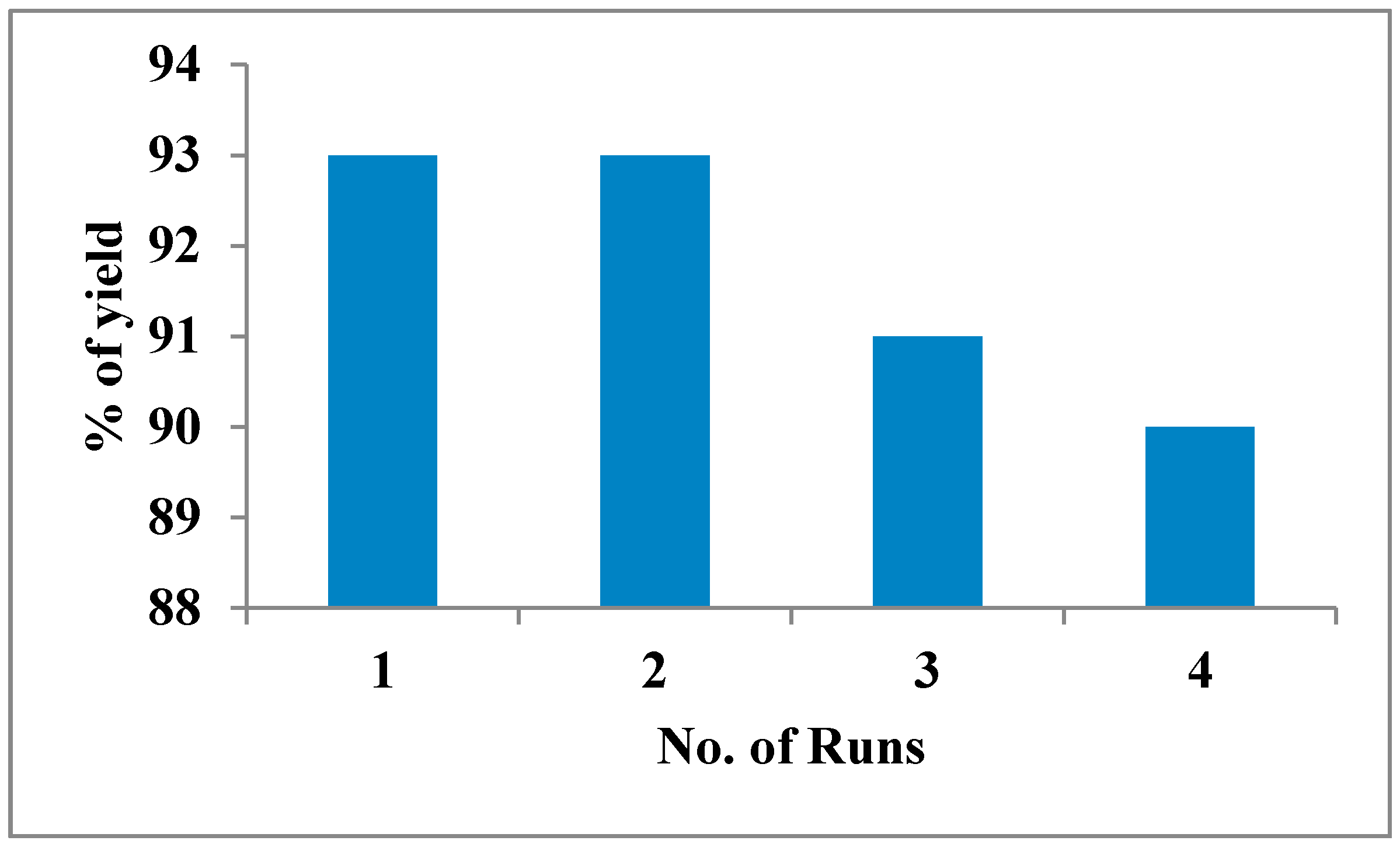
| Temperature Evaluation a | |||
|---|---|---|---|
| Entry | Temperature (°C) | Time (min) | Yield (%) c |
| 1 | 25 | 90 | 88 |
| 2 | 50 | 60 | 90 |
| 3 | 78 | 40 | 93 |
| Effect of Solvent b | ||
|---|---|---|
| Entry | Solvent | Yield (%) c |
| 1 | Ethanol | 93 |
| 2 | Methanol | 88 |
| 3 | Dichloromethane | 86 |
| 4 | Acetonitrile | 88 |
| Entry | R | Ph | Reaction Time (min) | Yield (%) b | mp (°C) | ||
|---|---|---|---|---|---|---|---|
| Conventional | US | Conventional | US | ||||
| 1 | -CH2Ph | Ph | 40 | 25 | 92 | 95 | 161–163 |
| 2 | -CH2Ph | 4-ClPh | 40 | 25 | 92 | 94 | 165–167 |
| 3 | -CH2Ph | 4-OC2H5Ph | 50 | 30 | 92 | 94 | 155–157 |
| 4 | -CH2Ph | 3,5-(OCH3)2Ph | 50 | 35 | 92 | 93 | 180–182 |
| 5 | -CH2Ph | 3-Cl Ph | 40 | 25 | 92 | 93 | 144–146 |
| 6 | 4-CH3Ph | 4-OH-3-OC2H5Ph | 45 | 25 | 90 | 94 | 180–182 |
| 7 | 4-CH3Ph | 4-C2H5Ph | 55 | 30 | 90 | 93 | 212–214 |
| 8 | 4-CH3Ph | 4-OHPh | 40 | 25 | 93 | 94 | >275 |
| 9 | 4-CH3Ph | 3,5-(OCH3)2Ph | 45 | 30 | 91 | 94 | 140–142 |
| 10 | 4-CH3Ph | 3,4,5-(OCH3)3Ph | 55 | 35 | 93 | 93 | 102–104 |
| 11 | 4-CH3Ph | 2-Thienyl | 40 | 25 | 89 | 92 | 200–201 |
| 12 | 4-OCH3Ph | 3,4,5-(OCH3)3Ph | 60 | 45 | 91 | 92 | 123–125 |
| 13 | 4-ClPh | 4-C2H5Ph | 55 | 35 | 92 | 93 | 181–182 |
| 14 | 4-ClPh | 3,4,5-(OCH3)3Ph | 60 | 40 | 91 | 93 | 123–125 |
| 15 | 4-ClPh | 4-CNPh | 60 | 45 | 87 | 89 | 112–114 |
| 16 | 4-ClPh | AllyloxyPh | 60 | 50 | 91 | 91 | 98–100 |
| 17 | 4-ClPh | 4-BrPh | 50 | 35 | 93 | 91 | 80–82 |
| 18 | 4-IPh | 2,4-(Cl)2Ph | 45 | 25 | 89 | 92 | 109–111 |
| 19 | 4-IPh | 4-OH-3-OCH3Ph | 50 | 30 | 93 | 94 | 96–98 |
| 20 | 4-CH3Ph | 3-OHPh | 50 | 25 | 93 | 93 | 260–162 |
| 21 | 4-ClPh | 3-OHPh | 45 | 30 | 94 | 92 | 85–87 |
| 22 | 4-ClPh | 4-OH-3-OC2H5Ph | 40 | 30 | 94 | 94 | 169–170 |
3. Experimental Section
3.1. Chemicals and Apparatus
3.2. Preparation of Al2O3 NPs
4. Spectral Data
5. Reusability of the Catalyst
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grimmett, M.R.; Katritzky, A.R.; Rees, C.W. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: London, UK, 1984; pp. 374–498. [Google Scholar]
- Takle, A.K.; Brown, M.J.B.; Davies, S.; Dean, D.K.; Francis, G.; Gaiba, A.; Hird, A.W.; King, F.W.; Lovell, P.J.; Naylor, A.; et al. The identification of potent and selective imidazole-based inhibitors of B-Raf kinase. Bioorg. Med. Chem. Lett. 2006, 16, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Khanna, I.K.; Weier, R.M.; Yu, Y.; Xu, X.D.; Koszyk, F.J.; Collins, P.W.; Koboldt, C.M.; Veenhuizen, A.W.; Perkins, W.E.; Casler, J.J.; et al. 1,2-Diarylpyrroles as Potent and Selective Inhibitors of Cyclooxygenase-2. J. Med. Chem. 1997, 40, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.H.M.; Van-Stuivenberg, H.H.; Coolen, K.K.A.C.; Adolfs, T.J.P.; McCreary, A.C.; Keizer, H.G.; Wals, H.C.; Veerman, W.; Borst, A.J.M.; de Loof, P.C.; et al. Bioisosteric replacements of the pyrazole moiety of rimonabant, synthesis, biological properties, and molecular modeling investigations of thiazoles, triazoles, and imidazoles as potent and selective CB1 cannabinoid receptor antagonists. J. Med. Chem. 2005, 48, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.F.; Fier-Thompson, S.M.; Garigipati, R.S.; Sorenson, M.E.; Smietana, J.M.; Lee, D.; Bender, P.E.; Lee, J.C.; Laydon, J.T.; Griswold, D.E.; et al. 2,4,5-triarylimidazole inhibitors of IL-1 biosynthesis. Bioorg. Med. Chem. Lett. 1995, 5, 1171–1176. [Google Scholar] [CrossRef]
- Laufer, S.A.; Zimmermann, W.; Ruff, K.J. Tetrasubstituted imidazole inhibitors of cytokine release: Probing substituents in the N-1 position. J. Med. Chem. 2004, 47, 6311–6325. [Google Scholar] [CrossRef] [PubMed]
- De Laszlo, S.E.; Hacker, C.; Li, B.; Kim, D.; MacCoss, M.; Mantlo, N.; Pivnichny, J.V.; Colwell, L.; Koch, G.E.; Cascieri, M.A.; et al. Potent, orally absorbed glucagon receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 641–646. [Google Scholar] [CrossRef]
- Eyers, P.A.; Craxton, M.; Morrice, N.; Cohen, P.; Goedert, M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 1998, 5, 321–328. [Google Scholar] [CrossRef]
- Newman, M.J.; Rodarte, J.C.; Benbatoul, K.D.; Romano, S.J.; Zhang, C.; Krane, S.; Moran, E.J.; Uyeda, R.T.; Dixon, R.; Guns, E.S.; et al. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000, 60, 2964–2972. [Google Scholar] [PubMed]
- Misono, M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 1141–1152. [Google Scholar] [CrossRef]
- Black, J.W.; Durant, G.J.; Emmett, J.C.; Ganellin, C.R. Sulphurmethylene isosterism in the development of metiamide, a new histamine H2-receptor antagonist. Nature 1974, 248, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Ucucu, U.; Karaburun, N.G.; Iskdag, I. Synthesis and analgesic activity of some 1-benzyl-2-substituted-4, 5-diphenyl-1H-imidazole derivatives. IL Farm. 2001, 56, 285–290. [Google Scholar] [CrossRef]
- Balalaei, S.; Arabanian, A. One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem. 2000, 2, 274–276. [Google Scholar] [CrossRef]
- Karimi, A.R.; Alimohammadi, Z.; Azizian, J.; Mohammadi, A.A.; Mohmmadizade, M.R. Solvent-free synthesis of tetrasubstituted imidazoles on silica gel/NaHSO4 support. Catal. Commun. 2006, 7, 728–732. [Google Scholar] [CrossRef]
- Kidwai, M.; Mothsra, P.; Bansal, V.; Somvanshi, R.K.; Ethayathulla, A.S.; Dey, S.; Singh, T.P. One-pot synthesis of highly substituted imidazoles using molecular iodine: A versatile catalyst. J. Mol. Catal. A Chem. 2 2007, 265, 177–182. [Google Scholar] [CrossRef]
- Nagarapu, L.; Apuri, S.; Kantevari, S. Potassium dodecatugstocobaltate trihydrate (K5CoW12O40•3H2O): A mild and efficient reusable catalyst for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiation. J. Mol. Catal. A Chem. 2007, 266, 104–108. [Google Scholar] [CrossRef]
- Heravi, M.M.; Derikvand, F.; Bamoharram, F.F. Highly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalysts. J. Mol. Catal. A Chem. 2007, 263, 112–114. [Google Scholar] [CrossRef]
- Kantevari, S.; Vuppalapati, S.V.N.; Biradar, D.O.; Nagarapu, L. Synthesis of 1,2,4,5-tetrasubstituted imidazoles using silica-bonded propylpiperazine N-sulfamic acid as a recyclable solid acid catalyst. J. Mol. Catal. A Chem. 2007, 266, 109–113. [Google Scholar] [CrossRef]
- Sharma, S.D.; Hazarika, P.; Konwar, D. An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by InCl3•3H2O. Tetrahedron Lett. 2008, 49, 2216–2220. [Google Scholar] [CrossRef]
- Sharma, G.V.; Jyothi, Y.; Lakshmi, P.S. Efficient room-temperature synthesis of tri- and tetrasubstituted imidazoles catalyzed by ZrCl4. Synth. Commun. 2006, 36, 2991–3000. [Google Scholar] [CrossRef]
- Sadeghi, B.; Mirjalili, B.F.; Hashemi, M.M. BF3•SiO2 an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron Lett. 2008, 49, 2575–2577. [Google Scholar] [CrossRef]
- Murthy, S.N.; Madhav, B.; Nageswar, Y.V.D. DABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multi-component condensation strategy. Tetrahedron Lett. 2010, 51, 5252–5257. [Google Scholar] [CrossRef]
- Wang, X.C.; Gong, H.P.; Quan, Z.J.; Li, L.; Ye, H.L. PEG-400 as an efficient reaction medium for the synthesis of 2,4,5-triaryl-1H-imidazoles and 1,2,4,5-tetraaryl-1H-imidazoles. Chin. Chem. Lett. 2009, 20, 44–47. [Google Scholar] [CrossRef]
- Niknam, K.; Deris, A.; Naeimi, F.; Majleci, F. Synthesis of 1,2,4,5-tetrasubstituted imidazoles using silica-bonded propylpiperazine Nsulfamic acid as a recyclable solid acid catalyst. Tetrahedron Lett. 2011, 52, 4642–4645. [Google Scholar] [CrossRef]
- Zielínski, P.A.; Schulz, R.; Kaliaguine, S.; van Neste, A. Structural transformations of alumina by high energy ball milling. J. Mater. Res. 1993, 8, 2985–2992. [Google Scholar] [CrossRef]
- Hanawa, T.; Kaga, M.; Itoh, Y.; Echizenya, T.; Oguchi, H.; Ota, M. Cytotoxicities of oxides, phosphates and sulphides of metals. Biomaterials 1992, 13, 20–24. [Google Scholar] [CrossRef]
- Dey, S.; Bakthavatchalu, V.; Tseng, M.T.; Wu, P.; Florence, R.L.; Grulke, E.A.; Yokel, R.A.; Sanjit, K.D.; Yang, H.S.; Chen, Y.; et al. Interactions between SIRT1 and AP-1 reveal a mechanistic insight into the growth promoting properties of alumina (Al2O3) nanoparticles in mouse skin epithelial cells. Carcinogenesis 2008, 29, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Oesterling, E.; Chopra, N.; Gavalas, V.; Arzuaga, X.; Lim, E.J.; Sultana, R.; Butterfield, L.; Bachas, D.A.; Hennig, B. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol. Lett. 2008, 178, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Cordingley, R.; Kohan, L.; Ben-Nissan, B.; Pezzotti, G. Alumia and Zirconia bioceramics in orthopaedic applications. J. Aust. Ceram. Soc. 2003, 39, 20–28. [Google Scholar]
- Guevara-Lara, A.; Bacaud, R.; Vrinat, M. Highly active NiMo/TiO2-Al2O3 catalysts: Influence of the preparation and the activation conditions on the catalytic activity. Appl. Catal. A Gen. 2007, 328, 99–108. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, B.P.; Vijayakumar, V.; Arasu, M.V.; Al-Dhabi, N.A. γ-Alumina Nanoparticle Catalyzed Efficient Synthesis of Highly Substituted Imidazoles. Molecules 2015, 20, 19221-19235. https://doi.org/10.3390/molecules201019221
Reddy BP, Vijayakumar V, Arasu MV, Al-Dhabi NA. γ-Alumina Nanoparticle Catalyzed Efficient Synthesis of Highly Substituted Imidazoles. Molecules. 2015; 20(10):19221-19235. https://doi.org/10.3390/molecules201019221
Chicago/Turabian StyleReddy, Bandapalli Palakshi, Vijayaparthasarathi Vijayakumar, Mariadhas Valan Arasu, and Naif Abdullah Al-Dhabi. 2015. "γ-Alumina Nanoparticle Catalyzed Efficient Synthesis of Highly Substituted Imidazoles" Molecules 20, no. 10: 19221-19235. https://doi.org/10.3390/molecules201019221
APA StyleReddy, B. P., Vijayakumar, V., Arasu, M. V., & Al-Dhabi, N. A. (2015). γ-Alumina Nanoparticle Catalyzed Efficient Synthesis of Highly Substituted Imidazoles. Molecules, 20(10), 19221-19235. https://doi.org/10.3390/molecules201019221






