In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Assays-Trolox Equivalent Antioxidant Capacity (TEAC) and Folin-Ciocalteu Assays
| Extracts | TP | TEAC |
|---|---|---|
| mg GAE per g DCE | mmol TE per g DCE | |
| M1 | 228.10 ± 2.40 | 2.43 ± 0.06 |
| M2 | 245.15 ± 9.69 | 2.11 ± 0.14 |
2.2. Identification of Phenolic Compounds in Aqueous Extract of S. Macrophylla Leaves
| Compounds | Empirical Formula | RT (min) | [M + H]+ Calcd | [M + H]+ obsd | Error (ppm) |
|---|---|---|---|---|---|
| Benzoic acid | C7H5O2 | 0.41 | 123.0446 | 123.0446 | 0.00 |
| p-Hydroxybenzoic acid | C7H6O3 | 1.73 | 139.0395 | 139.0390 | 3.59 |
| Cinnamic acid | C9H8O2 | 3.36 | 149.0602 | 149.0600 | 1.34 |
| Gentisic acid | C7H6O4 | 1.26 | 155.0344 | 155.0343 | 1.29 |
| Protocatechuic acid | C7H6O4 | 1.28 | 155.0344 | 155.0342 | 1.29 |
| Synaptic acid | C9H8O3 | 2.22 | 165.0551 | 165.0548 | 1.81 |
| p-Coumaric acid | C9H7O3 | 2.23 | 165.0551 | 165.0548 | 1.81 |
| Gallic acid | C7H5O5 | 0.81 | 171.0293 | 171.0291 | 1.16 |
| Syringic acid | C9H9O5 | 2.90 | 199.0606 | 199.0607 | 0.50 |
| Resveratrol | C14H12O3 | 3.99 | 229.0864 | 229.0864 | 0.00 |
| Chrysin | C15H10O5 | 1.16 | 271.0606 | 271.0611 | 1.84 |
| Apigenin | C15H9O5 | 3.78 | 273.0762 | 273.0765 | 1.09 |
| Naringenin | C15H12O5 | 3.88 | 273.0762 | 273.0766 | 1.46 |
| Luteolin | C15H9O6 | 4.38 | 287.0555 | 287.0555 | 0.00 |
| Kaempferol | C15H10O6 | 3.98 | 287.0555 | 287.0554 | 0.34 |
| Epicatechin | C15H14O6 | 2.23 | 291.0868 | 291.0873 | 1.71 |
| Catechin | C15H14O6 | 2.83 | 291.0868 | 291.0868 | 0.00 |
| Quercetin | C15H9O7 | 3.76 | 303.0504 | 303.0506 | 0.66 |
| Epigallocatechin | C15H14O7 | 1.07 | 307.0817 | 307.0824 | 2.27 |
| Gallocatechin | C15H13O7 | 1.16 | 307.0817 | 307.0823 | 1.95 |
| Myricetin | C15H10O8 | 2.38 | 319.0822 | 319.0822 | 0.00 |
| Apigenin-7-O-glucoside | C21H21O10 | 3.84 | 435.1291 | 435.1289 | 0.45 |
| Kaempferol-3-O-glucoside | C21H20O11 | 4.06 | 449.1083 | 449.1077 | 1.33 |
| Quercetin-3-O-rhaminoside | C21H19O12 | 3.71 | 465.1033 | 465.1027 | 1.29 |
| Quercetin-3-O-glucoside | C21H19O12 | 3.76 | 465.1033 | 465.1028 | 1.07 |
| Kaempferol-3-O-rutinoside | C27H30O15 | 4.06 | 595.1662 | 595.1660 | 0.35 |
| Quercetin-3-O-rutinoside | C27H30O16 | 3.76 | 611.1612 | 611.1609 | 0.49 |

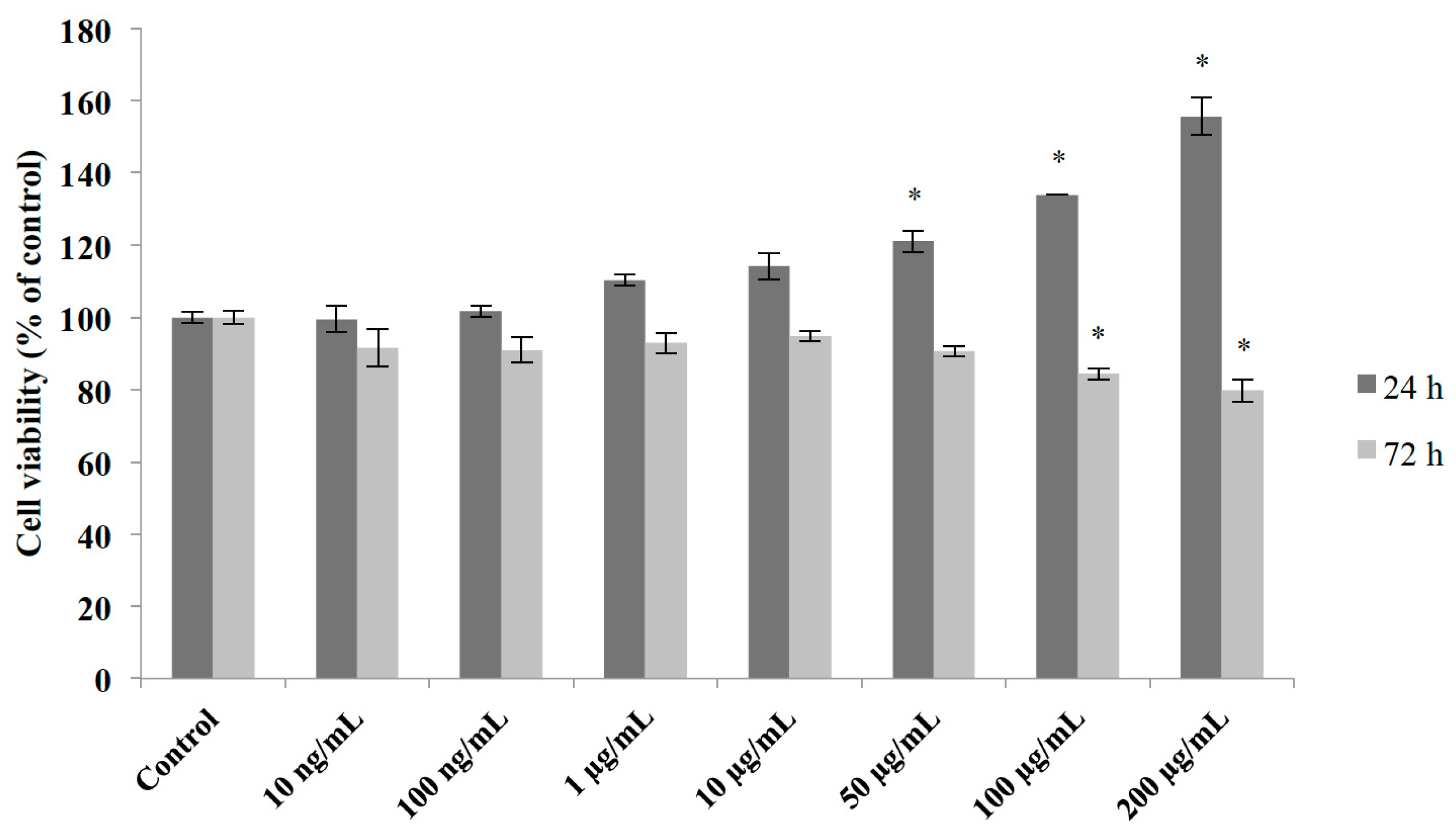
2.3. Effects of M1 Extract on Cell Viability of Cerebellar Primary Cultures
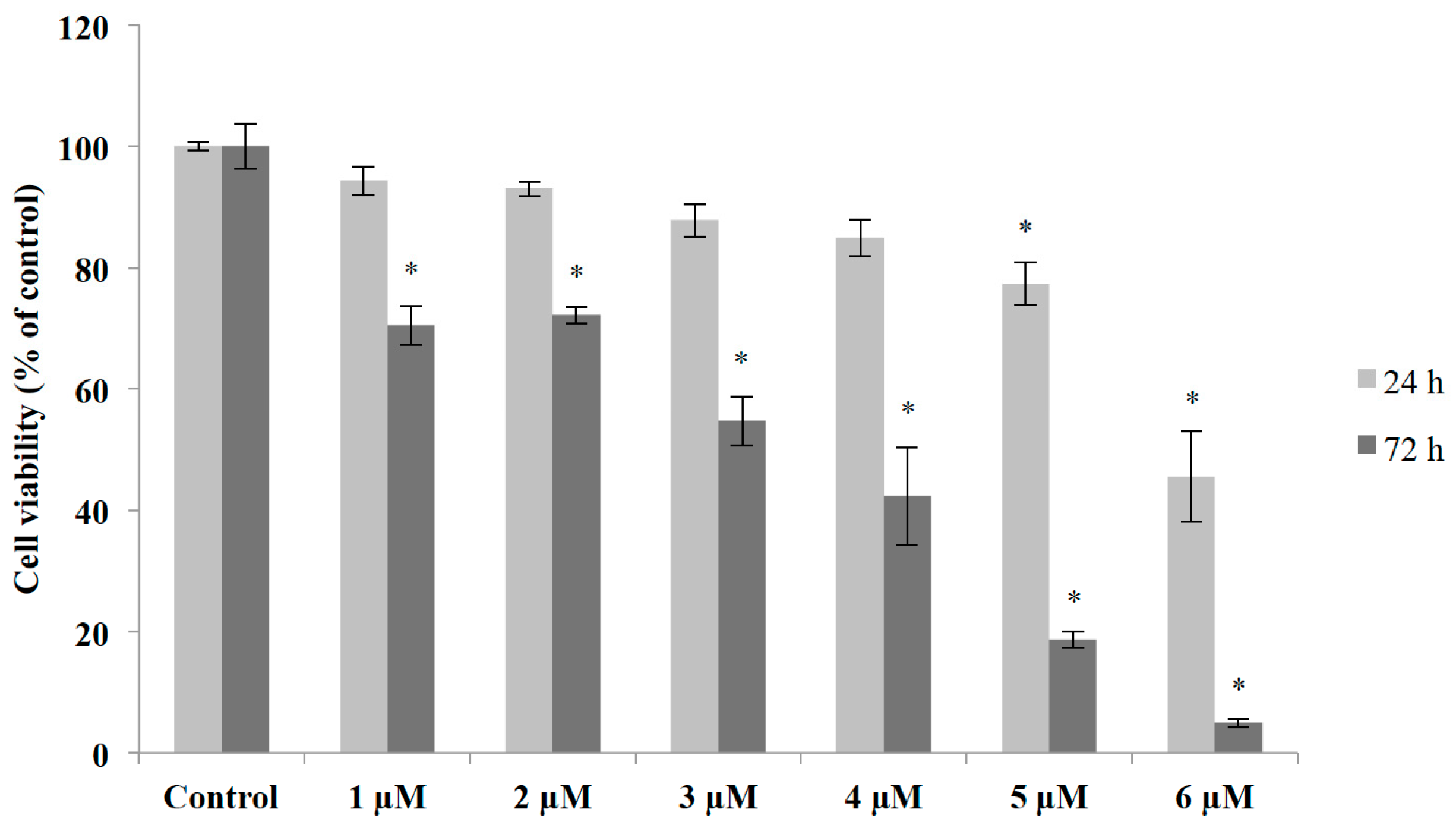
2.4. Cytoprotective Effect of an Aqueous Extract of Mahogany Leaves against Methyl Mercury-Induced Neurotoxicity
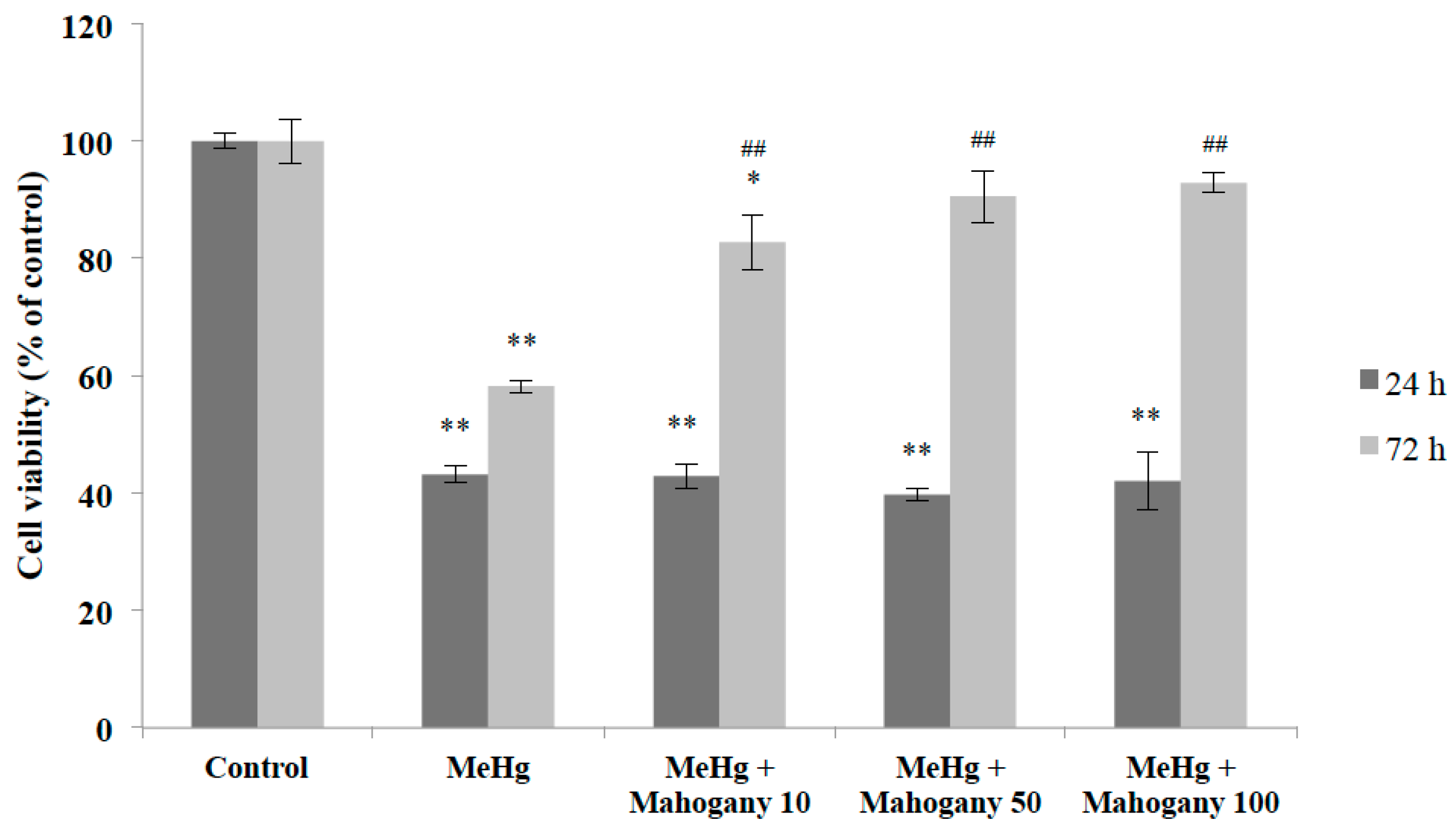
3. Experimental Section
3.1. Reagent and Standards
3.2. Plant Material
3.3. Preparation of S. Macrophylla Leaf Tea
3.4. TEAC Assay
3.5. Folin-Ciocalteau Assay
3.6. Analytical LC-MS/MS
3.7. Cell Culture
3.8. Methyl Mercury-Induced Oxidative Damage in Primary Cerebellar Cultures
3.9. Treatment with Aqueous Extract of Swietenia Macrophylla to Neuroprotective Assays
3.10. Analysis of Cell Viability
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar]
- Surmeier, D.J.; Guzman, J.N. The Origins of Oxidant Stress in Parkinson’s Disease and Therapeutic Strategies. Antioxid. Redox Signal. 2011, 7, 1289–1301. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Avila, D.S.; da Rocha, J.B.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 5, 575–594. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Yamashita, S.; Katakura, Y.; Shimizu, K. In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino. Molecules 2015, 20, 4813–4832. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Jennings, S.; Clements, T. The ecology, silviculture and biogeography of mahogany (Swietenia macrophylla): A critical review of the evidence. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 37–49. [Google Scholar] [CrossRef]
- Lemes, M.R.; Gribel, R.; Proctor, J.; Grattapaglia, D. Population genetic structure of mahogany (Swietenia macrophylla King, Meliaceae) across the Brazilian Amazon, based on variations at microsatellite loci: Implications for conservation. Mol. Ecol. 2003, 12, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Falah, S.; Suzuki, T.; Katayama, T. Chemical constituents from Swietenia macrophylla bark and their antioxidant activity. Pak. J. Biol. Sci. 2008, 11, 2007–2012. [Google Scholar] [PubMed]
- Da Silva, M.N.; Arruda, M.S.P.; Castro, K.C.F.; da Silva, M.F.; Das, G.F.; Fernandes, J.B.; Vieira, P.C. Limonoids of the phragmalin type from Swietenia macrophylla and their chemotaxonomic significance. J. Nat. Prod. 2008, 71, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Mootoo, B.S.; Ali, A.; Motilal, R.; Pingal, R.; Ramlal, A.; Khan, A. Limonoids from Swietenia macrophylla and S. aubrevilleana. J. Nat. Prod. 1999, 62, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huang, S.S.; Liao, C.H.; Wei, D.C.; Sung, P.J.; Wang, T.C.; Cheng, M.J. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010, 120, 379–384. [Google Scholar]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A 2003, 1, 29–40. [Google Scholar] [CrossRef]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous Determination of All Polyphenols in Vegetables, Fruits, and Teas. J. Agric. Food Chem. 2003, 3, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Re, R.; Pelligrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Arun, R.; Sravya, R.B.; Roja, C. A review on standardisation of herbal formulation. Inter. J. Phytother. 2012, 2, 74–88. [Google Scholar]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Chemical Constituents of Three Allium Species from Romania. Molecules 2013, 18, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Almajano, M.P.; Carbo, R.; Jiménez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Singleton, V.L.; Othofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ahlemeyer, B.; Baumgart-Vogt, E. Optimized protocols for the simultaneous preparation of primary neuronal cultures of the neocortex, hippocampus and cerebellum from individual newborn (P0.5) C57B1/6J mice. J. Neurosci. Methods 2005, 149, 110–120. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamplona, S.; Sá, P.; Lopes, D.; Costa, E.; Yamada, E.; Silva, C.E.; Arruda, M.; Souza, J.; Da Silva, M. In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla. Molecules 2015, 20, 18777-18788. https://doi.org/10.3390/molecules201018777
Pamplona S, Sá P, Lopes D, Costa E, Yamada E, Silva CE, Arruda M, Souza J, Da Silva M. In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla. Molecules. 2015; 20(10):18777-18788. https://doi.org/10.3390/molecules201018777
Chicago/Turabian StylePamplona, Sônia, Paulo Sá, Dielly Lopes, Edmar Costa, Elizabeth Yamada, Consuelo E Silva, Mara Arruda, Jesus Souza, and Milton Da Silva. 2015. "In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla" Molecules 20, no. 10: 18777-18788. https://doi.org/10.3390/molecules201018777
APA StylePamplona, S., Sá, P., Lopes, D., Costa, E., Yamada, E., Silva, C. E., Arruda, M., Souza, J., & Da Silva, M. (2015). In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla. Molecules, 20(10), 18777-18788. https://doi.org/10.3390/molecules201018777