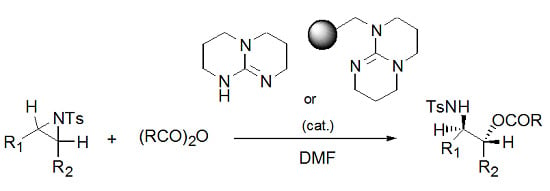
A Mild and Regioselective Ring-Opening of Aziridines with Acid Anhydride Using TBD or PS-TBD as a Catalyst
Abstract
:1. Introduction
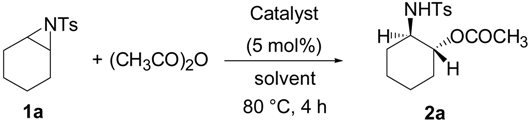
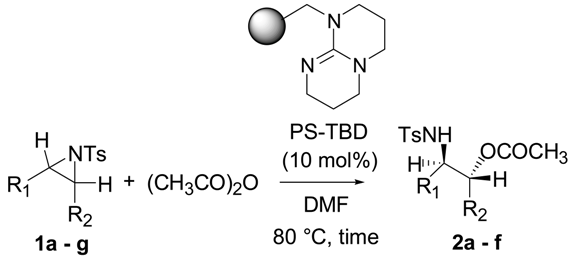
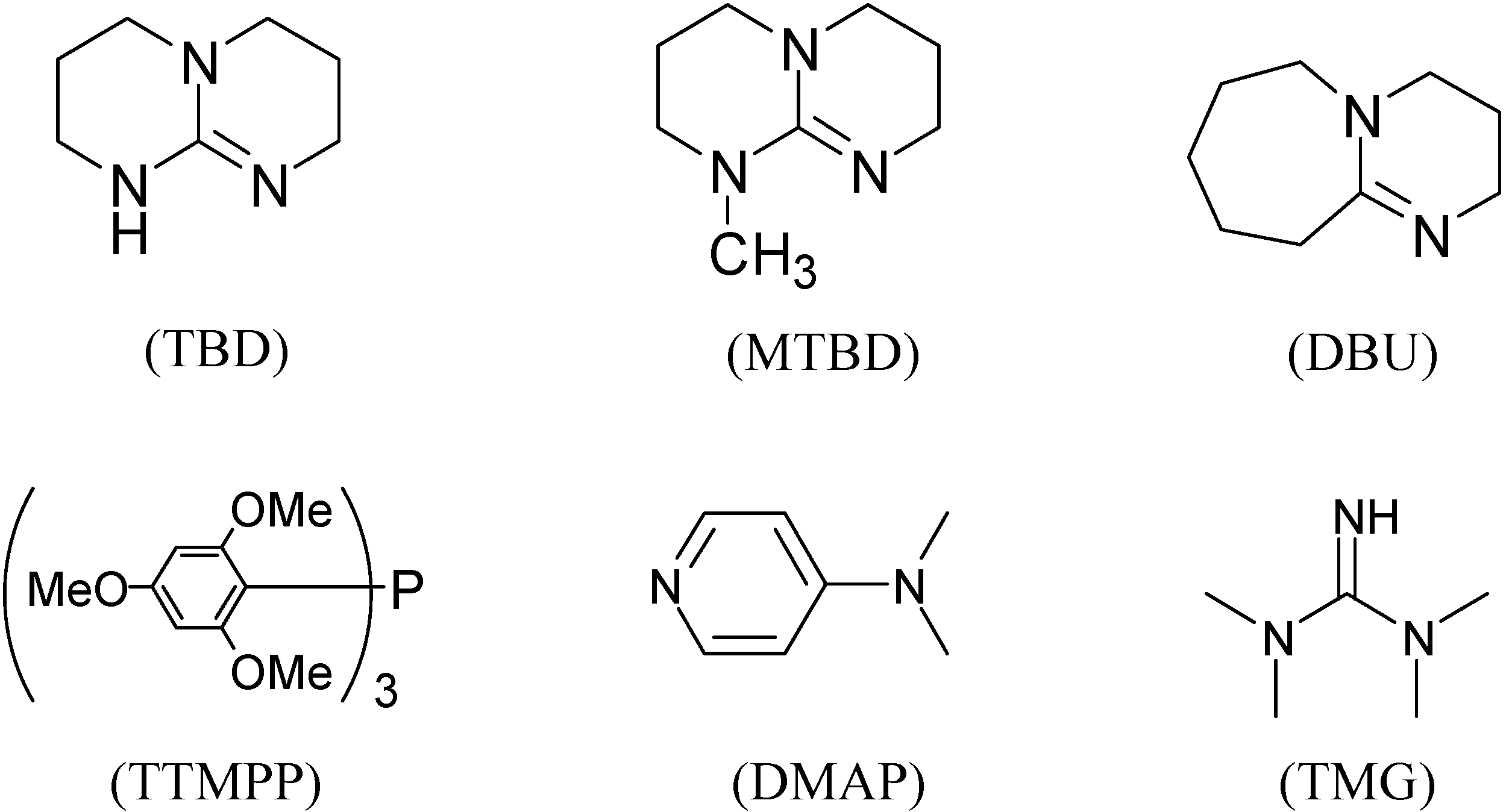
2. Results and Discussion
| Entry | Catalyst | Solvent | Yield (%) |
|---|---|---|---|
| 1 | TBD | DMF a,b | 78 |
| 2 | DMF | 94 | |
| 3 | DMF c | 90 | |
| 4 | THF b,d | 45 | |
| 5 | MeCN b,e | 10 | |
| 6 | toluene b | 71 | |
| 7 | DBU | DMF b | 55 |
| 8 | TMG | DMF b | 33 |
| 9 | DMAP | DMF b | 34 |
| 10 | TTMPP | DMF | 61 |
| 11 | MTBD | DMF b | 80 |
| Entry | Aziridine | R | Time | Product | Yield (%) a |
|---|---|---|---|---|---|
| 1 | 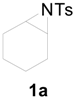 | CH3 | 4 h | 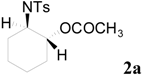 | 94 |
| 2 | C2H5 | 4 h | 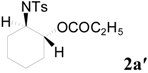 | 89 | |
| 3 | C6H5 | 8 h | 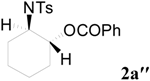 | 91 | |
| 4 b |  | CH3 | 12 h | 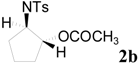 | 85 |
| 5 | C2H5 | 18 h | 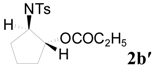 | 83 | |
| 6 |  | CH3 | 4 h |  | 86 |
| 7 | C2H5 | 6 h | 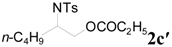 | 87 | |
| 8 | C6H5 | 12 h | 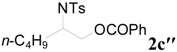 | 88 | |
| 9 | 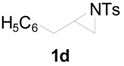 | CH3 | 4 h | 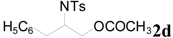 | 92 |
| 10 | C2H5 | 8 h | 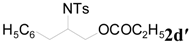 | 98 | |
| 11 | 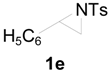 | CH3 | 1 h | 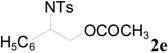 | 97 b |
| 12 | C2H5 | 4 h |  | 95 c | |
| 13 |  | CH3 | 1 h | 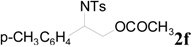 | 98 d |
| 14 | C2H5 | 4 h | 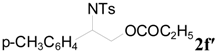 | 96 e |
| Entry | Aziridine | Product | Time | Yield (%) a |
|---|---|---|---|---|
| 1 | 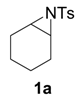 | 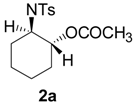 | 10 h | 85 |
| 2 | 10 h | 88 b | ||
| 3 | 10 h | 80 c | ||
| 4 | 10 h | 82 d | ||
| 5 | 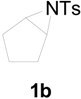 |  | 24 h | 89 |
| 6 |  | 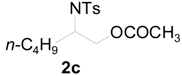 | 16 h | 78 |
| 7 | 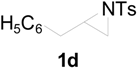 |  | 4 h | 92 |
| 8 | 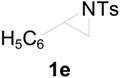 | 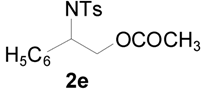 | 8 h | 86 |
| 9 | 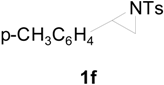 | 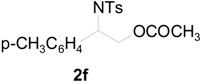 | 8 h | 80 |
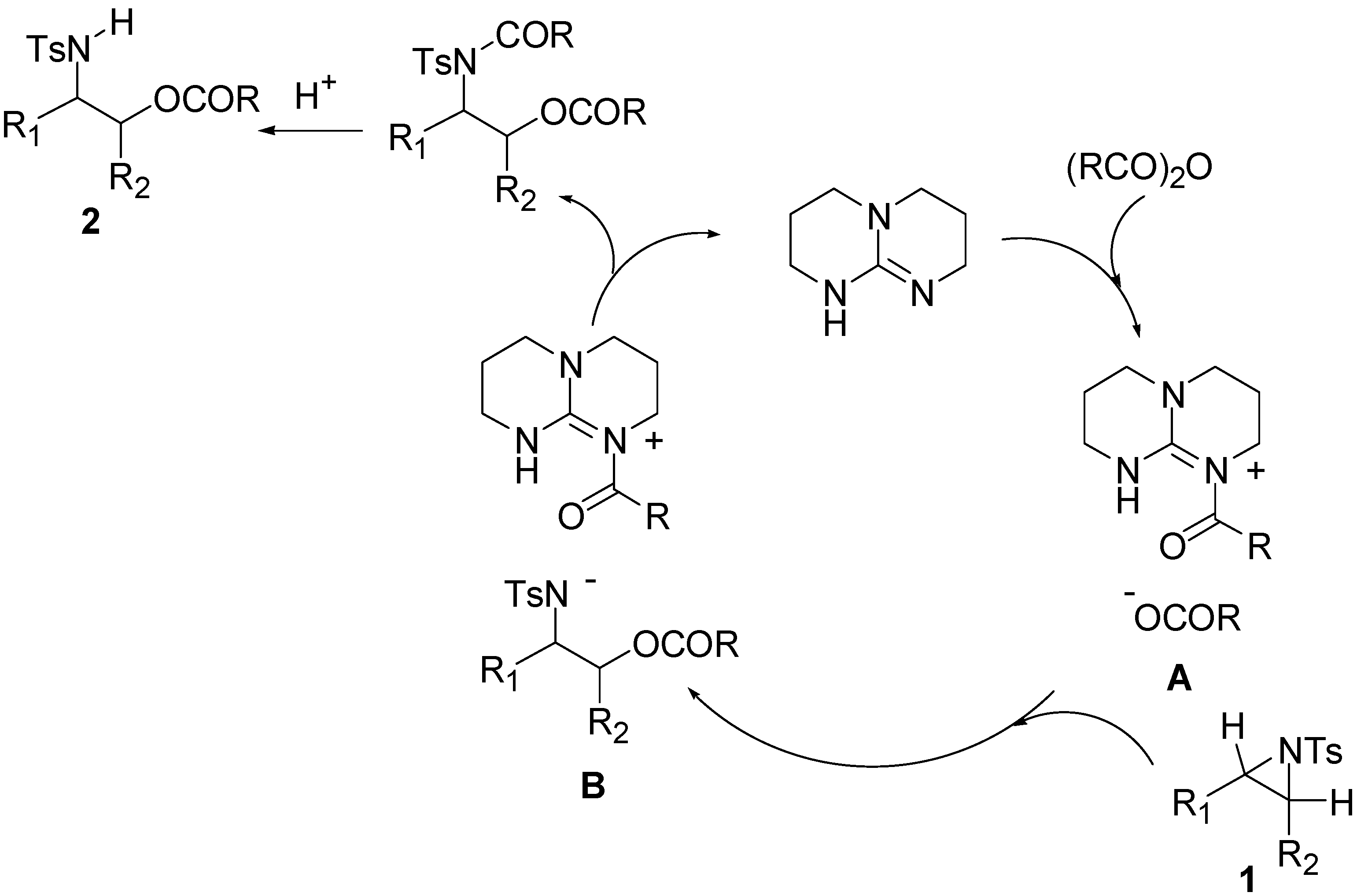
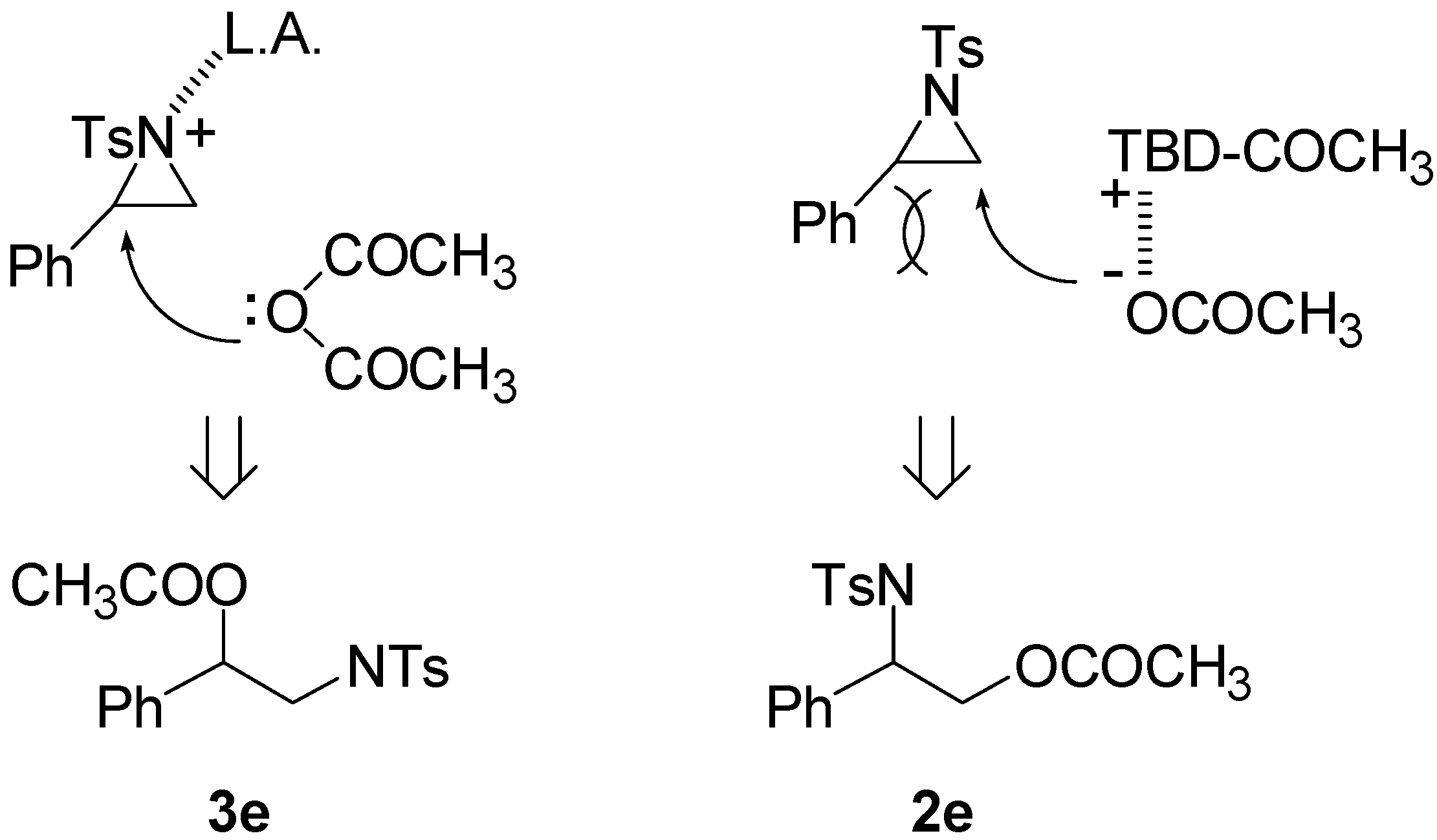
3. Experimental Section
3.1. General
3.2. Method
3.2.1. General Procedure for TBD-Catalyzed Ring-Opening of Aziridines with Acid Anhydride
3.2.2. General Procedure for PS-TBD Catalyzed Ring-Opening of Aziridines with Acid Anhydride
3.3. General Characterization of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Sweeney, J.B. Aziridines: Epoxides’ ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Pearson, W.H.; Lian, B.N.; Bergmeier, S.C. Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: New York, NY, USA, 1996; Volume 1a, pp. 1–96. [Google Scholar]
- Tanner, D. Chiral Aziridines—Their Synthesis and Use in Stereoselective Transformations. Angew. Chem. Int. Ed. Engl. 1994, 33, 599–619. [Google Scholar] [CrossRef]
- Hu, X.E. Nucleophilic ring opening of aziridines. Tetrahedron 2004, 60, 2701–2743. [Google Scholar] [CrossRef]
- Watson, I.D.G.; Yu, L.; Yudin, A.K. Advances in nitrogen transfer reactions involving aziridines. Acc. Chem. Res. 2006, 39, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; D’hooghe, M.; Kimpe, N.D. Aziridines synthesis and reactivity of C-heteroatom-substituted aziridines. Chem. Rev. 2007, 107, 2080–2135. [Google Scholar] [CrossRef] [PubMed]
- Lu, P. Recent developments in regioselective ring opening of aziridines. Tetrahedron 2010, 66, 2549–2560. [Google Scholar] [CrossRef]
- Chawla, R.; Singh, A.K.; Yadav, L.D.S. Organocatalysis in synthesis and reactions of epoxides and aziridines. RSC Adv. 2013, 3, 11385–11403. [Google Scholar] [CrossRef]
- Llaveria, J.; Beltran, A.; Díaz-Requejo, M.M.; Matheu, M.I.; Castillon, S.; Perez, P.J. Efficient Silver-Catalyzed Regio- and Stereospecific Aziridination of Dienes. Angew. Chem. Int. Ed. 2010, 49, 7092–7095. [Google Scholar] [CrossRef] [PubMed]
- Llaveria, J.; Beltran, A.; Sameera, W.M.C.; Locati, A.; Diaz-Requejo, M.M.; Matheu, M.I.; Castillon, S.; Maseras, F.; Perez, P.J. Chemo-, Regio-, and Stereoselective Silver-Catalyzed Aziridination of Dienes: Scope, Mechanistic Studies, and Ring-Opening Reactions. J. Am. Chem. Soc. 2014, 136, 5342–5350. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.A.; Zhou, P. Asymmetric synthesis of the antibiotic (+)-thiamphenicol using cis-N-(p-toluenesulfinyl)aziridine 2-carboxylic acids. Tetrahedron 1994, 35, 7525–7528. [Google Scholar] [CrossRef]
- Davis, F.A.; Reddy, G.V. Aziridine-2-carboxylic acid mediated asymmetric synthesis of d-erythro- and l-threo-sphingosine from a common precursor. Tetrahedron Lett. 1996, 37, 4349–4352. [Google Scholar] [CrossRef]
- Cardillo, G.; Gentilucci, L.; Tolomelli, A.; Tomasini, C. Ring expansion of N-acyl aziridine-2-imides to oxazoline-4-imides, useful precursors of pure beta-hydroxy alpha-aminoacids. Tetrahedron Lett. 1997, 39, 6953–6956. [Google Scholar] [CrossRef]
- Prasad, P.A.B.; Sekar, G.V.; Singh, K. An efficient method for the cleavage of aziridines using hydroxyl compound. Tetrahedron Lett. 2000, 41, 4677–4679. [Google Scholar] [CrossRef]
- Olofsson, B.; Khamrai, U.; Somfai, P. A regio- and stereodivergent synthesis of vic-amino alcohols. Org. Lett. 2000, 2, 4087–4089. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Nandy, J.P.; Shukla, S.; Siddiqui, I.; Iqbal, J. Stereoselective synthesis of beta-substituted phenylalanine-beta-phenylisoserine-derived tripeptides using N-cinnamoyl-l-proline as template: Synthesis of structural analogues of HIV protease inhibitors. J. Org. Chem. 2002, 67, 7858–7860. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.A.B.; Sanghi, R.; Singh, K. Studies on ring cleavage of aziridines with hydroxyl compound. Tetrahedron 2002, 58, 7355–7363. [Google Scholar] [CrossRef]
- Olofsson, B.; Somfai, P. A regio- and stereodivergent route to all isomers of vic-amino alcohols. J. Org. Chem. 2002, 67, 8574–8583. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.K.; Ghosh, A.; Raju, T.V. Efficient ring opening reactions of N-tosyl aziridines with amines and water in presence of catalytic amount of cerium(IV) ammonium nitrate. Chem. Lett. 2003, 32, 82–83. [Google Scholar] [CrossRef]
- Concellon, J.M.; Riego, E. Ring opening of nonactivated 2-(1-aminoalkyl) aziridines: Unusual regio- and stereoselective C-2 and C-3 cleavage. J. Org. Chem. 2003, 68, 6407–6410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.T.; Xu, Z.B.; Qu, J. Hot water-promoted ring-opening of epoxides and aziridines by water and other nucleopliles. J. Org. Chem. 2008, 73, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ni, B.; Chang, H.-H.; Wei, W.-L. NaHSO3-promoted ring openings of N-tosylaziridines and epoxides with H2O. Heterocycles 2014, 89, 1009–1016. [Google Scholar]
- Yadav, J.S.; Reddy, B.V.; Sadashiv, K.; Harikishan, K. Indium triflate-catalyzed ring opening of aziridines with carboxylic acid. Tetrahedron Lett. 2002, 43, 2099–2101. [Google Scholar] [CrossRef]
- Fan, R.-H.; Hou, X.-L. Tributylphosphine-catalyzed ring-opening reaction of epoxides and aziridines with acetic anhydride. Tetrahedron Lett. 2003, 44, 4411–4413. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Sadashiv, K.; Harikishan, K.; Narsaiah, A.V. Acylative cleavage of aziridines with acid anhydrides catalyzed by Scandium triflate. J. Mol. Catal. A 2004, 220, 153–157. [Google Scholar] [CrossRef]
- Das, B.; Reddy, V.S.; Tehseen, F. A mild, rapid and highly regioselective ring-opening of epoxides and aziridines with acetic anhydride under solvent-free conditions using ammonium-12-molybdophosphate. Tetrahedron Lett. 2006, 47, 6865–6868. [Google Scholar] [CrossRef]
- Sun, X.; Ye, S.; Wu, J. N-Heterocyclic Carbene: An efficient catalyst for the ring-opening reaction of aziridine with acid anhydride. Eur. J. Org. Chem. 2006, 4787–4790. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Chang, H.; Zhang, Y.; Wei, W. Tetrabutylammonium bromide-mediated ring opening reactions of N-tosylaziridines with carboxylic acids in DMF. RSC Adv. 2014, 4, 6490–6495. [Google Scholar] [CrossRef]
- Schroeder, G.; Łeska, B.; Jarczewski, A.; Nowak-Wydra, B.; Brzezinski, B. FTIR, NMR and kinetic studies of proton transfer reactions from nitro-substituted diarylmethanes to N-bases with guanidine character. J. Mol. Struct. 1995, 344, 77–88. [Google Scholar] [CrossRef]
- Coles, M.P. Bicyclic-guanidines, -guanidinates and -guanidinium salts: Wide ranging applications from a simple family of molecules. Chem. Commun. 2009, 3659–3676. [Google Scholar] [CrossRef] [PubMed]
- Simoni, D.; Rondanin, R.; Morini, M.; Baruchello, R.; Invidata, F.P. 1,5,7-Triazabicyclo [4.4.0]dec-1-ene (TBD), 7-methyl-TBD (MTBD) and the polymer-supported TBD (P-TBD): Three efficient catalysts for the nitroaldol (Henry) reaction and for the addition of dialkyl phosphites to unsaturated systems. Tetrahedron Lett. 2000, 41, 1607–1610. [Google Scholar] [CrossRef]
- Simoni, D.; Rossi, M.; Rondanin, R.; Mazzali, A.; Baruchello, R.; Malagutti, C.; Roberti, M.; Invidata, F.P. Strong bicyclic guanidine base-promoted Wittig and Horner-Wadsworth-Emmons reactions. Org. Lett. 2000, 2, 3765–3768. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Xu, J.; Tan, C.-T.; Tan, C.-H. 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) catalyzed Michael reactions. Tetrahedron Lett. 2005, 46, 6875–6878. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Waymouth, R.A.; Hedric, J.A. Triazabicyclodecene: A simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2006, 129, 4556–4557. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, Y.; Ye, W.; Tan, C.-H. P–C Bond formation via direct and three-component conjugate addition catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD). Tetrahedron Lett. 2007, 48, 51–54. [Google Scholar] [CrossRef]
- Sabot, C.; Kumar, K.A.; Meunier, S.; Mioskowski, C. A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) under solvent-free conditions. Tetrahedron Lett. 2007, 48, 3863–3866. [Google Scholar] [CrossRef]
- Sabot, C.; Kumar, K.A.; Antheaume, C.; Mioskowski, C. Triazabicyclodecene: An Effective Isotope Exchange Catalyst in CDCl3. J. Org. Chem. 2007, 72, 5001–5004. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Sabot, C.; Mioskowski, C.; Baati, R. TBD-Catalyzed Direct 5- and 6-enolexo Aldolization of Ketoaldehydes. Eur. J. Org. Chem. 2008, 4104–4108. [Google Scholar] [CrossRef]
- Mahe, O.; Frath, D.; Dez, I.; Marsais, F.; Levacher, V.; Briere, J.-F. TBD-organocatalysed synthesis of pyrazolines. Org. Biomol. Chem. 2009, 7, 3648–3651. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Rindone, B. Organocatalyzed synthesis of ureas from amines and ethylene carbonate. Tetrahedron Lett. 2010, 51, 6301–6304. [Google Scholar] [CrossRef]
- Poladura, B.; Martínez-Castañeda, Á.; Rodríguez-Solla, H.; Concellón, C.; del Amo, V. TBD-catalyzed α-sulfenylation of cyclic ketones: Desymmetrization of 4-substituted cyclohexanone. Tetrahedron 2012, 68, 6438–6446. [Google Scholar] [CrossRef]
- Lanari, D.; Rosati, O.; Curini, M. A solvent-free protocol for the synthesis of 3-formyl-2H-chromenes via domino oxa Michael/aldol reaction. Tetrahedron Lett. 2014, 55, 1752–1755. [Google Scholar] [CrossRef]
- Deredas, D.; Huben, K.; Maniukiewicz, W.; Krawczyk, H. Highly syn-diastereoselective Michael addition of enolizable ketones to 3-(diethoxyphosphoryl)coumarin. Tetrahedron 2014, 70, 8925–8929. [Google Scholar] [CrossRef]
- Simón, L.; Goodman, J.M. The Mechanism of TBD-catalyzed ring-opening polymerization of cyclic esters. J. Org. Chem. 2007, 72, 9656–9662. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, M.K.; Scholten, M.; Kirn, N.; Weber, R.L.; Hedrick, J.L.; Waymouth, R.M. Cyclic guanidine organic catalysts: What is magic about triazabicyclodecene? J. Org. Chem. 2009, 74, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Hammar, P.; Ghobril, C.; Antheaume, C.; Wagner, A.; Baati, R.; Himo, F. Theoretical Mechanistic Study of the TBD-Catalyzed Intramolecular aldol reaction of ketoaldehydes. J. Org. Chem. 2010, 75, 4728–4736. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tan, C.-H. Mechanistic considerations of guanidine-catalyzed reactions. Chem. Commun. 2011, 47, 8210–8222. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, S.; Harada, T.; Takahashi, H. TBD-catalyzed ring opening of aziridines with silylated nucleophiles. Synth. Commun. 2013, 43, 406–414. [Google Scholar] [CrossRef]
- Matsukawa, S.; Takahashi, H.; Takahashi, S. TBD-catalyzed trifluoromethylation of carbonyl compounds with (trifluoromethyl)trimethylsilane. Synth Commun. 2013, 43, 1523–1529. [Google Scholar] [CrossRef]
- Tomoi, M.; Kato, Y.; Kakiuchi, H. Polymer-supported bases, 2. Polystyrene-supported 1,8-diazabicyclo[5.4.0]undec-7-ene as reagent in organic syntheses. Makromol. Chem. 1984, 185, 2117–2124. [Google Scholar] [CrossRef]
- Iijima, K.; Fukuda, W.; Tomoi, M. Polymer-supported bases. XI. Esterification and alkylation in the presence of polymer-supported bicyclic amidine or guanidine moieties. J. Macromol. Sci. A Pure Appl. Chem. 1992, 29, 249–261. [Google Scholar] [CrossRef]
- Xu, W.; Mohan, R.; Morrissey, M.M. Polymer supported bases in combinatorial chemistry: Synthesis of aryl ethers from phenols and alkyl halides and aryl halides. Tetrahedron. Lett. 1997, 38, 7337–7340. [Google Scholar] [CrossRef]
- Boisnard, S.; Chastanet, J.; Zhu, J. A high throughput synthesis of aryl triflate and aryl nonaflate promoted by a polymer supported base (PTBD). Tetrahedron Lett. 1999, 40, 7469–7472. [Google Scholar] [CrossRef]
- McNamara, C.A.; Dixon, M.J.; Bradley, M. Recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem. Rev. 2002, 102, 3275–3300. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-supported organic catalysts. Chem. Rev. 2003, 103, 3401–3429. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, F. Immobilization of organic catalysts: When, why, and how. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Ikegami, S.; Hamamoto, H. Novel recycling system for organic synthesis via designer polymer-gel catalysts. Chem. Rev. 2009, 109, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Toy, P.H. Organic polymer supports for synthesis and for reagent and catalyst immobilization. Chem. Rev. 2009, 109, 815–838. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.E.; Hansen, T. Polymer-supported chiral organocatalysts: Synthetic strategies for the road towards affordable polymeric immobilization. Eur. J. Org. Chem. 2010, 17, 3179–3204. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pizzo, F.; Vittoriani, C.; Vaccaro, L. Polystyryl-supported TBD as an efficient and reusable catalyst under solvent-free conditions. Chem. Commun. 2004, 2756–2757. [Google Scholar] [CrossRef] [PubMed]
- Fringuelli, F.; Pizzo, F.; Vittoriani, C.; Vaccaro, L. Polystyrene-supported 1,5,7-triazabicyclo[4.4.0]dec-5-ene as an efficient and reusable catalyst for the thiolysis of 1,2-epoxides under solvent-free conditions. Eur. J. Org. Chem. 2006, 1231–1236. [Google Scholar] [CrossRef]
- Lanari, D.; Balini, R.; Palmieri, A.; Pizzo, F.; Vaccaro, L. Diastereoselective three-step route to o-(6-nitrocyclohex-3-en-1-yl)phenol and tetrahydro-6H-benzo[c]chromen-6-ol derivatives from salicylaldehydes. Eur. J. Org. Chem. 2011, 2874–2884. [Google Scholar] [CrossRef]
- Matsukawa, S.; Fujikawa, S. Polystyrene-supported TBD as an efficient and reusable organocatalyst for cyanosilylation of aldehydes, ketones, and imines. Tetrahedron Lett. 2012, 53, 1075–1077. [Google Scholar] [CrossRef]
- Matsukawa, S.; Harada, T.; Yasuda, S. Polystyrene-supported TBD catalyzed ring-opening of N-tosylaziridines with silylated nucleophiles. Org. Biomol. Chem. 2012, 10, 4886–4890. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, S.; Tsukamoto, K.; Harada, T.; Yasuda, S. An efficient method for opening N-tosyl aziridines with silylated nucleophiles using polystyrene-supported TBD as a reusable organocatalyst. Synthesis 2013, 45, 2959–2965. [Google Scholar] [CrossRef]
- Thakur, V.V.; Sudalai, A. N-Bromoamides as versatile catalysts for aziridination of olefins using chloramine-T. Tetrahedron Lett. 2003, 44, 989–992. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2a–2f, 2aʹ–2fʹ, 2aʹʹ, 2cʹʹ are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsukawa, S.; Mouri, Y. A Mild and Regioselective Ring-Opening of Aziridines with Acid Anhydride Using TBD or PS-TBD as a Catalyst. Molecules 2015, 20, 18482-18495. https://doi.org/10.3390/molecules201018482
Matsukawa S, Mouri Y. A Mild and Regioselective Ring-Opening of Aziridines with Acid Anhydride Using TBD or PS-TBD as a Catalyst. Molecules. 2015; 20(10):18482-18495. https://doi.org/10.3390/molecules201018482
Chicago/Turabian StyleMatsukawa, Satoru, and Yasutaka Mouri. 2015. "A Mild and Regioselective Ring-Opening of Aziridines with Acid Anhydride Using TBD or PS-TBD as a Catalyst" Molecules 20, no. 10: 18482-18495. https://doi.org/10.3390/molecules201018482
APA StyleMatsukawa, S., & Mouri, Y. (2015). A Mild and Regioselective Ring-Opening of Aziridines with Acid Anhydride Using TBD or PS-TBD as a Catalyst. Molecules, 20(10), 18482-18495. https://doi.org/10.3390/molecules201018482