Abstract
Coumarin is a natural compound well known for its phytotoxic potential. In the search for new herbicidal compounds to manage weeds, three synthetic derivatives bearing the coumarin scaffold (1–3), synthesized by a carbonylative organometallic approach, were in vitro assayed on germination and root growth of two noxious weeds, Amaranthus retroflexus and Echinochloa crus-galli. Moreover, the synthetic coumarins 1–3 were also in vitro assayed on seedlings growth of the model species Arabidopsis thaliana to identify the possible physiological targets. All molecules strongly affected seed germination and root growth of both weeds. Interestingly, the effects of synthetic coumarins on weed germination were higher than template natural coumarin, pointing out ED50 values ranging from 50–115 µM. Moreover, all synthetic coumarins showed a strong phytotoxic potential on both Arabidopsis shoot and root growth, causing a strong reduction in shoot fresh weight (ED50 values ≤ 60 µM), accompanied by leaf development and a decrease in pigment content. Furthermore, they caused a strong alteration in root growth (ED50 values ≤ 170 µM) and morphology with evident alterations in root tip anatomy. Taken together, our results highlight the promising potential herbicidal activity of these compounds.
1. Introduction
Weeds are still the most important pest because they compete with crops for water and nutrient resources reducing yields and quality and, consequently, causing huge economic losses [1,2,3,4]. Although mechanical, chemical, biological and cultural practices for weed management have evolved over the past century, synthetic herbicides currently represent the most common, effective and economical method for their control among all major crops [5,6]. However, concerns for the negative impact of synthetic herbicides on the environment and human health along with the rapid evolution of weeds’ resistance made the search for new herbicides with a new mode of action (MOA) and/or a new weed management paradigm more pressing and stringent [7]. Large efforts should be undertaken to develop alternative methods for weed control, using eco-friendly, cost-effective and bioactive natural or natural-like products. These compounds could be used directly as bio-herbicides or as templates for the production of synthetic herbicides [8]. Although many natural products have been employed to develop different conventional pesticides (e.g., piretroids) and fungicides, few herbicide examples may be provided [9]. Among them, leptospermone, a natural triketone found in the bottlebrush plant extract (Callistemon citrinus spp.) [10], is able to repress growth of several weeds [11,12]. This compound was then utilized to synthetize a more potent derivative, mesotrione (Callisto® herbicide), successfully employed as a pre-emergence and post-emergence herbicide. Cinmethylin, a herbicidal analogue of 1,4-cineole, is a moderately effective growth inhibitor used for monocotyledonous weed control [13], whereas pelargonic acid [14], sarmentine [15] and citral [16] are patented natural compounds isolated from plants, known for their high phytotoxic potential against the most common and noxious weeds.
However, there are few natural product-based phytotoxins described in the academic and patent literature. The industry is realizing that many natural compounds could have high potential as template for commercially successful herbicides, offering several economic and environmental advantages.
In the present work, three synthetic coumarin derivatives 1–3 were synthesized by PdI2/KI-catalyzed dicarbonylation of 2-(1-hydroxyprop-2-ynyl)phenols, and their biological activity, have been evaluated. Coumarins are secondary metabolites containing a 2H-1-benzopyran-2-one or benzopyrone moiety [17], largely known for their phytotoxic activity in many plant species. They affect many physiological processes [18] including photosynthesis [19,20,21], nutrient uptake and metabolism [22,23], seed germination [24,25,26] and root growth [27,28,29]. In particular, coumarin, the most simple and representative compound of this class, shows a high phytotoxic potential [30], and its relatively simple chemical structure makes it an excellent template for the synthesis of new structural analogues to develop novel natural herbicides.
In this context, the aim of the present paper was: (1) to assess the phytotoxic potential of the synthetic coumarin derivatives 1–3 on the noxious weeds, Amaranthus retroflexus and Echinochloa crus-galli, in order to evaluate their use as promising natural-like herbicides; and (2) to evaluate the phytotoxicity of these molecules on the model species Arabidopsis thaliana in order to identify their possible physiological targets.
2. Results
2.1. Synthesis of 3-[(Methoxycarbonyl)methyl]coumarins 1–3
Methyl (6-methoxy-2-oxo-2H-chromen-3-yl)acetate (1), methyl (8-methoxy-2-oxo-2H-chromen-3-yl)acetate (2), and methyl (4-methyl-2-oxo-2H-chromen-3-yl)acetate (3) were synthesized by PdI2/KI-catalyzed carbonylation [31,32,33,34,35] of readily available 2-(1-hydroxyprop-2-ynyl)phenols, according to Scheme 1 [36]. Reactions were carried out with 0.5–1 mol % of PdI2 in conjunction with 10–50 mol % of KI, at room temperature in MeOH as the solvent and under 90 atm of carbon monoxide (see the Experimental Section for details). A possible pathway leading to the coumarin derivatives involves the formation of a palladium phenate stabilized by triple bond coordination (I), followed by CO insertion, intramolecular syn insertion of the triple bond and alkoxycarbonylation of the resulting vinylpalladium intermediate (II). This leads to the formation of an allylalcoholic intermediate (III) and an H-Pd-I species, which, according to a known reactivity, react to give an allypalladium intermediate (IV) with elimination of water. Protonolysis of IV by HI eventually yields the final coumarin product with regeneration of PdI2 (Scheme 2; anionic iodide ligands are omitted for clarity).
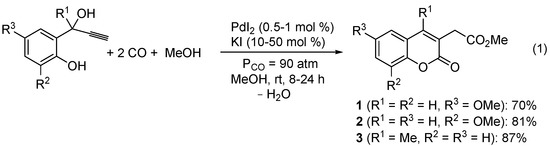
Scheme 1.
Synthesis of coumarin derivatives 1–3 by PdI2/KI-catalyzed dicarbonylation of 2-(1-hydroxyprop-2-ynyl)phenols [36].
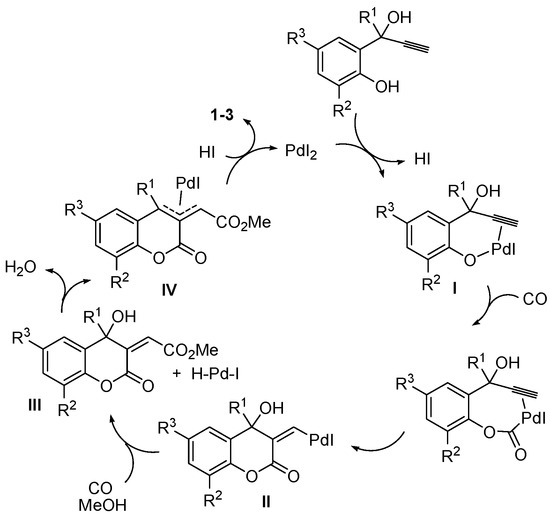
Scheme 2.
Possible reaction mechanism leading to coumarin derivatives 1–3 by PdI2/KI-catalyzed dicarbonylation of 2-(1-hydroxyprop-2-ynyl)phenols.
2.2. Bioassays on Weeds
2.2.1. Weed Germination Bioassay
All the synthetic coumarin derivatives 1–3 caused a strong inhibition of all the germination parameters on both weeds (Table 1). This effect was dose-dependent and statistically significant already at the lowest doses (Table 1). In particular, the total germination (GT %) was completely inhibited by 100 µM 1 and 200 µM 2 and 3 in A. retroflexus, while the same effect was observed with 100 µM 1 and 2 and 400 µM 3 in E. crus-galli (Table 1). In addition, germination speed (S) and speed of accumulated germination (AS) were strongly affected by all molecules in both species (Table 1).
The nonlinear regression fit of data related to GT (%) is characterized by a high statistical significance (p < 0.001) in both weeds. In A. retroflexus, the comparison of ED50 values confirmed the highest phytotoxicity of 1 and 2, able to cause 50% inhibition of GT at 54 and 58 µM concentrations, respectively, while 3 caused the same effect at 115 µM concentration (Table 1). On the other hand, 2 was the most bioactive molecule (72 µM) against E. crus-galli, followed by 1 and 3, which were both able to cause a 50% inhibition of GT at 105 µM concentration (Table 1).
In a complementary experiment, after each treatment, the un-germinated seeds of both weeds transferred in distilled water were able to recover seed germination ability underlining that the effect of all molecules, at all the concentrations assayed, was reversible.
2.2.2. Root Growth Bioassay
The effects of three synthetic coumarins (1–3) on root growth of A. retroflexus and E. crus-galli are reported in Table 1. All the molecules strongly inhibited root growth, and this effect increased along with the increase of molecule concentrations in both weeds (Table 1). The non-linear regression fit of data related to TRL show a high statistical significance (p < 0.001), as observed for seed germination. In A. retroflexus, 2 showed the most inhibitory effect (105 µM) followed by 3 (126 µM) and 1 (164 µM) (Table 1), whereas in E. crus-galli all the molecules assayed were able to cause a 50% of root growth inhibition at ~100 µM concentration (Table 1).

Table 1.
Effects of the coumarin derivatives 1–3 on seed germination (GT %), speed of germination (S), speed of accumulated germination (AS) and Total Root Length (TRL) of A. retroflexus and E. crus-galli.
| Physiological Process | A. retroflexus | E. crus-galli | ||||
|---|---|---|---|---|---|---|
| GT (%) | 1 | 2 | 3 | 1 | 2 | 3 |
| 0 µM | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a |
| 50 µM | 81.8 (3.6) b | 66 (5.1) b | 94 (4.1) a,b | 87.8 (4.9) b | 59.5 (7.9) b | 92 (4.9) ab |
| 100 µM | 0 (0.0) c | 8 (3.7) c | 84 (3.8) b | 54 (3.0) c | 46.5 (6.3) b | 54 (3.0) b |
| 200 µM | 0 (0.0) c | 0 (0.0) d | 0 (0.0) c | 0 (0.0) d | 0 (0.0) c | 10.2 (3.2) c |
| 400 µM | 0 (0.0) c | 0 (0.0) d | 0 (0.0) c | 0 (0.0) d | 0 (0.0) c | 0 (0.0) d |
| 800 µM | 0 (0.0) c | 0 (0.0) d | 0 (0.0) c | 0 (0.0) d | 0 (0.0) c | 0 (0.0) d |
| ED50 (µM) | 53.8 (1.3) a | 58 (1.6) a | 115.3 (5.8) b | 104.6 (2.4) b | 72.3 (7.6) a | 104.9 (3.4) b |
| S | ||||||
| 0 µM | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a |
| 50 µM | 42.5 (2.4) b | 37.2 (2.4) b | 57.3 (2.5) b | 65.5 (4.1) b | 42.2 (5.1) b | 57.5 (3.9) b |
| 100 µM | 0 (0.0) c | 3.6 (1.8) c | 41.9 (1.9) b | 35.2 (3.8) c | 23.9 (3.4) c | 25.8 (1.7) c |
| 200 µM | 0 (0.0) c | 0 (0.0) c | 0 (0.0) c | 0 (0.0) d | 0 (0.0) d | 4.7 (1.5) d |
| 400 µM | 0 (0.0) c | 0 (0.0) c | 0 (0.0) c | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d |
| 800 µM | 0 (0.0) c | 0 (0.0) c | 0 (0.0) c | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d |
| AS | ||||||
| 0 µM | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a |
| 50 µM | 31.1 (1.9) b | 29.7 (2.1) b | 51.3 (2.2) b | 55.5 (4.6) b | 38.5 (4.6) b | 47.2 (3.4) b |
| 100 µM | 0 (0.0) c | 2.1(1.0) c | 30.1 (1.3) c | 24.6 (3.9) c | 14.3 (2.4) c | 15.2 (1.4) c |
| 200 µM | 0 (0.0) c | 0 (0.0) c | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d | 2.7 (0.9) d |
| 400 µM | 0 (0.0) c | 0 (0.0) c | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d |
| 800 µM | 0 (0.0) c | 0 (0.0) | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d | 0 (0.0) d |
| TRL (cm) | ||||||
| 0 µM | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a | 100 (0.0) a |
| 50 µM | 76.9 (5.0) b | 67.1 (2.0) b | 83.9 (5.1) b | 69.5 (3.9) b | 74.9 (11.6) b | 75.3 (2.7) b |
| 100 µM | 63.5 (1.6) c | 47.5 (2.9) c | 51.2 (0.6) c | 56.8 (5.0) c | 47.8 (10.1) b | 49.8 (4.5) c |
| 200 µM | 46.4 (3.5) d | 41.6 (1.0) c | 36.8 (0.6) d | 25.1 (4.2) d | 21.6 (2.6) c | 26.3 (1.7) d |
| 400 µM | 27 (2.3) e | 20.8 (1.6) d | 22.6 (1.4) e | 9.4 (2.2) e | 6.9 (1.1) d | 14.2 (2.9) e |
| 800 µM | 8.7 (1.3) f | 7.1 (2.2) e | 7.7 (0.8) f | 4.2 (0.7) f | 4.7 (0.4) e | 4.7 (0.6) f |
| ED50 (µM) | 164 (9.4) c | 105.8 (8.9) a | 126.4 (9.4) b | 104 (6.3) a | 94.7 (7.4) a | 101.8 (4.3) a |
Data are expressed as percentage of the control. Different letters (a–f) along the columns, or along the row (ED50 parameter), indicate significant differences at p < 0.05. Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. Values within the brackets indicated the standard deviation (N = 5). All the dose response curves pointed out a significance level of p < 0.001.
2.3. Bioassays of Arabidopsis thaliana
2.3.1. Effects of the Synthetic Coumarins on Fresh Weight, Leaf Number and Pigments Content
All the molecules significantly affected shoot fresh weight (SFW) of Arabidopsis seedlings already at the lowest concentration (50 µM), causing 66%, 46% and 50% of reduction with 1, 2 and 3 treatments, respectively (Figure 1). Furthermore, this effect was most evident along with increasing of concentrations, reaching almost the complete inhibition at the highest concentration. The non-linear regression fits of the dose-response curves are characterized by a high statistical significance (p ≤ 0.001) and all the molecules showed very low ED50 values. In particular, comparing the ED50 values, 1 seemed to be more harmful showing a higher ED50 (26 µM) compared to 2 (53 µM) and 3 (59.3 µM), which did not significantly vary (Table 2).

| Molecules | ED50 (µM) | |||
|---|---|---|---|---|
| SFW | TRL | NLR | RHD | |
| 1 | 26.01 (0.16) a | 111.29 (19.3) ab | 42.49 (0.75) a | 55.36 (0.28) a |
| 2 | 53.07 (0.76) b | 74.24 (10.1) a | 43.56 (0.89) a | 56.18 (0.78) a |
| 3 | 59.27 (0.22) b | 173 (18.78) b | 43.34 (0.12) a | 49.27 (0.46) a |
Different letters (a–b) along the columns indicate significant differences at p < 0.05. Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. N = 5. Values within the brackets indicated the standard deviation (N = 5). All the dose-response curves pointed out a significance level of p < 0.001.
Conversely, LN was significantly affected only by 2 and 3, which were able to reduce it already at 200 µM (26% of inhibition), reaching the highest level of inhibition (76%) with both molecules at 800 µM (Figure 2). Furthermore, all the molecules strongly affected leaf area (LA) starting from the lowest concentration (50 µM) (data not shown). Interestingly, similar strong inhibitory effects were observed on pigment content coupled with a chlorotic appearance in Arabidopsis leaves treated with the lowest concentration (50 µM). These effects, observed at naked eye, were also confirmed by the experimental data which pointed out a strong dose-dependent reduction in all the pigments quantified (Figure 3).
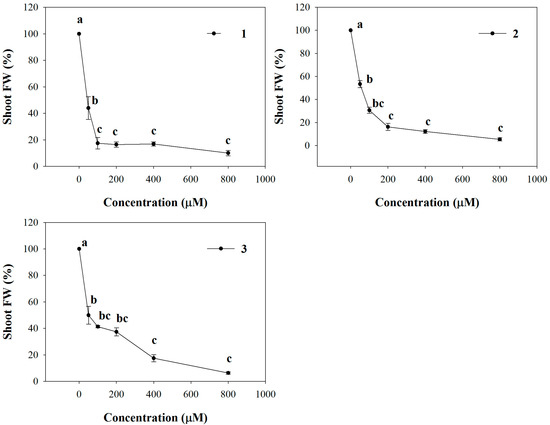
Figure 1.
Dose-response curves of shoot fresh weight (SFW) of A. thaliana seedlings treated with 1–3 for 15 days. Data are expressed as percentage of the control. Different letters (a–c) along the curves indicate significant differences at p ≤ 0.05). Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. N = 5.
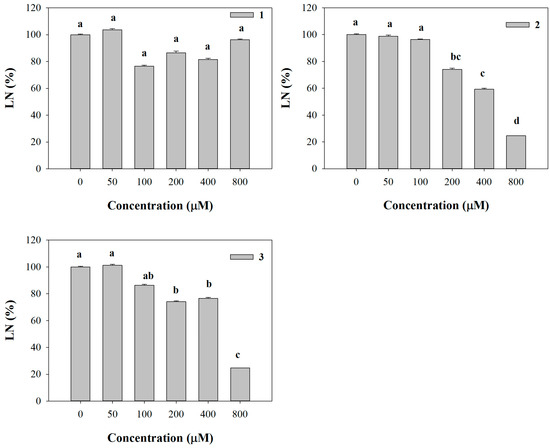
Figure 2.
Leaf number (LN) of A. thaliana seedlings treated with synthetic coumarins 1–3 Data are expressed as percentage of the control. Different letters (a–d) along the bars indicate significant differences at p ≤ 0.05). Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. N = 5.
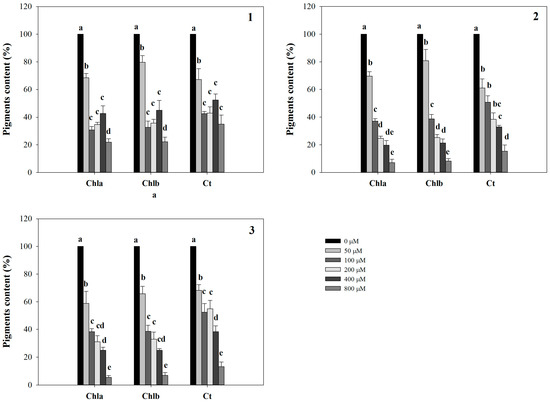
Figure 3.
Pigments content in A. thaliana shoots treated with synthetic coumarins 1–3. Data are expressed as percentage of the control. Different letters (a–e) along the bars indicate significant differences at p ≤ 0.05). Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. N = 5.
2.3.2. Root Growth and Morphology
All the molecules strongly affected A. thaliana root morphology showing a dose-dependent inhibitory effect on primary root growth. In particular, 100 µM of 1, 2 and 3 caused 60%, 77% and 44% of TRL inhibition, respectively, and this effect increased as their concentrations increased, reaching 90% of inhibition at the highest concentration (Figure 4A–C). The ED50 values were equal to 111, 74 and 173 µM (R2 = 0.98) for 1, 2 and 3, respectively (Table 2). Interestingly, NLR and RHD root traits were also strongly affected by all synthetic coumarins and the percentage of inhibition showed a similar trend for all the molecules (Figure 4D–I). In detail, Arabidopsis seedlings showed 90% of NLR inhibition already at the lowest concentration (50 µM), reaching 100% of inhibition at all higher concentrations (Figure 4D–F). A marked reduction of the RHD was observed already at the lowest concentration (50 µM) which reached a complete inhibition at 100 µM and this effect was confirmed by the ED50 values (Figure 4, Table 2).
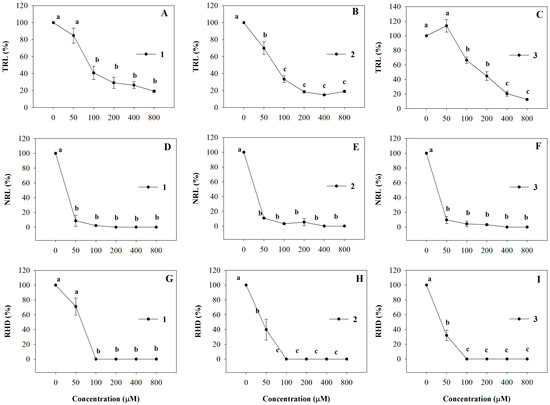
Figure 4.
Dose-response curves of root morphology of A. thaliana seedlings treated with 1–3 for 15 days: (A–C) Total Root Length (TRL); (D–F) Lateral Root Number (NLR); (G–I) Root Hair Density (RHD). Data are expressed as percentage of the control. Different letters (a–c) along the curves indicate significant differences at p ≤ 0.05). Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. N = 5.
However, root structure and organization were only modified by 1 and 3, which were able to cause a loss of the gravitropic response already at low concentrations (50 µM) (Figure 5). This effect became evident at 100 µM together with a strong root deformation causing a corkscrew-shape. Moreover, 3 treatment also determined a circumnutation phenomenon (Figure 5).

Figure 5.
Root apex of A. thaliana grown in vitro and treated with different concentrations of 1–3. The anticlockwise torsion of the cell files and the absence of root hairs (when treated with molecules 1 and 3) are evident.
2.3.3. Number of Mitotic Sites
All the synthetic coumarins affected lateral root initiation (Figure 6). In particular, 1 strongly reduced the number of mitotic sites (62% of inhibition) along the primary root, whereas the higher concentrations completely inhibited mitotic sites’ formation (Figure 6). Fifty and 100 µM 2 concentrations already caused 85% of inhibition, reaching a complete inhibition of mitotic sites’ formation at the higher concentrations, as already observed for 1 (Figure 6). Conversely, 3 did not affect mitotic sites’ formation at the lowest concentration (50 µM), whereas at the higher ones it exhibited the same trend of 1 and 2 (Figure 6).
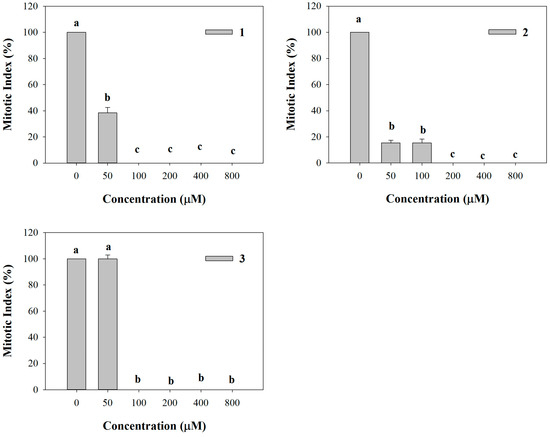
Figure 6.
Mitotic sites number evaluated along the primary root of A. thaliana seedlings treated with the synthetic coumarins 1–3. Data are expressed as percentage of the control. Different letters (a–c) along the curves indicate significant differences at p ≤ 0.05). Homoscedastic data were analyzed by ANOVA with Tukey’s test, whereas the heteroscedastic ones were analyzed with Tamhane’s T2. N = 5.
3. Discussion
Because of their multifaceted biological activity and broad spectrum of action, coumarins are currently of great interest as a source for new chemical structures with potential utilization in pharmacology, chemical and agronomy fields. Focusing on their potential application in agriculture, the development of new synthetic methods for natural herbicide production starting from the coumarin nucleus as a natural template could provide interesting tools for the development of natural herbicide models. Coumarin, the simplest compound of this class, is well known for its phytotoxicity in germination and growth of many species [24,25,37,38,39], and could represent a promising template for natural herbicides’ production. In this paper, we have evaluated the phytotoxic potential among widespread noxious weeds and Arabidopsis model plant of three coumarin derivatives, methyl (6-methoxy-2-oxo-2H-chromen-3-yl)acetate (1), methyl (8-methoxy-2-oxo-2H-chromen-3-yl)acetate (2), and methyl (4-methyl-2-oxo-2H-chromen-3-yl)acetate (3).
Similarly to the natural coumarin template [24,25], all the synthetic molecules exerted a dose-dependent inhibitory effect on seed germination of both noxious weeds. Interestingly, they showed a stronger biological activity compared to coumarin, which is generally considered the most active compound tested among structural analogues [40]. Indeed, 1 mM natural coumarin caused 60% and 40% inhibition in germination of E. crus-galli and A. retroflexus, respectively [41,42], and more than 60% in wheat seeds’ germination [24], while 1, 2 and 3 completely inhibited this process at lower concentrations. Similarly, Prabodh et al. [43] and Khanh et al. [44] observed that higher concentrations of template coumarin (above of 350 µM) were needed to reach 50% inhibition of Lactuca sativa, Lolium perenne and E. crus-galli germination process. Comparable results were also observed by Saleh and Abu El-Soud [45] on wheat germination. Also, other natural coumarins isolated from Meliaceae and Rutaceae, such as psoralen, showed lowest phytotoxic activity on germination process causing the complete inhibition at 1mM concentrations [46]. However, despite their potency, the effects of 1–3 were reverted after a careful seed washing of both weeds, allowing them to recover germination in the presence of deionized water, thereafter. This effect, observed also after treatment with natural coumarin [24], suggested that all the molecules did not cause an irreversible germination block. It has been demonstrated that exogenous application of phenols to weeds delayed but did not substantially inhibit germination [41]. The 1, 2 and 3 treatments also caused a strong dose-dependent inhibitory effect observed on root growth of both weeds. In accordance with coumarin effects on root growth of E. crus-galli [42], 100 µM of all the synthetic coumarin derivatives caused 50% inhibition of root growth on both weeds reaching 90% with the highest concentration (800 µM). Conversely, recent results demonstrated that 430 µM (L. perenne and L. sativa) and 690 µM (E. crusgalli) coumarin concentrations were needed to reach 50% inhibition of root growth of these species [43,44].
Given their high phytotoxicity on weeds, the effects of these synthetic coumarins were evaluated on shoot and root growth of model species Arabidopsis thaliana. As reported by Pennacchio et al. [47], this species is sensitive to different allelochemicals and satisfies all of the selection criteria for target species such as fast growth, short life cycle, and mutant availability useful to better identify sites target and mode of action of molecules [48].
All the molecules strongly affected A. thaliana growth causing an evident chlorosis, a reduction of leaf number (LN), shoot fresh weight (SFW) and leaf area (LA), together with several alterations in root growth and morphology. In particular, 1, at all concentrations, reduced SFW without a consistent reduction of leaf number, indicating that this molecule caused a biomass loss without interfering with leaf differentiation. Conversely, 2 and 3, at concentrations higher than 100 µM, caused a dose dependent LA and LN inhibition which matched SFW responses. Similar results were already observed by Sánchez-Moreiras et al. [49] in Arabidopsis treated with 2-3H-benzoxazolinone (BOA), an allelochemical mainly found in the Poaceae [50,51] and largely known for its phytotoxic effect [8,52,53]. Furthermore, similar to natural coumarin, the synthetic coumarin derivatives inhibited in a dose dependent-manner chlorophyll and carotenoids content of A. thaliana. Knypl [54,55] showed that coumarin was able to delay the loss of chlorophyll from leaves kept in the dark and to stimulate yellowing in light. Moreover, some natural 4-phenylcoumarins and imperatorin, a furanocoumarin, acted as photophosphorylation uncouplers or energy transfer inhibitors [21,56,57]. Macias et al. [58] demonstrated that several coumarins reduced chlorophyll content and photosynthetic activity in spinach chloroplast. Since the chlorophyll content is strictly related to plant biomass production [59], any reduction in leaf pigments would limit net photosynthesis altering the entire plant metabolism. However, if the reduction in chlorophyll content was due to a synthesis inhibition or a chemical-induced degradation was not clarified. Probably, the strong effect induced by these synthetic coumarins on root growth, essential for nutrient uptake, could be indirectly responsible for shortage of some nutrients involved in chlorophyll synthesis. Although natural coumarin increased nitrate uptake in wheat roots [22], many phenolic and synthetic diphenolic compounds markedly reduced net nutrient uptake such as nitrate, ammonium, potassium and phosphorus in different plant species [60,61,62].
All the synthetic molecules strongly affected the root system more than the natural coumarins [45,46] inducing modifications on root morphology and anatomy, such as unaligned cells, gravitropism loss and root hair inhibition. Similar results were observed by several authors in treated roots with oryzalin, taxol and colchicines, affecting microtubule organization [63,64,65], which could also justify root hair loss [66,67]. Furthermore, they caused a strong decrease in the number of lateral roots accompanied by a reduction in mitotic sites’ formation attributable to a biased hormonal balance. Indeed, the formation of lateral roots is dependent on the coordinated action of many factors among which auxin transport and redistribution together with ethylene played a key role [68,69]. These effects were also found in Arabidopsis roots after treatment with 3-(methoxycarbonylmethylene)isobenzofuran-1-imines, molecules synthetized from simple substrates by a catalytic carbonylation approach that have showed a very promising potential herbicidal activity [70].
4. Experimental Section
4.1. General Information
Chemicals were purchased from Sigma-Aldrich Italia (Milano, Italy) and were used as such without further purification. Melting points were taken on a Reichert Thermovar apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded at 25 °C in CDCl3 solutions with a Bruker DPX Avance 300 spectrometer (Bruker Italia, Milano, Italy) operating at 300 MHz and 75 MHz, respectively, with Me4Si as internal standard. IR spectra were taken with a JASCO FT-IR 4200 spectrometer (Jasco Europe s.r.l., Cremella, Lecco, Italy). Mass spectra were obtained using a Shimadzu QP-2010 GC-MS apparatus (Shimadzu Italia, Milano, Italy) at 70 eV ionization voltage. Microanalyses were carried out with a Carlo Erba Elemental Analyzer Mod. 1106 (Carlo Erba, Cornaredo, Milano, Italy). All reactions were analyzed by TLC (Merck Italy, Vimodrone, Milano, Italy) on silica gel 60 F254 (Merck Italy) or on neutral alumina (Merck Italy) and by GLC using a Shimadzu GC-2010 gas chromatograph (Shimadzu Italia, Milano, Italy) and capillary columns with polymethylsilicone + 5% polyphenylsilicone as the stationary phase (HP-5). Column chromatography was performed on silica gel 60 (Merck Italy, 70–230 mesh). Evaporation refers to the removal of solvent under reduced pressure.
Synthetic coumarins 1–3 were prepared and characterized as we previously reported [31].
4.2. Bioassays on Weeds
4.2.1. Seed Germination Bioassay
The potential phytotoxic activity of the three synthetic coumarin derivatives (1–3) was assayed on seed germination of two noxious weeds: Amaranthus retroflexus and Echinochloa crus-galli. These species has been chosen not only for their economic importance [71,72] but also because they are representatives of the two different classes Liliopsida and Magnoliopsida. For the experiments, all the molecules were firstly dissolved in 0.1% EtOH (v/v) and then diluted in deionized water to reach the final concentrations: 0, 50, 100, 200, 400, and 800 µM. The same amount of ethanol employed to solubilize the molecules was added in the control.
Seeds were surface sterilized for 15 min using a 15% (v/v) NaClO solution and then rinsed three times with sterile deionized water. Ten seeds for each species were distributed into a Petri dish (6 cm diameter) on a double layer of filter paper moistened with 2 mL of each concentration and molecule. Control treatments received 2 mL of distilled water. Petri dishes were then placed in a growth chamber at 25 ± 1 °C, 70% relative humidity, in dark conditions. Seeds germination was recorded every 24 h for five consecutive days. The total germination index [GT (%)], germination speed (S), and speed of accumulated germination (AS) were calculated as reported by Chiapusio et al. [73]. At the end of treatments, the un-germinated seeds were carefully washed, placed in Petri dishes containing filter paper, wetted with deionized water and checked daily for three days in order to evaluate the reversible or irreversible inhibition of the seed germination process [74].
4.2.2. Root Elongation Bioassay
Five pre-germinated seeds of A. retroflexus and E. crus-galli, selected for uniformity in root length (1 mm), were placed in Petri dishes (6 cm diameter) on a double layer of filter paper moistened with 2 mL of the synthetic coumarin solutions at the same concentrations applied for seed germination bioassay (Section 2.2.1). Petri dishes were then placed in a growth chamber at 25 ± 1 °C and 70% relative humidity. After 48 h of exposure, Total Root Length (TRL) was measured according to Araniti et al. [75].
4.3. Bioassays of Arabidopsis thaliana
4.3.1. Seedling Growth Bioassays
Arabidopsis thaliana (L.) Heynh. seeds ecotype Columbia (Col-0) were surface sterilized for 3 min in 50% EtOH and NaOCl 0.5% with Triton X-100 at 0.01% and then rinsed three times in distilled water. After sterilization, seeds were maintained in 0.1% agar at 4 °C for 72 h to promote the synchronization of germination. Then, 24 sterilized seeds were sown in Petri dishes (100 × 150 mm) containing agar medium (0.8% w/v) enriched with micro- and macronutrients (Murashige-Skoog, Sigma-Aldrich) and supplemented with 1% sucrose. According to Araniti et al. [76] plates were placed vertically in the growth chamber (22 ± 2 °C temperature and 75 mol·m−2·s−1 light intensity) to promote geotropic root growth.
Once germinated, 24 seedlings (7 day old) were transferred to a single plate and grown for 14 days in the same medium containing the synthetic coumarin derivatives at the same concentrations previously reported (Section 2.2.1). All the molecules were previously dissolved in 0.1% EtOH (v/v), and then autoclaved. The same amount of EtOH was added to the control plates.
After 14 days of treatments, seedlings were collected and separated into shoot and root. Shoot Fresh Weight (SFW) and Leaf Number (LN) were evaluated. Whole root system was imaged by scanning (STD 1600, Régent Instruments Inc., Quebec, QC, Canada) and Total Root Length (TRL), Number of Lateral Roots (NLR) and Lateral Root Length (LRL), using WinRhizo Pro system v. 2002a (Instruments Régent Inc., Quebec, Canada) were measured. Root Hair Density (RHD) was analyzed by using a stereoscopic microscopy (Olympus SZX9, Jackson, MS, USA).
4.3.2. Determination of Mitotic Sites
The number of mitotic sites was evaluated as described by Canellas et al. [77], with some modifications. After 14 days of treatment with synthetic coumarin derivatives, roots were washed in 50 mM phosphate buffer (pH 7.4) and then warmed at 75 °C in 0.5% KOH (w/v) solution for 20 min. The roots were carefully rinsed in 50 mM phosphate buffer (pH 7.4) and then stained in haematoxylin solution containing 0.025 g haematoxylin, 0.0125 g ferric ammonium sulphate and 1.5 mL of 45% (v/v) acetic acid for 16 h, in dark conditions. Finally, the roots were distained in 80% lactic acid (w/v) at 75 °C for 20 s, and the mitotic sites were counted by stereoscopic microscopy (Olympus SZX9).
4.3.3. Pigment Quantification
Chlorophyll a (Chla), chlorophyll b (Chlb), and carotenoids (Ct) quantification was carried out on A. thaliana seedlings treated with each molecule for 14 days (see Section 2.1). One hundred milligrams of leaf tissue for each replicate and treatment were ground in liquid nitrogen and extracted with 1.5 mL methanol. The extract was then centrifuged at 170 g for five minutes and, successively, 500 µL of supernatant was mixed with 500 µL methanol. The absorbance of the extracts was determined at 470, 653, 666 and 750 nm. The pigment content (mg·g−1 of SFW) was evaluated according to the following Equations proposed by Wellburn [78]:
where DO is the optical density, V is the volume of methanol used (mL) and (X + C) the sum of carotenoids and xanthophylls.
4.4. Statistical Analysis
A completely random design with 5 replications was adopted to evaluate the effects of synthetic coumarin derivatives on seed germination and root growth of both weeds and seedlings growth of A. thaliana. Data were evaluated for normality (Kolmogorov-Smirnov test) and tested for homogeneity of variances (Levene’s test). The statistical significance of differences among group means were estimated by analysis of variance followed by Tukey test in case of homoscedastic data, and by Tamhane’s T2 test in the case of heteroscedastic data (p ≤ 0.05). All statistical analyses were performed by using SPSS ver. 6.1 software (Insightful Corporation, Seattle, WA, USA). According to Araniti et al. [79] the SFW, TRL, NLR, RHD responses to different doses of all molecules were evaluated by a nonlinear regression model using a log-logistic function that allowed to estimate the ED50 parameter, the dose required to reduce 50% of the total response. The phytotoxicity comparison among the three molecules was performed by one-way ANOVA using the ED50 as a variable with the molecule as main factor. The ED50 data were first checked for deviations from normality (Kolmogorov-Smirnov test) and tested for homogeneity (Leven Median test). The statistical significance of differences among group means were estimated by analysis of variance followed by Tukey test in case of homoscedastic data, and by Tamhane’s T2 test in the case of heteroscedastic data (p ≤ 0.05).
5. Conclusions
In conclusion, all the synthetic coumarins 1–3 exhibited a strong phytotoxic activity among Arabidopsis seedlings affecting shoot and root growth as well as root morphology, seed germination and root growth of two noxious weeds higher than the natural coumarin. These results suggested that the three synthetic coumarins could be promising phytotoxic molecules employable as new natural-like herbicides. In perspective, the potential effectiveness of these coumarin derivatives as herbicides will have to be tested in field conditions, where the soil properties and microbial interactions come into play.
Acknowledgments
This research work was partially supported by MIUR PON R & C 2007-2013 Project “DIRECT FOOD” cod. PON01_00878 (research grant to R.M.) and partially supported by MIUR SIR-2014 cod. RBSI14L9CE (MEDANAT).
Author Contributions
F.A., R.M., F.S., B.G. and M.R.A. conceived and designed the experiments. R.M., S.V.G. and B.G. synthetized the molecules. F.A., R.M. and A.L. performed the experiments. F.A., F.S. and M.R.A. analyzed the data. F.A., R.M., F.S., B.G. and M.R.A. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [PubMed]
- Gressel, J. Global advances in weed management. J. Agric. Sci. 2011, 149, 47–53. [Google Scholar]
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. Bio-Control 2001, 46, 229–260. [Google Scholar]
- Rajcan, I.; Swanton, C.J. Understanding maize–weed competition: Resource competition, light quality and the whole plant. Field Crops Res. 2001, 71, 139–150. [Google Scholar] [CrossRef]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the art: I. Weed control research and safener technology: The path to modern agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Phipps, R.; Strange, A.; Grey, P. Environmental and human health impacts of growing genetically modified herbicide-tolerant sugar beet: A life-cycle assessment. Plant Biotechbol. J. 2004, 2, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The future of natural products in pest management. In Abstracts of Papers, Proceedings of the 230th ACS National Meeting, Washington, DC, USA, 31 August 2005.
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.L.; Prisbylla, M.P.; Cromartie, T.H.; Dagarin, D.P.; Howard, S.W.; Provan, W.M.; Mutter, L.C. The discovery and structural requirements of inhibitors of p-hydroxyphenylpyruvate dioxygenase. Weed Sci. 1997, 45, 601–609. [Google Scholar]
- Cornes, D. Callisto: A very successful maize herbicide inspired by allelochemistry. In Proceedings of the Fourth World Congress on Allelopathy, Wagga Wagga, NSW, Australia, 21–26 August 2005; Volume 2, p. 2008.
- Duke, S.O. The role of natural products in crop protection. Planta Med. 2014, 80, 751. [Google Scholar] [CrossRef]
- Grossmann, K.; Hutzler, J.; Tresch, S.; Christiansen, N.; Looser, R.; Ehrhardt, T. On the mode of action of the herbicides cinmethylin and 5-benzyloxymethyl-1, 2-isoxazolines: Putative inhibitors of plant tyrosine aminotransferase. Pest Manag. Sci. 2012, 68, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Sasaki, M. Pelargonic Acid-Containing Liquid Agents for Weed Control. Patent JP 2011001337, 7 March 2011. [Google Scholar]
- Huang, H.; Asolkar, R. Use of Sarmentine and Its Analogs for Controlling Plant Pests. Patent WO 2011011415, 27 January 2011. [Google Scholar]
- Bessette, S. Pesticidal Compositions Containing Plant Essential Oils Against Beetles. Patent US 20030091661, 13 December.
- Zobel, A.M.; Brown, S.A. Coumarins in the interaction between plants and its environment. Allelopathy J. 1995, 2, 9–20. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Mahmood, K.; Khan, M.B.; Song, Y.Y.; Ye, M.; Baerson, S.R.; Zeng, R.S. Differential morphological, cytological, and biochemical responses of two rice cultivars to coumarin. Allelopathy J. 2013, 31, 281–296. [Google Scholar]
- Razavi, S.M. Plant coumarins as allelopathic agents. Int. J. Biol. Chem. 2011, 5, 86–90. [Google Scholar] [CrossRef]
- Calera, M.R.; Mata, R.; Lotina-Hennsen, B.; Anaya, A.L. Uncoupling behavior of the 4-phenylcoumarin in spinach chloroplasts: Structure-activity relationships. J. Agric. Food Chem. 1996, 44, 2966–2969. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; de Santis, C.; Sidari, M.; Sorgonà, A.; Badiani, M.; Cacco, G. Influence of coumarin on the net nitrate uptake in durum wheat. New Phytol. 2001, 150, 619–627. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Sorgonà, A.; Sidari, M.; Badiani, M.; Fuggi, A. Coumarin inhibits the growth of carrot (Daucus carota L. cv. Saint Valery) cells in suspension culture. J. Plant Physiol. 2003, 160, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, M.R.; Cacco, G.; Sorgonà, A.; Marabottini, R.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. The inhibitory effects of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, CV. Simeto) seeds. J. Chem. Ecol. 2006, 32, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Pergo, E.M.; Abrahim, D.; da Silva, P.C.S.; Kern, K.A.; da Silva, L.J.; Voll, E.; Ishii-Iwamoto, E.L. Bidens pilosa L. exhibits high sensitivity to coumarin in comparison with three other weed species. J. Chem. Ecol. 2008, 34, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gruber, M.Y.; Hegedus, D.D.; Lydiate, D.J.; Gao, M.J. Effects of a coumarin derivative, 4-methylumbelliferone, on seed germination and seedling establishment in Arabidopsis. J. Chem. Ecol. 2011, 37, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Kupidlowska, E.; Kowalec, M.; Sulkowski, G.; Zobel, A.M. The effect of coumarins on root elongation and ultrastructure of meristematic cell protoplast. Ann. Bot. 1994, 73, 525–530. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Sorgonà, A.; Albano, S.; Cacco, G. Coumarin differentially affects the morphology of different root types of maize seedlings. J. Chem. Ecol. 2004, 30, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, M.R.; Nicolò, A.; Lupini, A.; Oliva, S.; Sorgonà, A. Effects of different allelochemicals on root morphology of Arabidopsis thaliana. Allelopathy J. 2008, 22, 245–252. [Google Scholar]
- Brown, S.A. Biochemistry of plant coumarins. In The Shikimic Acid Pathway; Springer: New York, NY, USA, 1986; pp. 287–316. [Google Scholar]
- Gabriele, B.; Salerno, G.; Costa, M.; Chiusoli, G.P. Recent developments in the synthesis of heterocyclic derivatives by PdI2-catalyzed carbonylation reactions. J. Organomet. Chem. 2003, 687, 219–228. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M.; Chiusoli, G.P. Recent advances in the synthesis of carbonyl compounds by palladium-catalyzed oxidative carbonylation reactions of unsaturated substrates. Curr. Org. Chem. 2004, 8, 919–946. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M. PdI2-catalyzed synthesis of heterocycles. Synlett 2004, 14, 2468–2483. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M. Oxidative carbonylations. Top. Organomet. Chem. 2006, 18, 239–272. [Google Scholar]
- Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative carbonylation as a powerful tool for the direct synthesis of carbonylated heterocycles. Eur. J. Org. Chem. 2012, 35, 6825–6839. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Plastina, P. A novel palladium-catalyzed dicarbonylation process leading to coumarines. J. Org. Chem. 2008, 73, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, A.H.; Asadi, G.A.; Ghorbani, R. Herbicidal activity of coumarin when applied as a pre-plant incorporated into soil. Not. Sci. Biol. 2015, 7, 239–243. [Google Scholar]
- Chon, S.U.; Kim, Y.M. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agric. Crop Sci. 2004, 190, 145–150. [Google Scholar] [CrossRef]
- Mishra, A. Allelopathy: Natural and an environment-friendly unique tool for weed control. Int. J. Adv. Res. Eng. Appl. Sci. 2015, 4, 26–31. [Google Scholar]
- Haig, T.J.; Haig, T.J.; Seal, A.N.; Pratley, J.E.; An, M.; Wu, H. Lavender as a source of novel plant compounds for the development of a natural herbicide. J. Chem. Ecol. 2009, 35, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.D.; Hoagland, R.E. The effects of naturally occurring phenolic compounds on seed germination. Weed Sci. 1982, 30, 206–212. [Google Scholar]
- Chon, S.U.; Choi, S.K.; Jung, S.; Jang, H.G.; Pyo, B.S.; Kim, S.M. Effects of alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology of alfalfa and barnyard grass. Crop Prot. 2002, 21, 1077–1082. [Google Scholar] [CrossRef]
- Prabodh, S.; Prajwal, P.; Ananad, K.; Bimala, L.; Noura, S.D.; Debra, M.M.; William, N.S. Bioactivities of volatile components from Nepalese Artemisia species. Nat. Prod. Commun. 2012, 7, 1651–1658. [Google Scholar]
- Khanh, T.D.; Chung, I.M.; Tawata, S.; Xuan, T.D. Weed suppression by Passiflora edulis and its potential allelochemicals. Weed Res. 2006, 46, 296–303. [Google Scholar] [CrossRef]
- Saleh, A.M.; El-Soud, W.A. Evidence for “gibberellin-like” activity of coumarin. S. Afr. J. Bot. 2015, 100, 51–57. [Google Scholar] [CrossRef]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.; Sampaio, O.M.; Severino, V.G.; Cazal, C.M.; das Graças Fernandes, M.F.; Fernandes, J.B.; Macias, F.A. Phytotoxicity of alkaloids, coumarins and flavonoids isolated from 11 species belonging to the Rutaceae and Meliaceae families. Phytochem. Lett. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Pennacchio, M.; Jefferson, L.V.; Havens, K. Arabidopsis thaliana: A new test species for phytotoxic bioassays. J. Chem. Ecol. 2005, 31, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Michniewicz, M.; Brewer, P.B.; Friml, J. Polar auxin transport and asymmetric auxin distribution. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2007. [Google Scholar]
- Sánchez-Moreiras, A.M.; Martínez-Peñalver, A.; Reigosa, M.J. Early senescence induced by 2-3H-benzoxazolinone (BOA) in Arabidopsis thaliana. J. Plant Physiol. 2011, 168, 863–870. [Google Scholar] [CrossRef]
- Schulz, M.; Marocco, A.; Tabaglio, V.; Macias, F.A.; Molinillo, J.M. Benzoxazinoids in rye allelopathy-from discovery to application in sustainable weed control and organic farming. J. Chem. Ecol. 2013, 39, 154–174. [Google Scholar] [CrossRef]
- Pratt, K.; Kumar, P.; Chilton, W.S. Cyclic hydroxamic acids in dicotyledonous plants. Biochem. System. Ecol. 1995, 23, 781–785. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M. Optimization of benzoxazinones as natural herbicide models by lipophilicity enhancement. J. Agric. Food Chem. 2006, 54, 9357–9365. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M. Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Nat. Prod. Rep. 2009, 26, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Knypl, J.S. Control of RNA, protein, and chlorophyll synthesis in senescing leaf tissue of kale by coumarin and growth retardants. Physiol. Biochem. 1969, 160, 217–233. [Google Scholar]
- Knypl, J.S. Inhibition of chlorophyll synthesis by growth retardants and coumarin, and its reversal by potassium. Nature 1969, 1025–1026. [Google Scholar] [CrossRef]
- Mata, R.; Pereda-Miranda, R.; Lotina-Hennsen, B. Natural products from mexican plants as a source of potential herbicide agents. In Secondary Metabolites from Mexican Plants: Chemistry and Biological Properties; RodriguezHahn, L., Ed.; Research Signpost: Trivandum, India, 1996; pp. 59–68. [Google Scholar]
- Lotina-Hennsen, B.; Mata, R.; Calderon, J.S.; CéspedesAcuna, C.L.; Jiménez-Estrada, M. Secondary metabolites isolated from mexican plants: Target and mechanism of action on photosynthesis. Recent. Res. Dev. Agric. Food Chem. 1998, 2, 731–749. [Google Scholar]
- Macias, M.L.; Rojas, I.S.; Mata, R.; Lotina-Hennsen, B. Effect of selected coumarins on spinach chloroplast photosynthesis. J. Agric. Food Chem. 1999, 47, 2137–2140. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Schepers, J. Relationships between gross primary production, green LAI, and canopy chlorophyll content in maize: Implications for remote sensing of primary production. Remote Sens. Environ. 2014, 144, 65–72. [Google Scholar] [CrossRef]
- Zhou, X.; Song, H.; Wang, J. Effects of coumarin on net nitrate uptake and nitrogen metabolism in roots of alfalfa (Medicago sativa). Allelopathy J. 2013, 31, 377. [Google Scholar]
- Yu, J.Q.; Matsui, Y. Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on ion uptake by cucumber seedlings. J. Chem. Ecol. 1997, 23, 817–827. [Google Scholar] [CrossRef]
- Lehman, M.E.; Blum, U. Evaluation of ferulic acid uptake as a measurement of allelochemical dose: Effective concentration. J. Chem. Ecol. 1999, 25, 2585–2600. [Google Scholar] [CrossRef]
- Furutani, I.; Watanabe, Y.; Prieto, R.; Masukawa, M.; Suzuki, K.; Naoi, K.; Thitamadee, S.; Shikanai, T.; Hashimoto, T. The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development 2000, 127, 4443–4453. [Google Scholar]
- Sugimoto, K.; Himmelspach, R.; Williamson, R.E.; Wasteneys, G.O. Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 2003, 15, 1414–1429. [Google Scholar] [PubMed]
- Baskin, T.I.; Beemster, G.T.; Judy-March, J.E.; Marga, F. Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis. Plant Physiol. 2004, 135, 2279–2290. [Google Scholar] [CrossRef]
- Smith, R.D.; Wilson, J.E.; Walker, J.C.; Baskin, T.I. Protein phosphatase inhibitors block root hair development and alter cell shape in Arabidopsis roots. Planta 1994, 194, 516–524. [Google Scholar] [CrossRef]
- Baskin, T.I.; Wilson, J.E. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 1997, 113, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Nibau, C.; Gibbs, D.J.; Coates, J.C. Branching out in new directions: The control of root architecture by lateral root formation. New Phytol. 2008, 179, 595–614. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. The Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Mancuso, R.; Ziccarelli, I.; Sunseri, F.; Abenavoli, M.R.; Gabriele, B. 3-(Methoxycarbonylmethylene) isobenzofuran-1-imines as a new class of potential herbicides. Molecules 2014, 19, 8261–8275. [Google Scholar] [CrossRef] [PubMed]
- Iamonico, D. Biology, life-strategy and invasiveness of Amaranthus retroflexus L. (Amaranthaceae) in central Italy: Preliminary remarks. Bot. Serbica 2010, 34, 137–145. [Google Scholar]
- Kim, S.K.; Kim, S.Y.; Won, J.G.; Shin, J.H.; Kim, H.Y. Effect of densities of Echinochloa crus-galli and Cyperus serotinus in direct-seeding flooded rice on rice yield and quality, and economic threshold level of the weeds. Korean J. Weed Sci. 2012, 32, 44–51. [Google Scholar] [CrossRef]
- Chiapusio, G.; Sanchez, A.M.; Reigosa, M.J.; Gonzalez, L.; Pellissier, F. Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 1997, 23, 2445–2453. [Google Scholar]
- Araniti, F.; Marrelli, M.; Lupini, A.; Mercati, F.; Statti, G.A.; Abenavoli, M.R. Phytotoxic activity of Cachrys pungens Jan, a mediterranean species: Separation, identification and quantification of potential allelochemicals. Acta Physiol. Plant. 2014, 36, 1071–1083. [Google Scholar] [CrossRef]
- Araniti, F.; Sunseri, F.; Abenavoli, M.R. Phytotoxic activity and phytochemical characterization of Lotus ornithopodioides L., a spontaneous species of Mediterranean area. Phytochem. Lett. 2014, 8, 179–183. [Google Scholar] [CrossRef]
- Araniti, F.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M.; Abenavoli, M.R. Individual and joint activity of terpenoids, isolated from Calamintha nepeta extract, on Arabidopsis thaliana. Nat. Prod. Res. 2013, 27, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sorgonà, A.; Conforti, F.; Marrelli, M.; Statti, G.A.; Menichini, F.; Abenavoli, M.R. Allelopathic potential of Artemisia arborescens: Isolation, identification and quantification of phytotoxic compounds through fractionation-guided bioassays. Nat. Prod. Res. 2013, 27, 880–887. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).