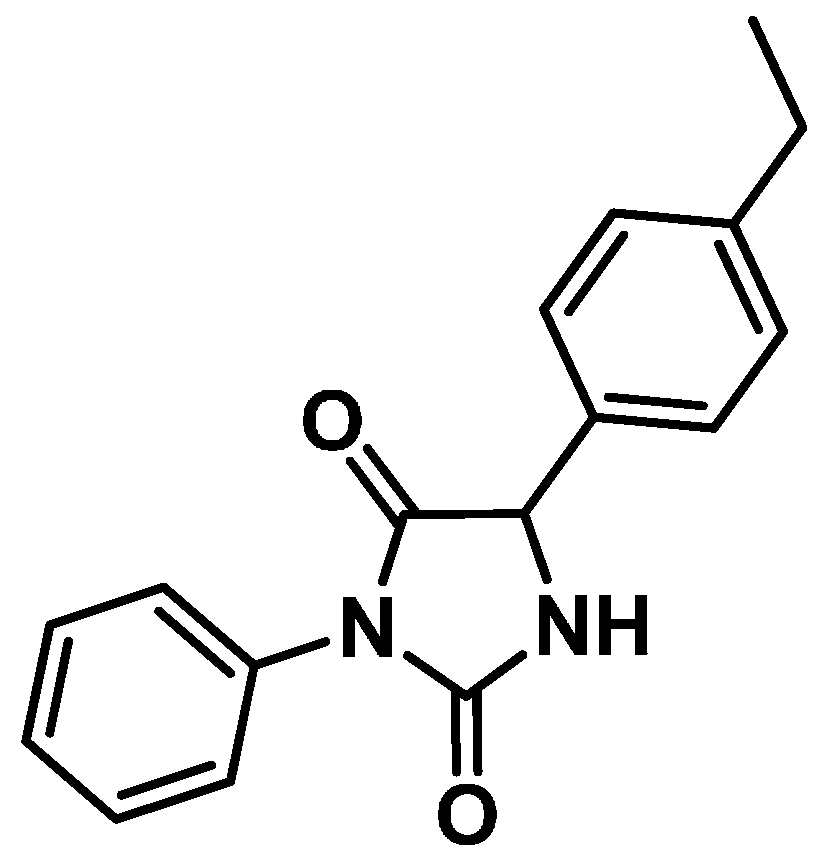
Antinociceptive Effect of Hydantoin 3-Phenyl-5-(4-ethylphenyl)-imidazolidine-2,4-dione in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acute Toxicity
2.2. Behavioral Pharmacological Screening
2.3. Rotarod Test
| Treatment | Dose (mg/Kg, i.p) | Time Spent on the Rotarod (Minutes) | ||
|---|---|---|---|---|
| 30 | 60 | 120 | ||
| Control (10 mL/kg) | - | 179.3 ± 0.7 | 167.8 ± 11.2 | 173.0 ± 6.0 |
| IM-3 | 50 | 136.4 ± 19.7 | 162.4 ± 15.5 | 161.4 ± 18.6 |
| 100 | 133.9 ± 18.9 | 159.4 ± 18.8 | 177.9 ± 1.4 | |
| 200 | 146.9 ± 18.0 | 163.6 ± 15.2 | 179.4 ± 0.6 | |
2.4. Elevated Plus-Maze Test
| Treatment | Dose (mg/kg, i.p.) | Open Arms | |
|---|---|---|---|
| Number of Entries | Duration (s) | ||
| Control (10 mL/kg) | - | 2.5 ± 0.8 | 41.2 ± 14.3 |
| IM-3 | 50 | 3.2 ± 0.6 | 41.5 ± 14.3 |
| 100 | 2.2 ± 0.8 | 22.0 ± 9.5 | |
| Diazepam | 0.5 | 6.4 ± 0.6 ** | 90.7 ± 14.2 ** |
2.5. Acetic Acid-Induced Writhing Test
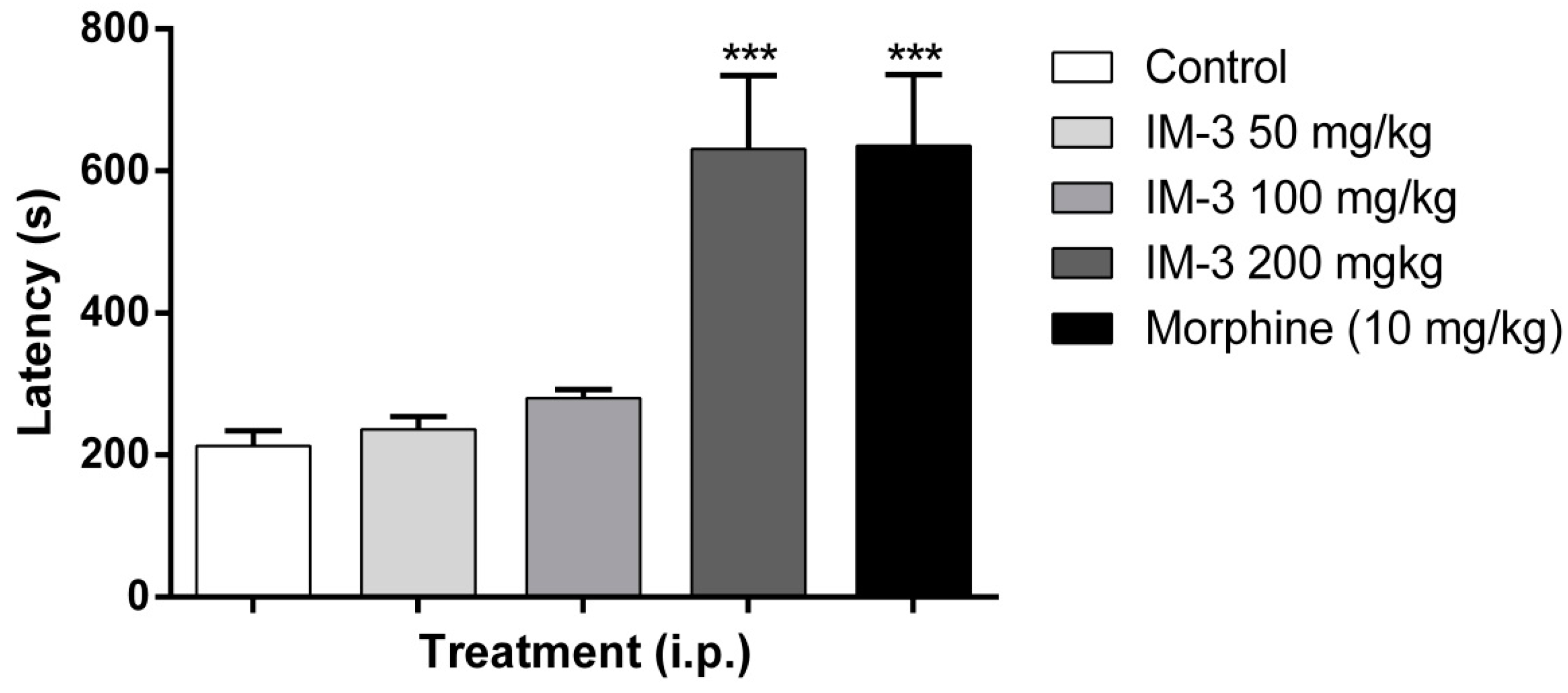
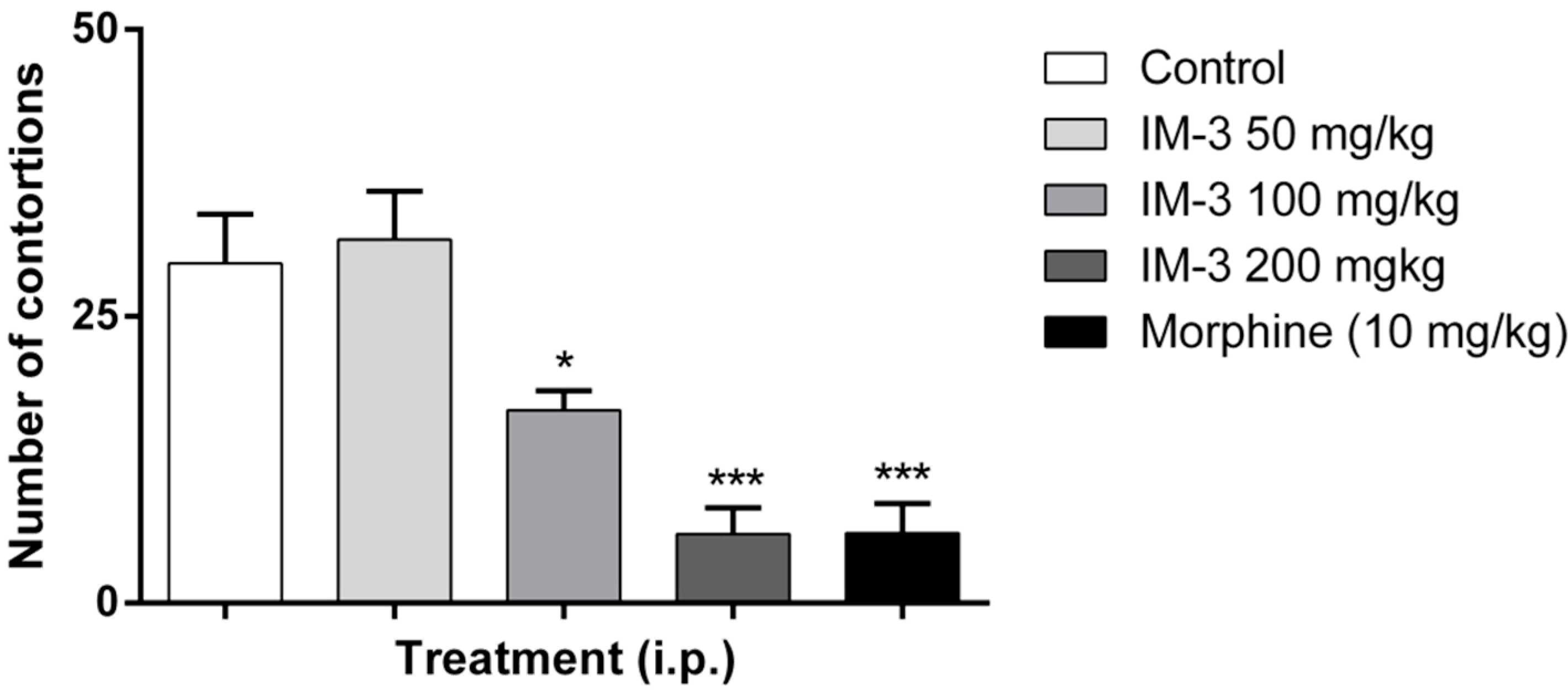
2.6. Formalin Test
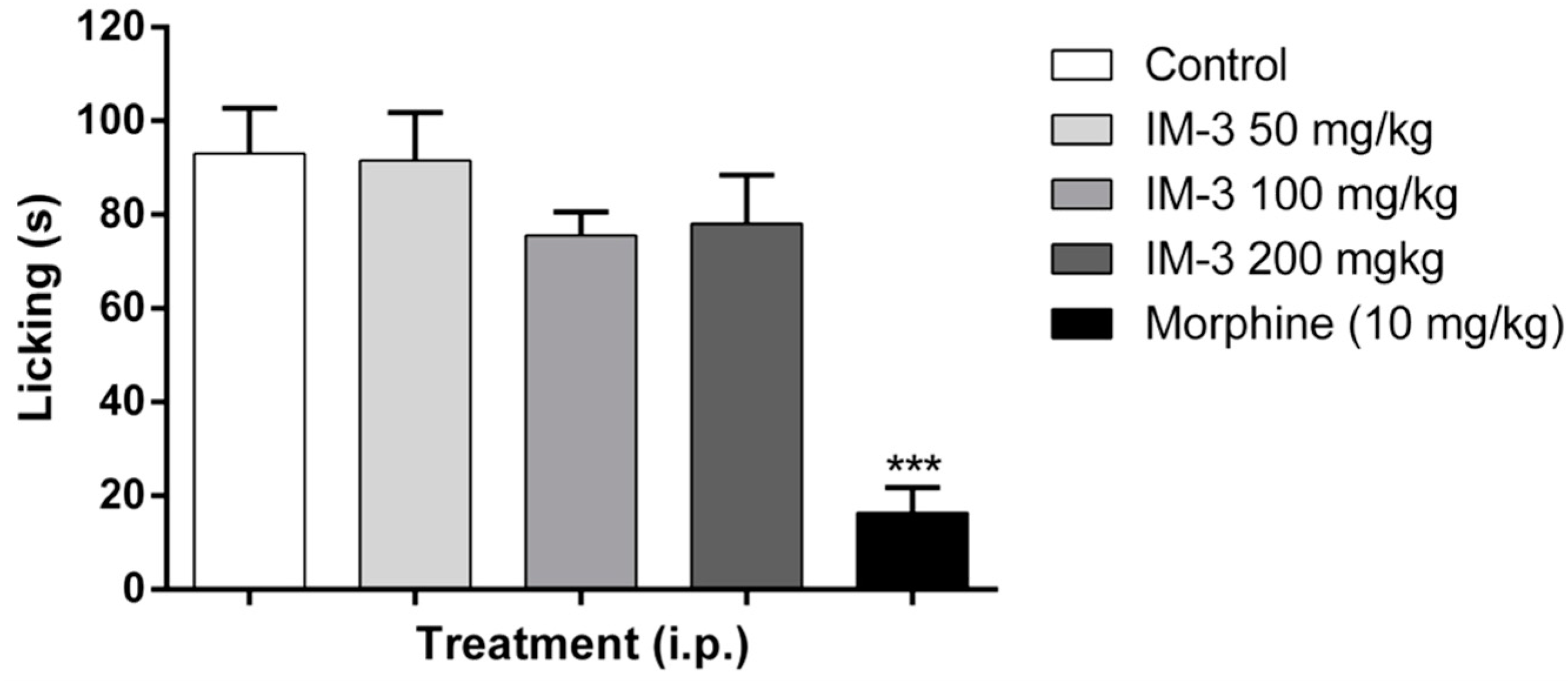
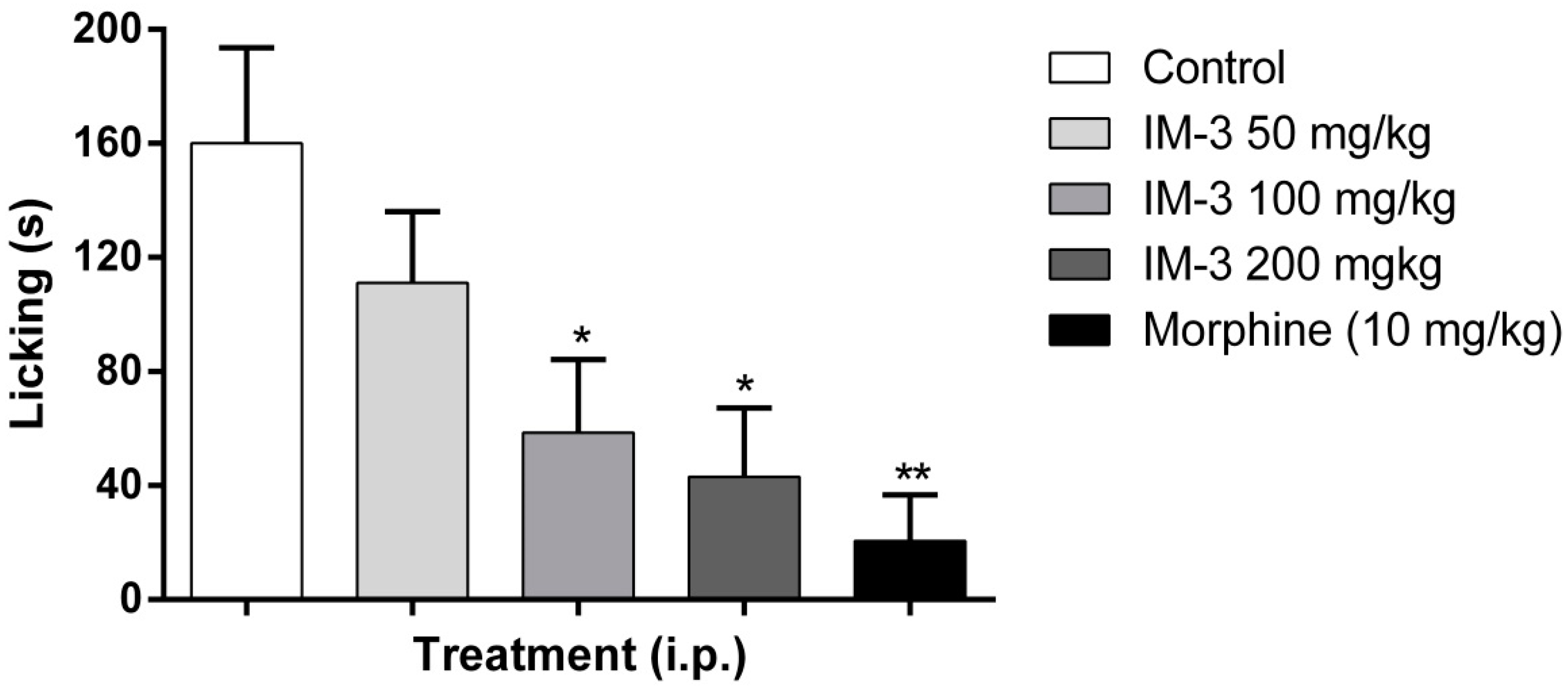
2.7. Hot Plate Test
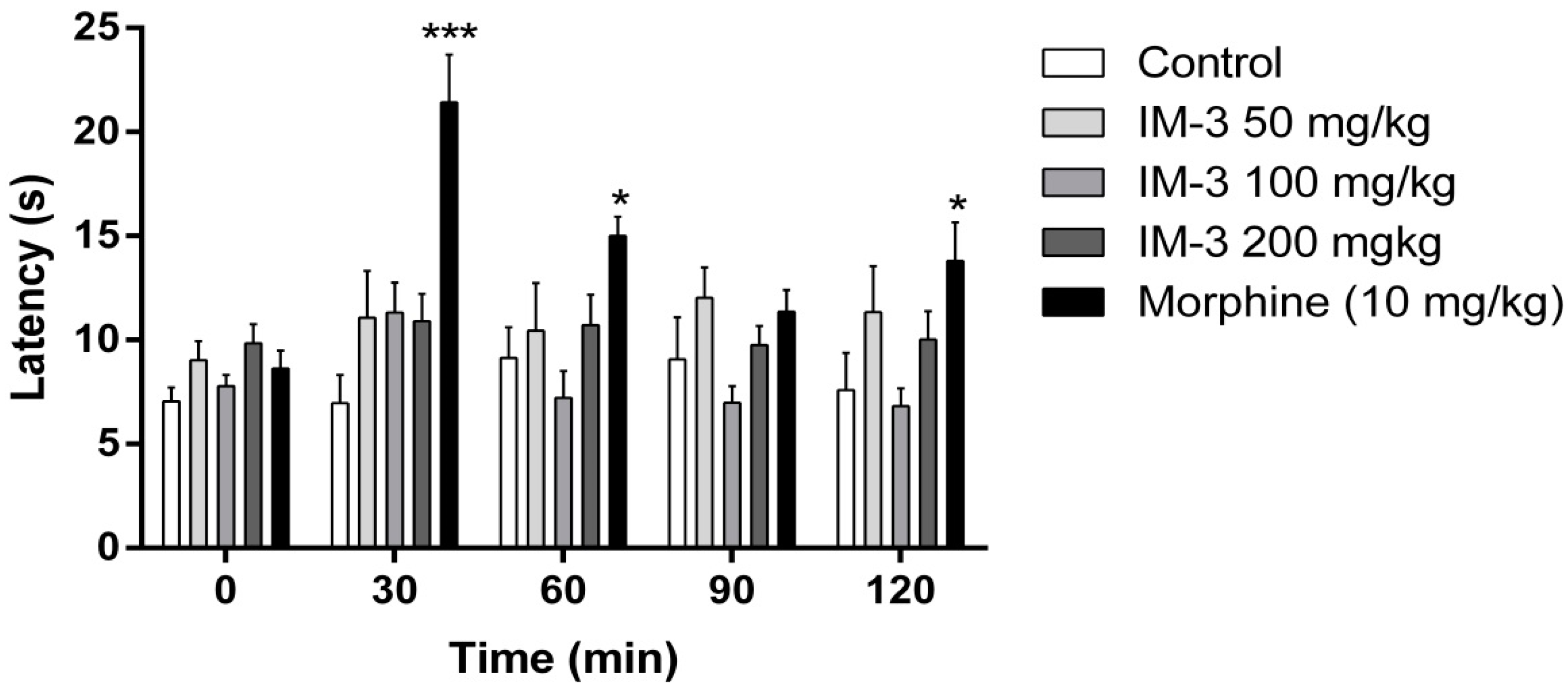
2.8. Discussion
3. Experimental Section
3.1. Animals
3.2. Drugs and Treatments
3.3. Acute Toxicity
3.4. Behavioral Pharmacological Screening
3.5. Motor Incoordination (Rotarod Test)
3.6. Anxiolytic Activity (Elevated Plus-Maze Test)
3.7. Antinociceptive Activity Evaluation of IM-3
3.7.1. Acetic Acid-Induced Writhing Test
3.7.2. Formalin Test
3.7.3. Hot Plate Test
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dohi, T.; Morita, K.; Kitayama, T.; Motoyama, N.; Morioka, N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol. Ther. 2009, 123, 54–79. [Google Scholar] [CrossRef] [PubMed]
- Miraucourt, L.S.; Dallel, R.; Voisin, D.L. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS One 2007, 2, e1116. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Rhudy, J.L.; Bartley, E.J.; Palit, S.; Kerr, K.L.; Kuhn, B.L.; Martin, S.L.; DelVentura, J.L.; Terry, E.L. Do sex hormones influence emotional modulation of pain and nociception in healthy women? Biol. Psychol. 2013, 94, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L.; Cruz-Almeida, Y.; Glover, T.L.; King, C.D.; Goodin, B.R.; Sibille, K.T.; Bartley, E.J.; Herbert, M.S.; Sotolongo, A.; Fessler, B.J.; et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J. Pain 2014, 15, 272–282. [Google Scholar]
- Craft, R.M. Modulation of pain by estrogens. Pain 2007, 132, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Horsham, S.; Chung, M.C. Investigation of the relationship between trauma and pain catastrophising: The roles of emotional processing and altered self-capacity. Psychiatry Res. 2013, 208, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.C. Individual differences in the subjective experience of pain: New insights into mechanisms and models. Headache 2010, 50, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Porreca, F. Challenges in the development of novel treatment strategies for neuropathic pain. NeuroRx 2005, 2, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Lira, B.F.; Athayde-Filho, P.F.; Miller, J.; Simas, A.M.; Dias, A.F.; Vieira, M.J. Synthesis and characterization of some new mesoionic 1,3-thiazolium-5-thiolates via cyclodehydration and in situ 1,3-dipolar cycloaddition/cycloreversion. Molecules 2002, 7, 791–800. [Google Scholar] [CrossRef]
- De Athayde-Filho, P.F.; Miller, J.; Simas, A.M.; Lira, B.F.; de Souza Luis, J.A.; Zuckerman-Schpector, J. Synthesis, characterization and crystallographic studies of three 2-aryl-3-methyl-4-aryl-1,3-thiazolium-5-thiolates. Synthesis (Stuttgart) 2003, 5, 685–690. [Google Scholar] [CrossRef]
- Lira, B.F.; Miller, J.; Simas, A.M.; Athayde-Filho, P.F.; Dias, A.F.; Silva, R.O.; Oliveira, V.C. Synthesis and complete assignments of 1H and 13C NMR spectra of mesoionic 2-(ptrifluoromethylphenyl)-3-methyl-4-(p-tolyl)-1,3-thiazolium-5-thiolate and 2-(p-chlorophenyl)-3-methyl–4-(p-isopropylphenyl)-1,3-thiazolium-5-thiolate. ARKIVOC 2004, 6, 12–21. [Google Scholar] [CrossRef]
- Bosco, C.A.C.; Maciel, G.S.; Rakov, N.; de Araújo, C.B.; Acioli, L.H.; Simas, A.M.; Athayde-Filho, P.F.; Miller, J. Probing the nuclear susceptibility of mesoionic compounds using two-beam coupling with chirp-controlled pulses. Chem. Phys. Lett. 2007, 449, 101–106. [Google Scholar] [CrossRef]
- Pilla, V.; de Araújo, C.B.; Lira, B.F.; Simas, A.M.; Miller, J.; Athayde-Filho, P.F. Nonlinear absorption of new mesoionic compounds. Opt. Commun. 2006, 264, 225–228. [Google Scholar] [CrossRef]
- Mantegazza, M.; Curia, G.; Biagini, G.; Ragsdale, D.S.; Avoli, M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010, 9, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, S.; Singh, G.P.; Singh, G.; Kumar, M. Phenytoin as a co-analgesic in cancer pain. J. Pain Symptom Manag. 1992, 7, 209–213. [Google Scholar] [CrossRef]
- Imam, S.H.; Landry, K.; Kaul, V.; Gambhir, H.; John, D.; Kloss, B. Free phenytoin toxicity. Am. J. Emerg. Med. 2014, 32, 1301.e3–1301.e4. [Google Scholar] [CrossRef]
- Guerra, A.S.; Malta, D.J.; Laranjeira, L.P.; Maia, M.B.; Colaço, N.C.; Lima, M.C.; Galdino, S.L.; Pitta, I.R.; Gonçalves-Silva, T. Anti-inflammatory and antinociceptive activities of indole-imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Gerona-Navarro, G.; González-Muñiz, R.; Fernández-Carvajal, A.; González-Ros, J.M.; Ferrer-Montiel, A.; Carreño, C.; Albericio, F.; Royo, M. Solid-phase synthesis of a library of amphiphatic hydantoins. Discovery of new hits for TRPV1 blockade. ACS Comb. Sci. 2011, 13, 458–465. [Google Scholar] [CrossRef]
- Luis, J.A.S.; Barbosa-Filho, J.M.; Lira, B.F.; Medeiros, I.A.; de Morais, L.C.S.M.L.; dos Santos, A.F.; de Oliveira, C.S.; Athayde-Filho, P.F. Synthesis of new imidazolidin-2,4-dione and 2-thioxoimidazolidin- 4-ones via C-phenylglycine derivatives. Molecules 2010, 15, 128–137. [Google Scholar] [CrossRef]
- Almeida, R.N.; Falcão, A.C.G.M.; Diniz, R.S.T.; Quintans-Júnior, L.J.; Polari, R.M.; Barbosa-Filho, J.M.; Agra, M.F.; Duarte, J.C.; Ferreira, C.D.; Antoniolli, A.R.; et al. Methodology for evaluation plants with activity in the central nervous system and some experimental data. Rev. Bras. Farm. 1999, 80, 72–76. [Google Scholar]
- Almeida, F.R.C.; Oliveira, F.S. Avaliação de drogas analgésicas de ação central. In Psicofarmacologia: Fundamentos Práticos; Almeida, R.N., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2006; pp. 179–188. [Google Scholar]
- Wong, P.T.; Tan, S.F. Pharmacological profiles of 5-phenylmethylenehydantoin and its methoxy substituted derivatives. Jpn. J. Pharmacol. 1989, 49, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Vogel, H.G.; Vogel, W.H. Drug Discovery and Evaluation, Pharmacological Assays; Springer: Berlin, Germmany, 1997; pp. 370–371. [Google Scholar]
- Deraedt, R.; Jouquey, S.; Delevalle, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.D.G.; Nakamura, M.; Ferreira, S.H. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz. J. Med. Biol. Res. 1988, 21, 341–343. [Google Scholar] [PubMed]
- Shibata, M.; Ohbubo, T.; Takahashi, H.; Inoki, R. Modified formalin test: Characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Tjölsen, A.; Hole, K. Animals models of analgesia. In The Pharmacology of Pain; Handbook of Experimental Pharmacology Series, v. 25; Dickenson, A.H., Besson, J.M., Eds.; Springer-Verlag: Berlin, Germany, 1997; pp. 1–20. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Woolfe, H.G.; MacDonald, A.D. The evaluation of the analgesic action of pethidine hydrochloride. J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Sudo, R.T.; Calasans-Maia, J.A.; Galdino, S.L.; Lima, M.C.; Zapata-Sudo, G.; Hernandes, M.Z.; Pitta, I.R. Interaction of morphine with a new alpha2-adrenoceptor agonist in mice. J. Pain 2010, 11, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F.A. Simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. 1957, 46, 207–208. [Google Scholar]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in mouse. Br. J. Pharmacol. 1968, 32, 295–310. [Google Scholar]
- Souza, M.M.; Madeira, A.; Berti, C.; Krogh, R.; Yunes, R.A.; Cechinel-Filho, V. Antinociceptive properties of the methanolic extract obtained from Ipomoea pes-caprae (L.) R. Br. J. Ethnopharmacol. 2000, 69, 85–90. [Google Scholar] [CrossRef]
- Silva, M.G.; Oliveira, F.S.; Quintans-Júnior, L.J.; Oliveira, T.M.L.; Diniz, M.F.F.M. Investigação do efeito analgésico central e anti-inflamatório de Conocliniopsis prasiifolia (DC) R.M. King & H. Robinson em Roedores. Acta Farm. Bonaer. 2005, 24, 533–537. [Google Scholar]
- Al-ghamdi, M.S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Sample availability: Samples of the compound hydantoin 3-phenyl-5-(4-ethylphenyl)-imidazolidine-2,4-dione are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Queiroz, R.B.; De Carvalho, F.L.; Fonsêca, D.V.d.; Barbosa-Filho, J.M.; Salgado, P.R.R.; Paulo, L.L.; De Queiroz, A.B.M.; Pordeus, L.C.d.M.; De Souza, S.A.; Souza, H.D.d.S.; et al. Antinociceptive Effect of Hydantoin 3-Phenyl-5-(4-ethylphenyl)-imidazolidine-2,4-dione in Mice. Molecules 2015, 20, 974-986. https://doi.org/10.3390/molecules20010974
De Queiroz RB, De Carvalho FL, Fonsêca DVd, Barbosa-Filho JM, Salgado PRR, Paulo LL, De Queiroz ABM, Pordeus LCdM, De Souza SA, Souza HDdS, et al. Antinociceptive Effect of Hydantoin 3-Phenyl-5-(4-ethylphenyl)-imidazolidine-2,4-dione in Mice. Molecules. 2015; 20(1):974-986. https://doi.org/10.3390/molecules20010974
Chicago/Turabian StyleDe Queiroz, Ronaldo Bezerra, Fabíola Lélis De Carvalho, Diogo Vilar da Fonsêca, José Maria Barbosa-Filho, Paula Regina Rodrigues Salgado, Luciano Leite Paulo, Ana Bárbara Maroja De Queiroz, Liana Clébia de Morais Pordeus, Severino Araújo De Souza, Helivaldo Diogenes da Silva Souza, and et al. 2015. "Antinociceptive Effect of Hydantoin 3-Phenyl-5-(4-ethylphenyl)-imidazolidine-2,4-dione in Mice" Molecules 20, no. 1: 974-986. https://doi.org/10.3390/molecules20010974
APA StyleDe Queiroz, R. B., De Carvalho, F. L., Fonsêca, D. V. d., Barbosa-Filho, J. M., Salgado, P. R. R., Paulo, L. L., De Queiroz, A. B. M., Pordeus, L. C. d. M., De Souza, S. A., Souza, H. D. d. S., Lira, B. F., & De Athayde-Filho, P. F. (2015). Antinociceptive Effect of Hydantoin 3-Phenyl-5-(4-ethylphenyl)-imidazolidine-2,4-dione in Mice. Molecules, 20(1), 974-986. https://doi.org/10.3390/molecules20010974





