2.1. Isolation of Apios Tuber Lectin (ATL)
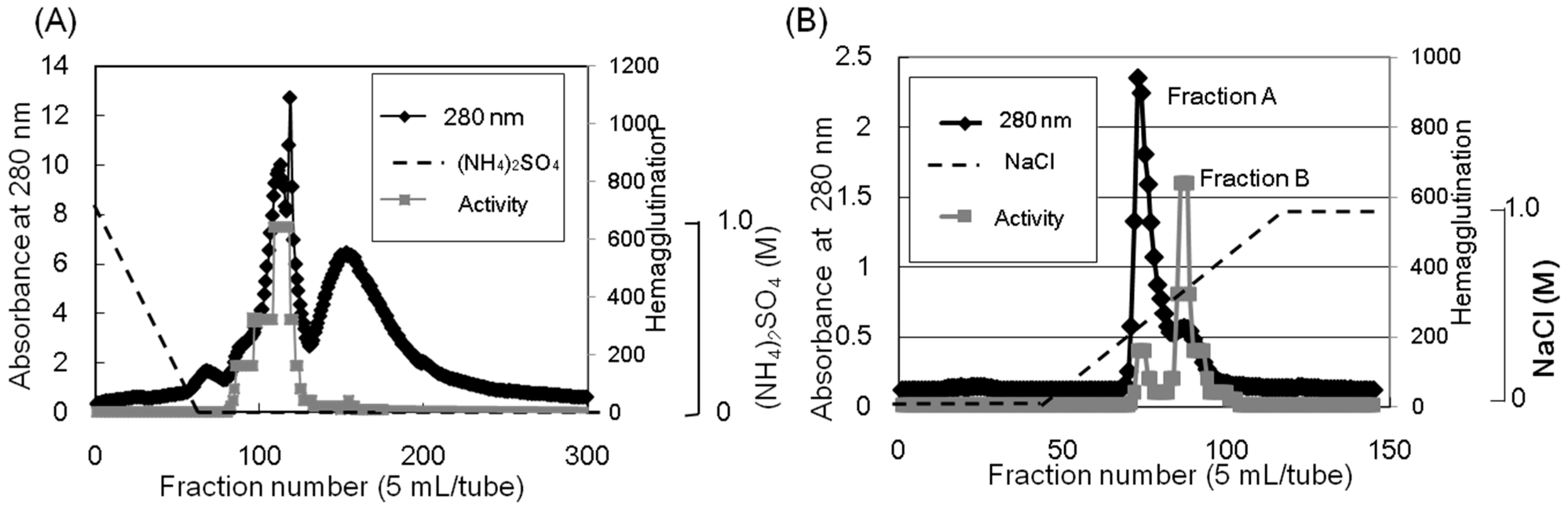
Apios tuber lectin was isolated by hydrophobic chromatography and anion exchange chromatography (
Figure 1). Two major peaks, Fractions A and B, were obtained by anion exchange chromatography with yields of 240 mg and 60 mg from 200 g of the tubers, respectively (
Table 1). Fraction B showed strong hemagglutination activity with the minimum concentration of 4 μg/mL required for the activity against rabbit erythrocytes. The isolated
Apios tuber lectin (Fraction B), named ATL, gave a single protein band of 28 kDa during SDS-PAGE in the presence and absence of 2-mercaptethanol (
Figure 2). Fraction A also gave a single band of 28 kDa during SDS-PAGE, but showed only marginal hemagglutination activity. As shown in
Figure 2, ATL and Fraction A protein (band c) were major components in the extract of
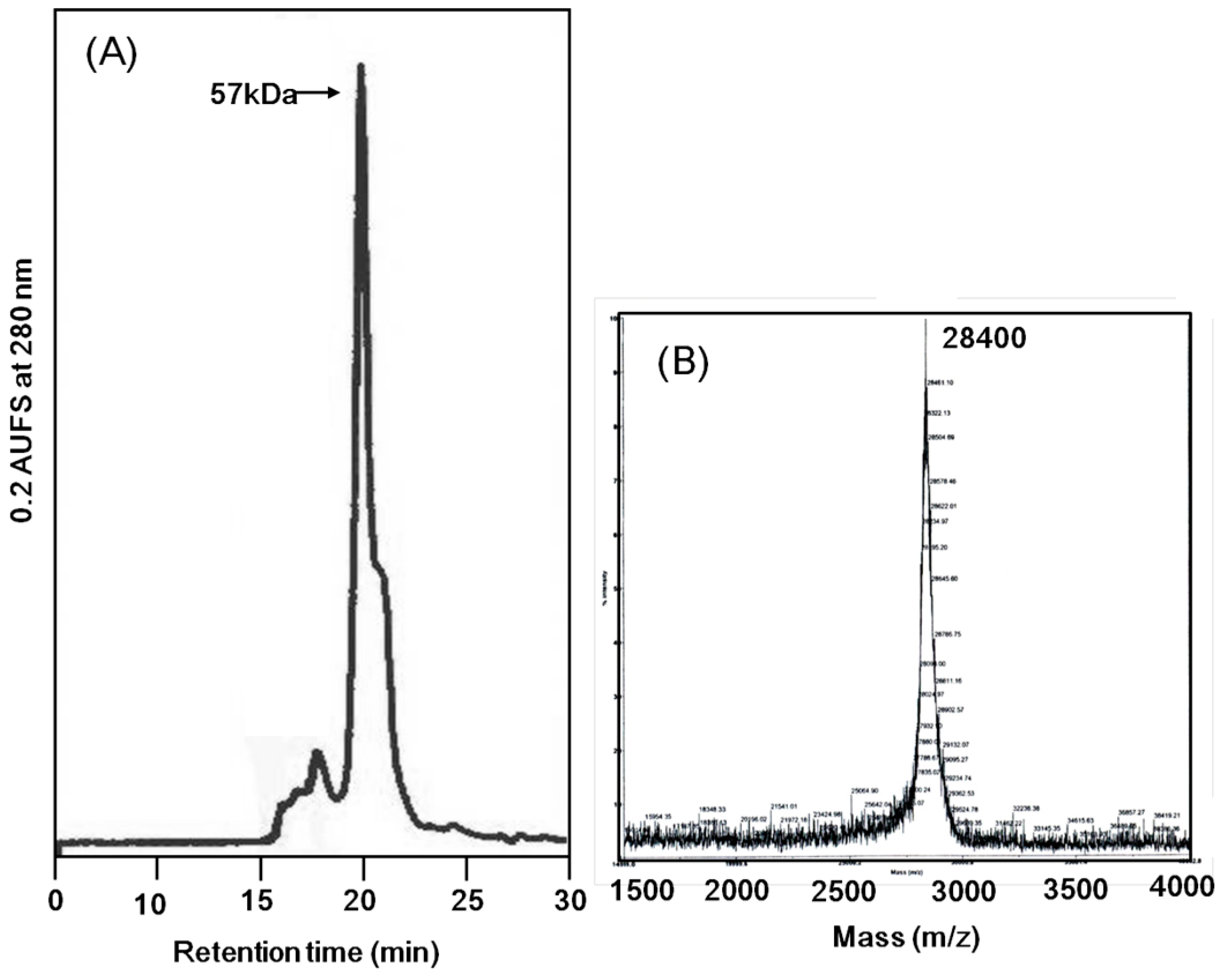
Apios tubers. The apparent molecular weight of ATL was estimated to be 57 kDa by size exclusion chromatography (
Figure 3A). The MALDI-TOF mass spectrometry of ATL gave a peak of 28.4 kDa (
Figure 3B). Fraction A protein showed a similar molecular mass. These results indicate that both ATL and Fraction A protein are composed of non-covalently bound homodimers.
Figure 1.
Purification of the Apios tuber lectin (ATL). (A) The protein fraction obtained by 40% saturated ammonium sulfate precipitation was separated by hydrophobic chromatography on a Toyopearl phenyl-650M column (2.8 × 16 cm) pre-equilibrated with 1.0 M ammonium sulfate in 50 mM Tris-HCl buffer (pH 8.0). Proteins were eluted with a decreasing linear gradient of ammonium sulfate (1.0–0 M) in the same buffer. Factions showing hemagglutination activity were collected and dialyzed against 50 mM Tris-HCl buffer (pH 8.0). (B) The dialysate was subjected to anion-exchange chromatography on a Toyopearl DEAE-650M column (2.8 × 32 cm) pre-equilibrated with 50 mM Tris-HCl (pH 8.0) and eluted with a linear gradient of NaCl (0 to 1.0 M) in the same buffer. Fractions A and B (ATL) were dialyzed against distilled water and lyophilized.
Figure 1.
Purification of the Apios tuber lectin (ATL). (A) The protein fraction obtained by 40% saturated ammonium sulfate precipitation was separated by hydrophobic chromatography on a Toyopearl phenyl-650M column (2.8 × 16 cm) pre-equilibrated with 1.0 M ammonium sulfate in 50 mM Tris-HCl buffer (pH 8.0). Proteins were eluted with a decreasing linear gradient of ammonium sulfate (1.0–0 M) in the same buffer. Factions showing hemagglutination activity were collected and dialyzed against 50 mM Tris-HCl buffer (pH 8.0). (B) The dialysate was subjected to anion-exchange chromatography on a Toyopearl DEAE-650M column (2.8 × 32 cm) pre-equilibrated with 50 mM Tris-HCl (pH 8.0) and eluted with a linear gradient of NaCl (0 to 1.0 M) in the same buffer. Fractions A and B (ATL) were dialyzed against distilled water and lyophilized.
Table 1.
Summary of the purification of Apios tuber lectin (ATL).
Table 1.
Summary of the purification of Apios tuber lectin (ATL).
| Purification Step | Protein (mg) | Total Activity (Units) | Recovery (%) |
|---|
| Extract | 12,000 | 307,200 | 100 |
| Ammonium sulfate precipitation | | | |
| 20% saturation | | 29,593 | 10 |
| 40% saturation | | 204,800 | 67 |
| Hydrophobic chromatography | 500 | 196,608 | 64 |
| Ion-exchange chromatography | | | |
| Fraction A | 240 | 10,752 | 4 |
| Fraction B (ATL) | 60 | 210,944 | 69 |
Figure 2.
SDS-PAGE patterns of Apios tuber proteins in 15% acrylamide gel under reducing condition. Lanes M: Protein markers, E: Apios protein fraction obtained by ammonium sulfate precipitation, A: Fraction A; B: Fraction B (Apios tuber lectin).
Figure 2.
SDS-PAGE patterns of Apios tuber proteins in 15% acrylamide gel under reducing condition. Lanes M: Protein markers, E: Apios protein fraction obtained by ammonium sulfate precipitation, A: Fraction A; B: Fraction B (Apios tuber lectin).
Figure 3.
Molecular mass determination of Apios tuber lectin. (A) Size exclusion chromatography on a PC200S (N) column (5 μm, 7.8 × 300 nm) in 50 mM HEPES (pH 6.9) containing 0.25 M NaCl and 5 mM CaCl2 as the mobile phase. Flow rate: 0.8 mL/min; UV detection: 280 nm. (B) MALDI-TOF mass spectral analysis.
Figure 3.
Molecular mass determination of Apios tuber lectin. (A) Size exclusion chromatography on a PC200S (N) column (5 μm, 7.8 × 300 nm) in 50 mM HEPES (pH 6.9) containing 0.25 M NaCl and 5 mM CaCl2 as the mobile phase. Flow rate: 0.8 mL/min; UV detection: 280 nm. (B) MALDI-TOF mass spectral analysis.
2.2. Hemagglutination Activity of ATL
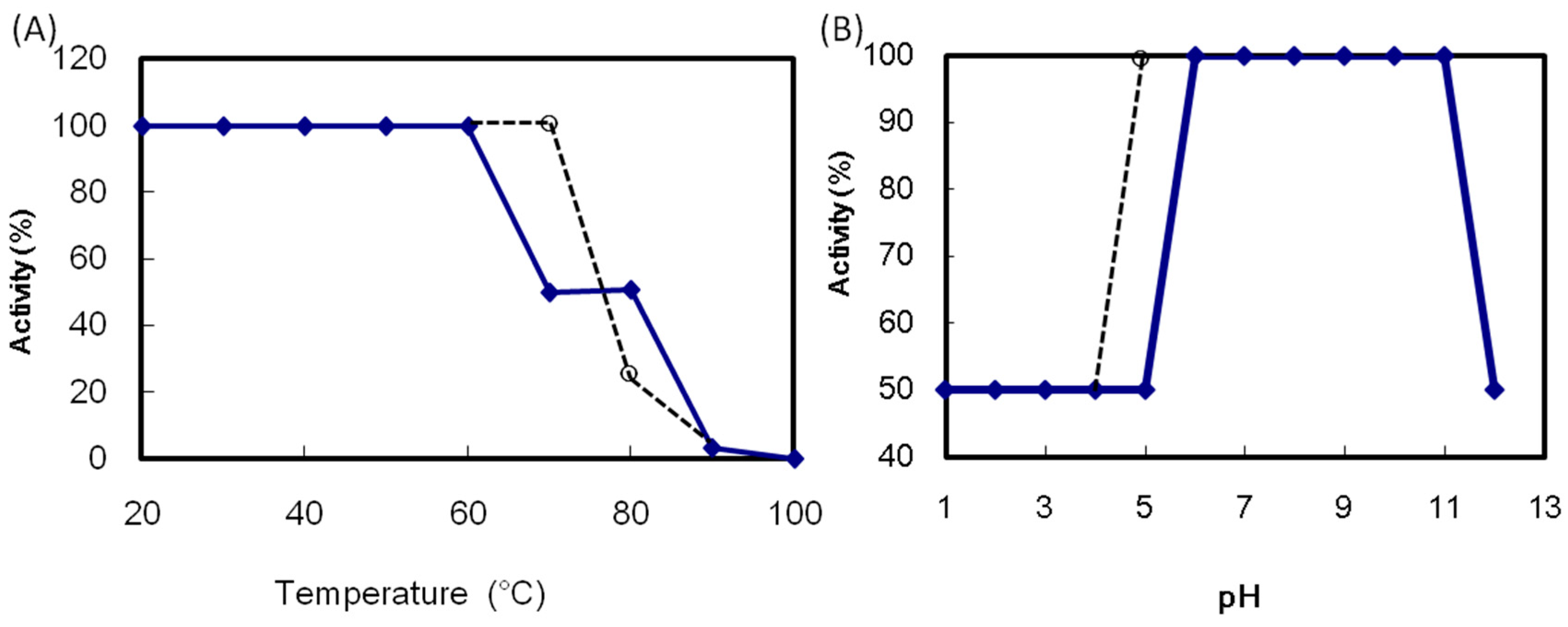
ATL maintained its maximum hemagglutination activity after incubation at 60 °C for 30 min (
Figure 4A). The activity decreased above 70 °C and was lost after heating at 100 °C for 30 min. The ATL activity in the pH 6.0 to 11.0 was maintained (
Figure 4B). The thermal and pH stability of ATL was comparable to those of SBA. The ATL hemagglutination activity was decreased by 75% after EDTA treatment (
Table 2). The addition of Ca
2+ led to the recovery of the maximum activity, indicating the requirement of Ca
2+ for the hemagglutination activity of ATL. On the other hands, Mg
2+ did not affect the activity. It is known that legume lectins are metalloproteins which contain Mn
2+ and Ca
2+ ion. Both divalent cations are required for the functional conformation of the monosaccharide binding site [
2]. The present result suggests that ALT does not require Mn
2+ for the activity but require Ca
2+ to express full activity.
Figure 4.
Effect of temperature and pH on the hemagglutination activity of Apios tuber lectin (ATL). (A) ATL was incubated at the indicated temperatures for 30 min; (B) ATL was incubated at various pH values overnight. After adjusting the pH to 7, the hemagglutination activity was measured. Solid line: ATL, broken line: Soybean lectin (SBA).
Figure 4.
Effect of temperature and pH on the hemagglutination activity of Apios tuber lectin (ATL). (A) ATL was incubated at the indicated temperatures for 30 min; (B) ATL was incubated at various pH values overnight. After adjusting the pH to 7, the hemagglutination activity was measured. Solid line: ATL, broken line: Soybean lectin (SBA).
Table 2.
Requirement of divalent metal ions for hemagglutination activity. The activity recovered was indicated when the non-treated activity was 100%.
Table 2.
Requirement of divalent metal ions for hemagglutination activity. The activity recovered was indicated when the non-treated activity was 100%.
| Metal Ion (10 mM) | EDTA | Activity (%) |
|---|
| (−) | (−) | 100 |
| (−) | (+) | 25 |
| Ca2+, Mg2+ | (+) | 100 |
| Ca2+ | (+) | 100 |
| Mg2+ | (+) | 25 |
2.3. N-Terminal Sequencing of Proteins Contained in Apios Tubers
Five major and a few minor bands could be detected when the extract of
Apios tubers was subjected to SDS-PAGE (
Figure 2, lane E). The N-terminal amino acid sequence of each protein band was analyzed by Edman degradation on a gas-phase protein sequencer after electroblotting onto a PVDF membrane. The N-terminal amino acid sequences of bands (a), (b), and (d) were EDNNELQNYVPVYVMLPLE, ERLNPGDIYVPVYVMLPLEL, and NPVLDMDGDLVQNGGAYYILPVIRGKGGGIERAVTGKETTPLYTVVQS, respectively. They are similar to those of β-amylase from soybean (85% homology), β-amylase from
Arabidopsis thaliana (80% homology), and Kunitz type trypsin inhibitor from soybean (72% homology), respectively. This is the first time that the presence of β-amylase- and Kunitz type trypsin inhibitor-like protein are shown in
Apios tubers, though the Bowman-Birk type trypsin inhibitor, corresponding to band e, has been reported by Zhang
et al. [
20,
21].
The first 30 amino acid residues of band (c) containing ATL and Fraction A protein were determined to be AKLPFFSFNLDRFFPNEPNLIFQGDAKASS, which was completely consistent with the deduced
N-terminal amino acid sequence of
Apios americana lectin (AAL) [
22]. Both ATL from SDS-PAGE and Fraction A protein separated by ion-exchange chromatography also showed the same
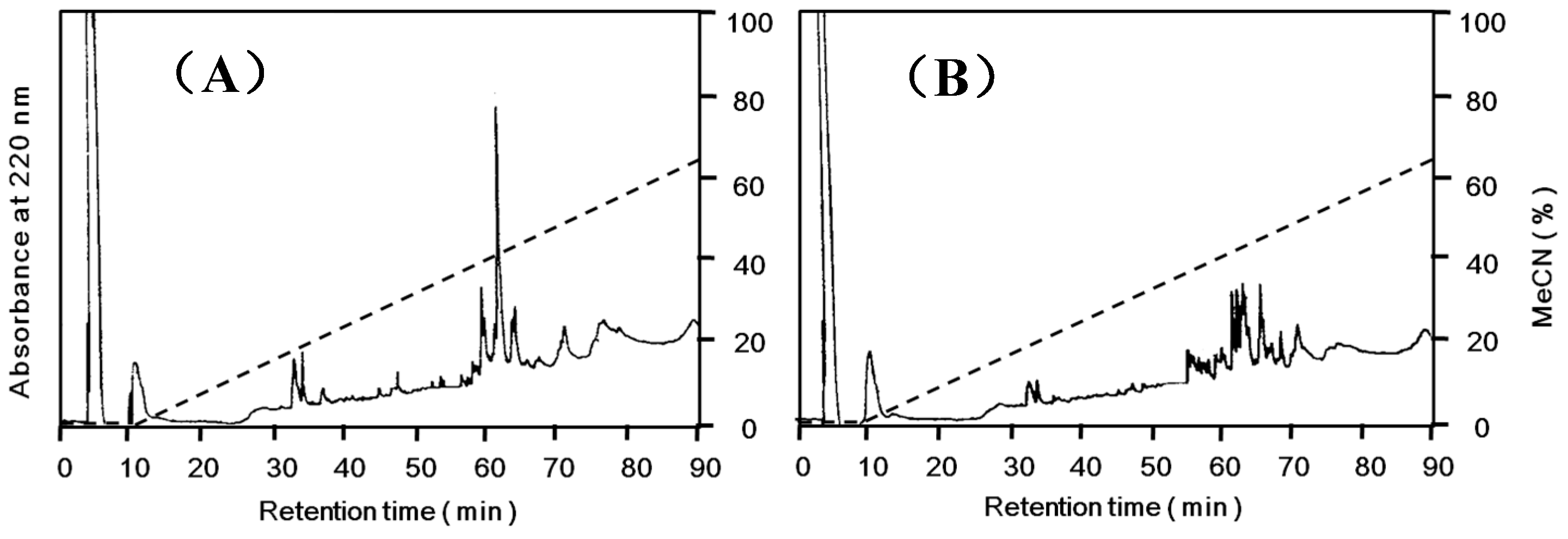
N-terminal amino acid sequences, however, they gave slightly different peptide maps (
Figure 5).
Figure 5.
Peptide maps of Apios tuber lectin (ATL) and Fraction A. Each protein was reduced and S-carboxamidomethylated, and digested with arginylendpeptidase. Peptides were separated by reversed-phase HPLC on TSKgel ODS 120T (5 μm, 4.6 × 250 mm) using a linear gradient of acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 1.0 mL/min. (A) ATL; (B) Fraction A.
Figure 5.
Peptide maps of Apios tuber lectin (ATL) and Fraction A. Each protein was reduced and S-carboxamidomethylated, and digested with arginylendpeptidase. Peptides were separated by reversed-phase HPLC on TSKgel ODS 120T (5 μm, 4.6 × 250 mm) using a linear gradient of acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 1.0 mL/min. (A) ATL; (B) Fraction A.
The amino acid sequence of
Apios tuber lectin (ATL) was determined by direct protein sequencing of peptides generated by digestion of
S-carboxamidomethylated (CAM)-ATL with several proteases,
Achromobacter lysyl-endopeptidase,
Staphylococcis aurous V8 protease, and mouse submandibular arginylendopeptidase. The digests were separated by RP-HPLC on a TSKgel ODS 120T column (4.6 × 250 mm) using a linear gradient of acetonitrile in 0.1% trifluoroacetic acid. The peptide fragments were subjected to protein sequencing by a protein sequencer and MALDI-TOF-MS spectrometry. The amino acid sequence of ATL (the sequences of the fragments are not shown) was consisted with the deduced amino acid sequence of
Apios tuber lectin (AAL) cDNA reported by Kouzuma
et al. [
22], though the
C-terminal amino acid sequence of ATL could not be determined in this study, due to the absence of the corresponding peptide fragments (
Figure 6).
ATL showed a significant sequence homology to other legume lectins, such as soybean lectin (SBA),
Concanavalin A (ConA), and
Ulex europeus agglutinin-II (UEA-II), with 30%–40% amino acid identity. The legume lectins show a characteristic four loop structures,
i.e., loops A, B, C and D, which are conserved among legume lectins and might be related to carbohydrate-binding properties. Loop D seems to be correlated with carbohydrate-binding specificity [
23]. The loop D of mannose-binding lectins (ConA) consists of 18 amino acid residues, whereas that of
N-acetyl galactosamine-binding lectins (SBA and UEA-II) consists of more than 18–19 amino acid residues. ATL, showing glucosamine- and galactosamine-binding activity, has 23 amino acid residues in loop D. These results demonstrate that the characteristic loop structures of the legume lectin family allow the formation of binding sites with a wide range of specificities.
Figure 6.
Sequence comparison of
Apios tuber lectin (AAL) with soybean lectin (SBA). Identical amino acid residues are underlined beneath the sequence of SBA. The broken lines under the sequence of AAL are the sequence which was confirmed by direct sequencing of ATL using a protein sequencer in this study. Loops A-D are indicated by boldface letters. Asp (D) 240, Asp (D) 243, and Ser (S) 246 are putative truncating sites of SBA [
24]. The amino acid sequence of AAL deduced from cDNA sequencing is cited from reference [
22].
Figure 6.
Sequence comparison of
Apios tuber lectin (AAL) with soybean lectin (SBA). Identical amino acid residues are underlined beneath the sequence of SBA. The broken lines under the sequence of AAL are the sequence which was confirmed by direct sequencing of ATL using a protein sequencer in this study. Loops A-D are indicated by boldface letters. Asp (D) 240, Asp (D) 243, and Ser (S) 246 are putative truncating sites of SBA [
24]. The amino acid sequence of AAL deduced from cDNA sequencing is cited from reference [
22].
Ion-exchange chromatography (
Figure 1B) revealed that the proteins contained in band c (
Figure 2) consisted of two protein fractions. They showed the same
N-terminal amino acid sequences and the same subunit structures, but different hemagglutination activities and different peptide maps. The ratio of the contents of
Apios tube lectin (ATL) and non-lectin component (Fraction A protein) contained in the fractions was 1:4. Kouzuma
et al. [
22] reported the purification and cDNA cloning of
Apios tuber lectin, named AAL. AAL required the minimal concentration of 125 μg/mL to agglutinate rabbit erythrocytes and the hemagglutinating activity was not inhibited by any monosaccharide. On the other hand, ATL isolated in this study required the minimal concentration of 4μg/mL to agglutinate the erythrocytes and the hemagglutinating activity was inhibited by 63 mM
d-glucosamine and
d-galactosamine. Furthermore, the molecular mass of AAL was estimated to be 30,110 Da by its cDNA sequence and SDS-PAGE, while for ATL a value of 28,400 Da was obtained by MALDI-TOF mass spectrometry. We do not have experimental results to explain these discrepancies between AAL and ATL. Since the major part of the amino acid sequence deduced by cDNA sequencing could be confirmed in this study, the lack of the C-terminal part of ATL may result in the smaller molecular mass. In fact, we could not find the peptide fragments corresponding to the
C-terminal part from the proteolytic digests prepared in this study. One plausible explanation is the truncation of AAL to ATL at the
C-terminal segment. Legume lectins are generally synthesized as a precursor, which subsequently is post-translationally processed into a mature protein. This post-translational processing may consist of proteolytic cleavage of the precursor chain,
C-terminal trimming, removal of covalent carbohydrate, and even ligation of the original
C- and
N-termini [
25]. SBA,
Phaseolus vulgaris lectin (PHA-E), and the lectins from
Dolichos biflorus have been known to contain ragged
C-terminal ends. SBA consisted of at least five isolectins. The subunits of the isolectins, SBA-I, SBA-II, and SBA-III, consisted of 240, 243, 246 amino acid residues. They were generated by cleaving at Asp240, Asp243, and Ser246, respectively, of the intact SBA consisting of 253 amino acid residues [
25].
In addition, the presence of the protein corresponding to the Fraction A protein has not been reported in
Apios tubers. The lack of hemagglutinating activity of Fraction A protein might be due to the different internal amino acid sequence as indicated by peptide mapping (
Figure 5), because the substitution of an amino acid such as a cysteine residue sometimes abolishes the activity even in the case of legume lectins [
22]. Kouzuma
et al. [
22] also reported the purification and cDNA cloning of a lectin-like protein (AALP) from
Apios tubers as a minor side fraction of AAL. AALP had no hemagglutinating activity with a homologous amino acid sequence to AAL. However, the Fraction A protein is a distinct molecule from AALP, because the
N-terminal amino acid sequences (ADSLSFSFKEFTADPEDLIF) and molecular mass (26,305 Da) of AALP are quite different from those of Fraction A protein. Taken together, the discrepancy between the characteristics of ATL and AAL might be caused by the truncation of AAL to ATL at the
C-terminal segment.
2.4. Sugar Binding Specificity
The sugar binding specificity of ATL was examined by the hemagglutination inhibition assay with rabbit erythrocytes (
Table 3). The hemagglutination activity of ATL was inhibited by rather high concentrations of
d-galactosamine and
d-glucosamine at 63 mM,
d-galactose (Gal) and
d-ribose at 125 mM,
d-mannose (Man) at 250 mM, and
d-glucose (Glc), Me-α-
d-mannopyranoside, and
l-fucose (Fuc) at 500 mM. No inhibition was observed with
N-Ac-glucosamine (GlcNAc) and
N-Ac-galactosamine (GalNAc) even at a concentration of 1.0 M.
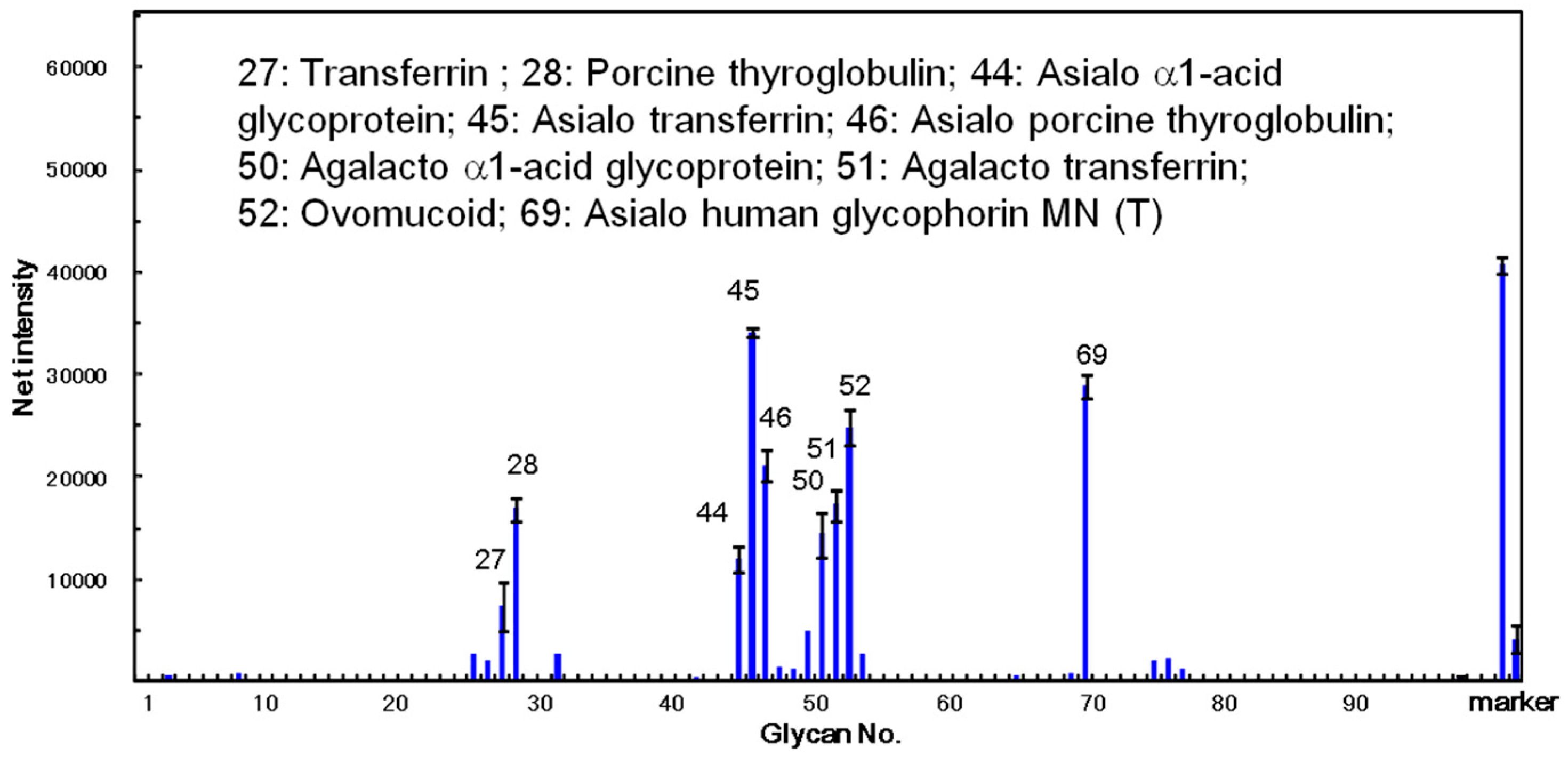
The carbohydrate-binding specificity of ATL was investigated using glycoconjugate microarray assays where 96 glycoconjugates were analyzed at the same time. The fluorescent image data analyzed with the Array Pro analyzer Ver. 4.5 (Media Cybernetics, Inc., Rockville, MD, USA), are shown in
Figure 7. The results clearly demonstrated that ATL mainly bound to the asparagine (
N)-linked sugar chain including its desialylated and agalactosylated glycoproteins, such as α1-acid glycoprotein, transferrin, and glycophorin MN (T). The lectin also showed high affinity against ovomucoid. These results indicate that ATL has a high binding activity against glycoproteins containing various complex sugar chains.
Table 3.
Inhibition of hemagglutination activity of ATL by saccharides.
Table 3.
Inhibition of hemagglutination activity of ATL by saccharides.
| Sugar a | mM |
|---|
| d-Galactosamine | 63 |
| d-Glucosamine | 63 |
| d-Galactose | 125 |
| d-Ribose | 125 |
| d-Mannose | 250 |
| d-Glucose | 500 |
| d-Me-α-d-mannopyranoside | 500 |
| L-Fucose | 500 |
| N-Ac-d-galactosamine | >1000 |
| N-Ac-d-glucosamine | >1000 |
Figure 7.
Specificity profiling of
Apios tuber lectin (ATL). Scan images of ATL (12 ng/well) were analyzed with the Array Pro analyzer ver. 4.5. The net intensity value for each spot was determined as the signal intensity minus the background value. Data are the average ± S.D. of triplicate determinations. Detailed information on the glycans can be found in reference [
26].
Figure 7.
Specificity profiling of
Apios tuber lectin (ATL). Scan images of ATL (12 ng/well) were analyzed with the Array Pro analyzer ver. 4.5. The net intensity value for each spot was determined as the signal intensity minus the background value. Data are the average ± S.D. of triplicate determinations. Detailed information on the glycans can be found in reference [
26].
Many legume lectins contain, in addition to their carbohydrate binding site, one or more binding sites for hydrophobic ligands such as adenine and adenine-related plant hormone [
24]. The broad binding specificity of legume lectins is explained by the fact that substitutions of amino acids involved in sugar binding activity and variations in the length of a particular loop, profoundly change the structure of the binding site without affecting the overall three-dimensional structure of lectin [
27]. The interaction with simple sugars takes place in the monosaccharide-binding site located at the surface of the lectin. On the other hand, the oligosaccharides of glycoproteins interact with the extended carbohydrate-binding site comprising a number of adjacent residues beside a central monosaccharide-binding site. This characteristic feature of the binding sites may be correlated with the unique binding specificity of ATL.
2.5. Effect of ATL on Caco-2 Cell Monolayers
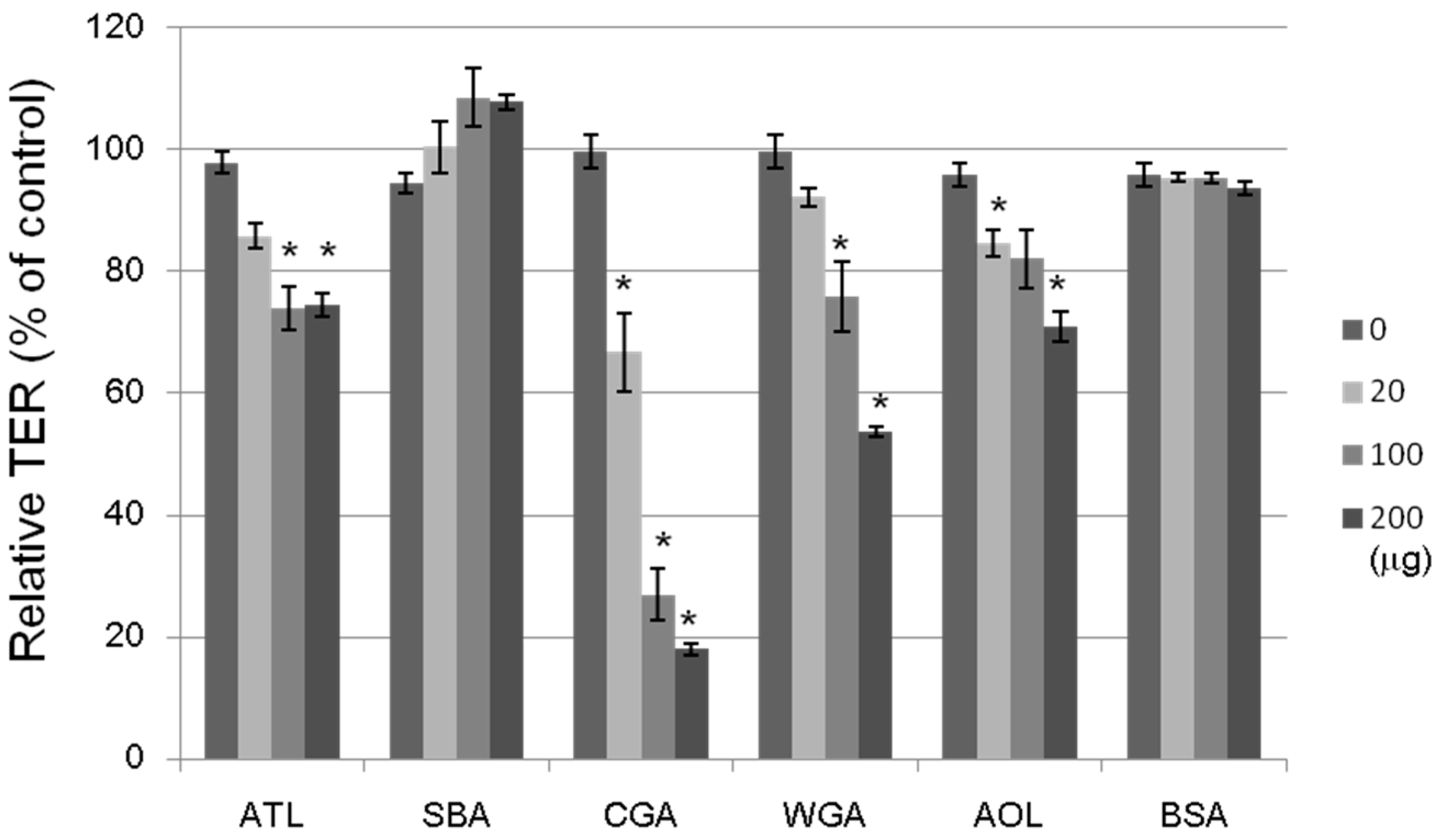
The tightness of the intercellular junctions was evaluated by transepithelial electrical resistance (TER) measurements; a decrease in TER would indicate an increase in paracellular transport, or vice versa (
Figure 8). The non-lectin protein, bovine serum albumin (BSA; 20–200 μg/mL), had no effect on the TER value. The ATL, Japanese jack bean lectin (CGA), wheat germ lectin (WGA), and
Aspergillus oryzae lectin (AOL) treatments at 100 μg/mL each decreased the TER value by 20%–70% after a 2-h incubation period, whereas SBA treatment did not change at all. We investigated the effects of 16 lectins with varying sugar-binding specificities on the transport system across Caco-2 cell monolayers by using four fluorescent markers, whose transport pathways were known. ATL decreased the TER value, indicating an increase in paracellular transport. It has been also shown that other lectins having distinct sugar binding specificities: e.g., CGA for Man, WGA for GlnNAc, and AOL for L-fucose (Fuc) exhibited similar effects. Although the precise mechanisms underlying these modulating effects remain unclear, lectins may modulate the transport system through intracellular signaling, controlling the expression of various proteins, or assembling and disassembling cytoskeletal proteins, as has been reported for other food components.
Figure 8.
Effects of lectins on the TER values of Caco-2 monolayers. The cell monolayer TER value was measured after incubating for 2 h with a lectin (20–200 μg/mL). ATL: Apios tuber lectin, SBA: Soybean lectin, CGA: Japanese jack bean lectin, WGA: Wheat germ lectin, and AOL: Aspergillus oryzae lectin. Bovine serum albumin (BSA) (20–200 μg/mL) was used as a reference. Results are expressed as the percentage of the control value without a lectin at 0 h, and are the mean ± S.D. of three different determinations. * p < 0.05, compared with the control value.
Figure 8.
Effects of lectins on the TER values of Caco-2 monolayers. The cell monolayer TER value was measured after incubating for 2 h with a lectin (20–200 μg/mL). ATL: Apios tuber lectin, SBA: Soybean lectin, CGA: Japanese jack bean lectin, WGA: Wheat germ lectin, and AOL: Aspergillus oryzae lectin. Bovine serum albumin (BSA) (20–200 μg/mL) was used as a reference. Results are expressed as the percentage of the control value without a lectin at 0 h, and are the mean ± S.D. of three different determinations. * p < 0.05, compared with the control value.
The absorption of nutrients and food factors across the intestinal epithelium occurs because of one or more different transport pathways such as passive paracellular transport, passive transcellular transport, and carrier-mediated transport. We have shown that several lectins, including SBA, CGA, and WGA, increased the transport of isoflavones but not their aglycones. The TER across a Caco-2 cell monolayer reflects any effects on the TJ-mediated paracellular pathways. ATL, which decreased the TER, may have any effect on the intestinal transport system if its active form can reach the intestine.