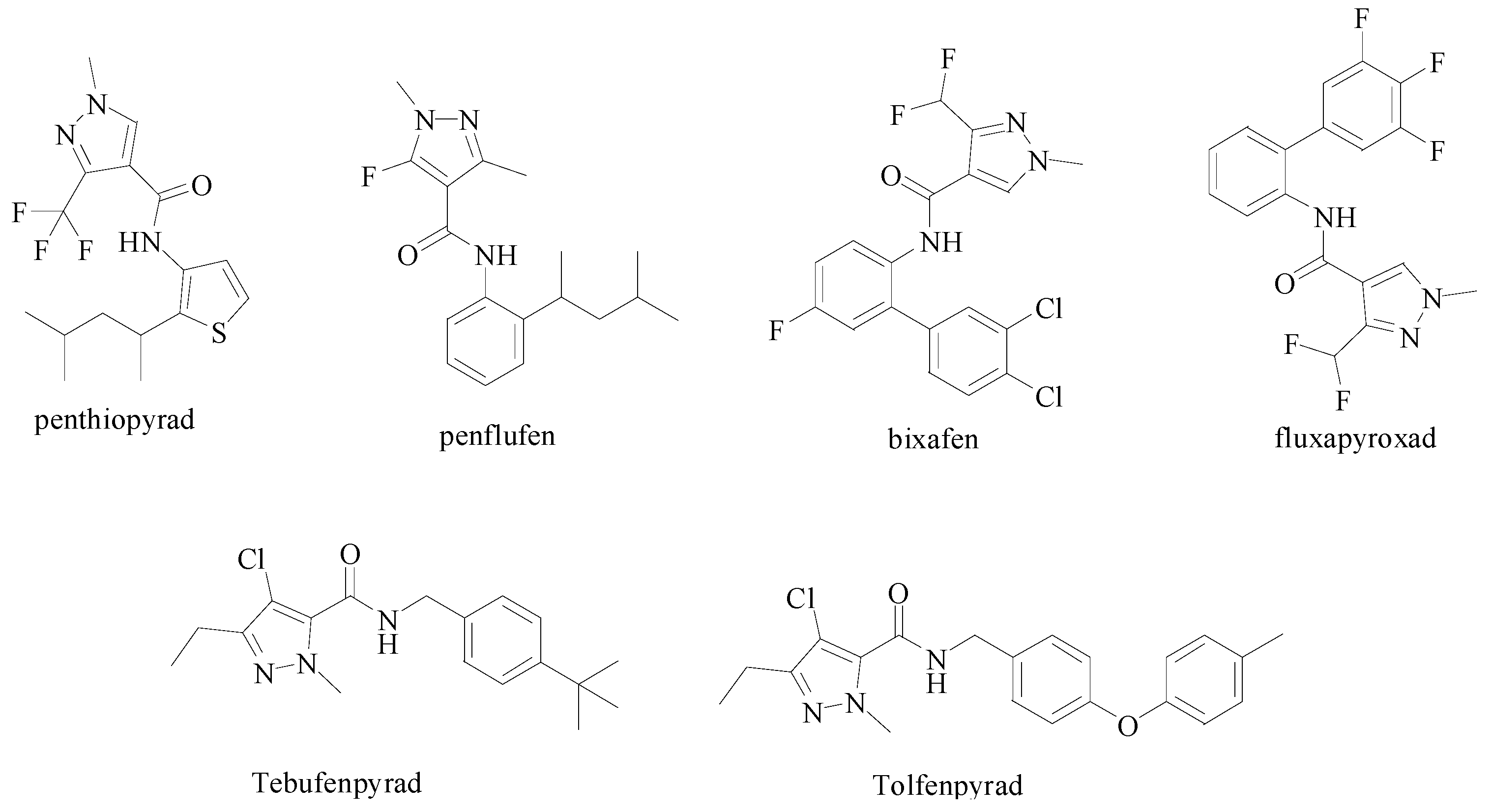
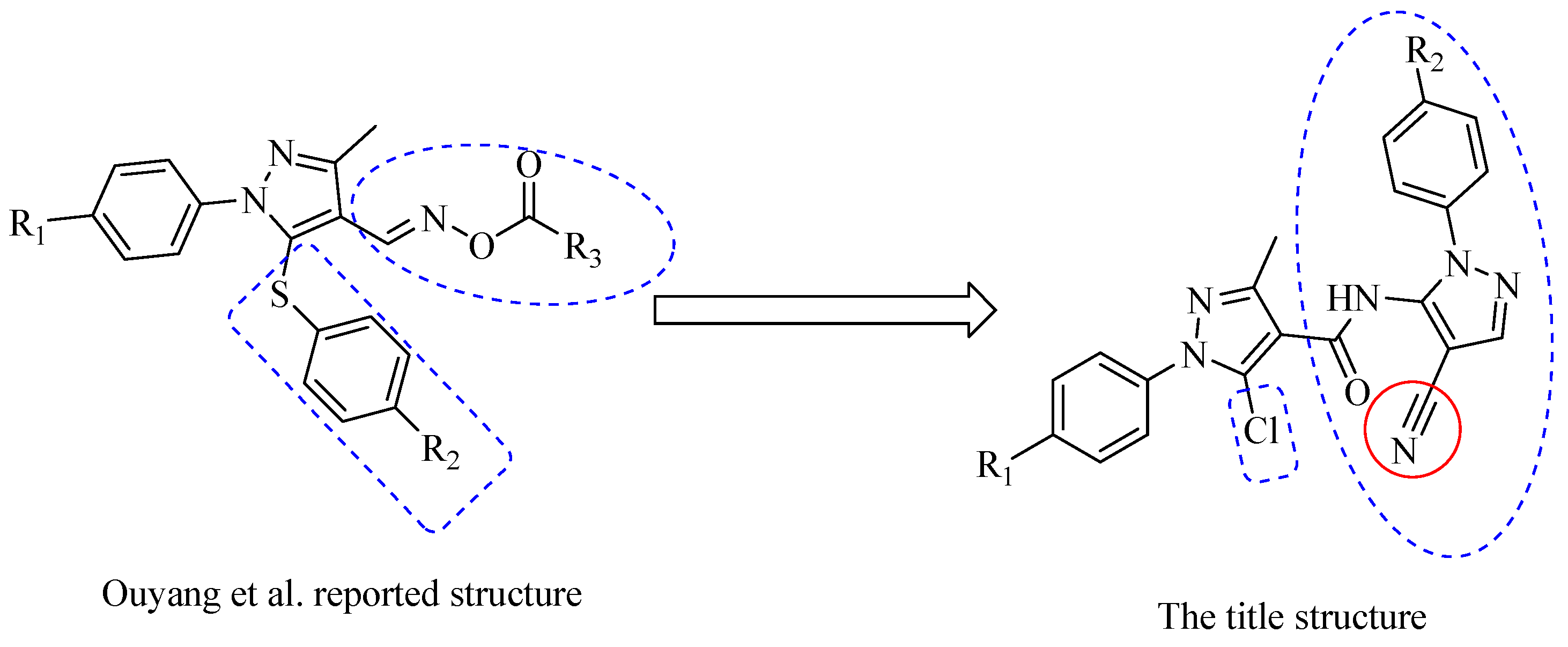
Design, Synthesis and Anti-Tobacco Mosaic Virus (TMV) Activity of 5-Chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide Derivatives
Abstract
:1. Introduction
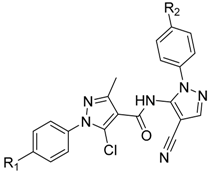
| Compound NO. | R1 | R2 |
|---|---|---|
| 3a | H | H |
| 3b | H | Cl |
| 3c | H | CH3 |
| 3d | H | F |
| 3e | Cl | H |
| 3f | Cl | Cl |
| 3g | Cl | CH3 |
| 3h | Cl | F |
| 3i | CH3 | H |
| 3j | CH3 | Cl |
| 3k | CH3 | CH3 |
| 3l | CH3 | F |
| 3m | F | H |
| 3n | F | Cl |
| 3o | F | CH3 |
| 3p | F | F |
2. Results and Discussion
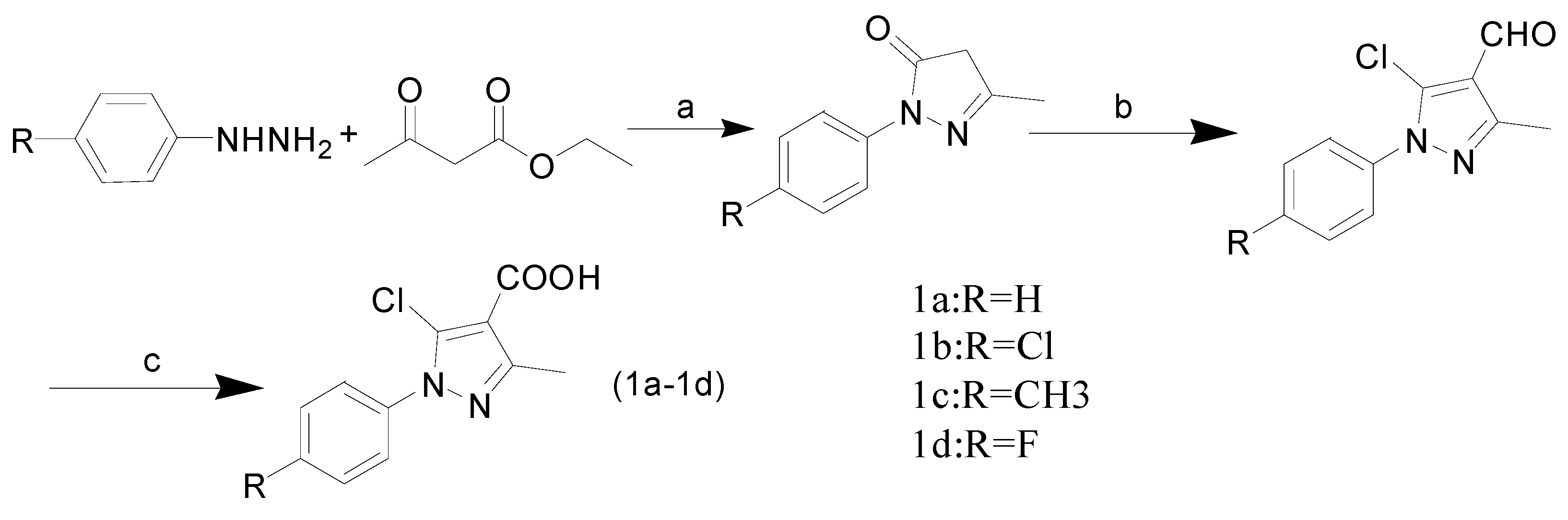
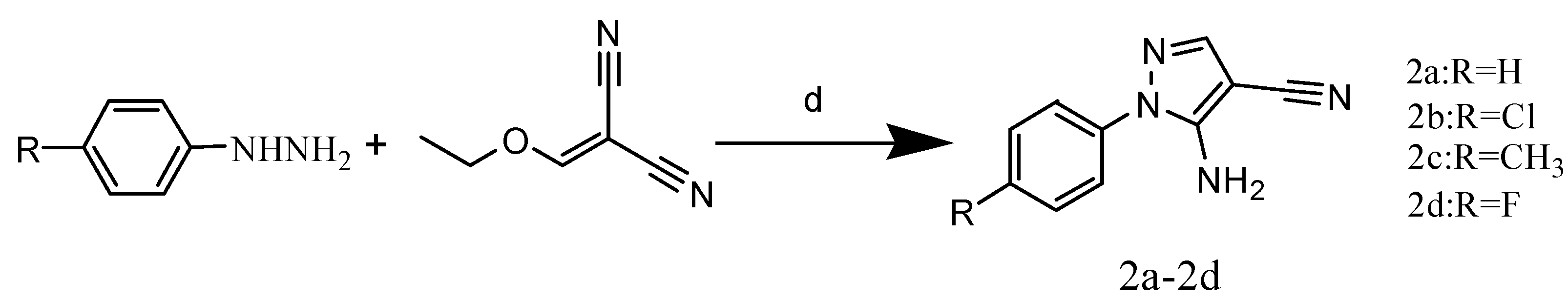
2.1. Chemistry

2.2. Bioactivity
| Compound NO. | In Vitro Inhibition Rate (%) | In Vivo Inhibition Rate (%) | ||
|---|---|---|---|---|
| Protection Effect | Inactivation Effect | Curative Effect | ||
| 3a | 54.8 ± 1.11 | 32.4 ± 1.45 | 43.2 ± 4.01 | 47.3 ± 0.88 |
| 3b | 66.4 ± 0.78 | 48.4 ± 0.23 | 54.2 ± 2.11 | 54.5 ± 1.03 |
| 3c | 52.1 ± 1.20 | 43.1 ± 2.33 | 42.6 ± 1.09 | 42.2 ± 2.12 |
| 3d | 68.1 ± 2.33 | 60.2 ± 2.11 | 53.1 ± 2.09 | 55.1 ± 2.15 |
| 3e | 67.2 ± 0.48 | 49.3 ± 1.03 | 58.4 ± 0.97 | 56.5 ± 0.55 |
| 3f | 83.2 ± 1.16 | 76.7 ± 2.54 | 74.2 ± 1.58 | 78.5 ± 0.82 |
| 3g | 64.3 ± 0.66 | 33.8 ± 3.56 | 52.3 ± 3.12 | 41.2 ± 3.20 |
| 3h | 81.6 ± 0.72 | 78.9 ± 1.21 | 66.4 ± 4.23 | 75.3 ± 1.31 |
| 3i | 53.3 ± 0.51 | 22.6 ± 1.36 | 43.3 ± 1.24 | 52.1 ± 2.11 |
| 3j | 42.3 ± 0.48 | 45.2 ± 1.97 | 52.6 ± 0.65 | 44.2 ± 5.12 |
| 3k | 50.2 ± 1.33 | 42.1 ± 1.29 | 22.8 ± 6.03 | 38.9 ± 4.33 |
| 3l | 64.6 ± 4.11 | 41.2 ± 7.03 | 45.3 ± 7.34 | 42.1 ± 1.43 |
| 3m | 74.4 ± 2.08 | 52.1 ± 3.02 | 64.6 ± 0.63 | 45.9 ± 3.32 |
| 3n | 84.3 ± 1.52 | 77.5 ± 3.51 | 74.5 ± 2.13 | 78.9 ± 1.65 |
| 3o | 72.1 ± 1.18 | 61.3 ± 1.30 | 67.8 ± 0.92 | 52.1 ± 2.06 |
| 3p | 86.5 ± 4.96 | 77.2 ± 6.03 | 84.3 ± 0.52 | 81.5 ± 1.03 |
| Ningnanmycin | 82.1 ± 3.31 | 62.4 ± 7.20 | 78.4 ± 5.14 | 55.2 ± 6.12 |
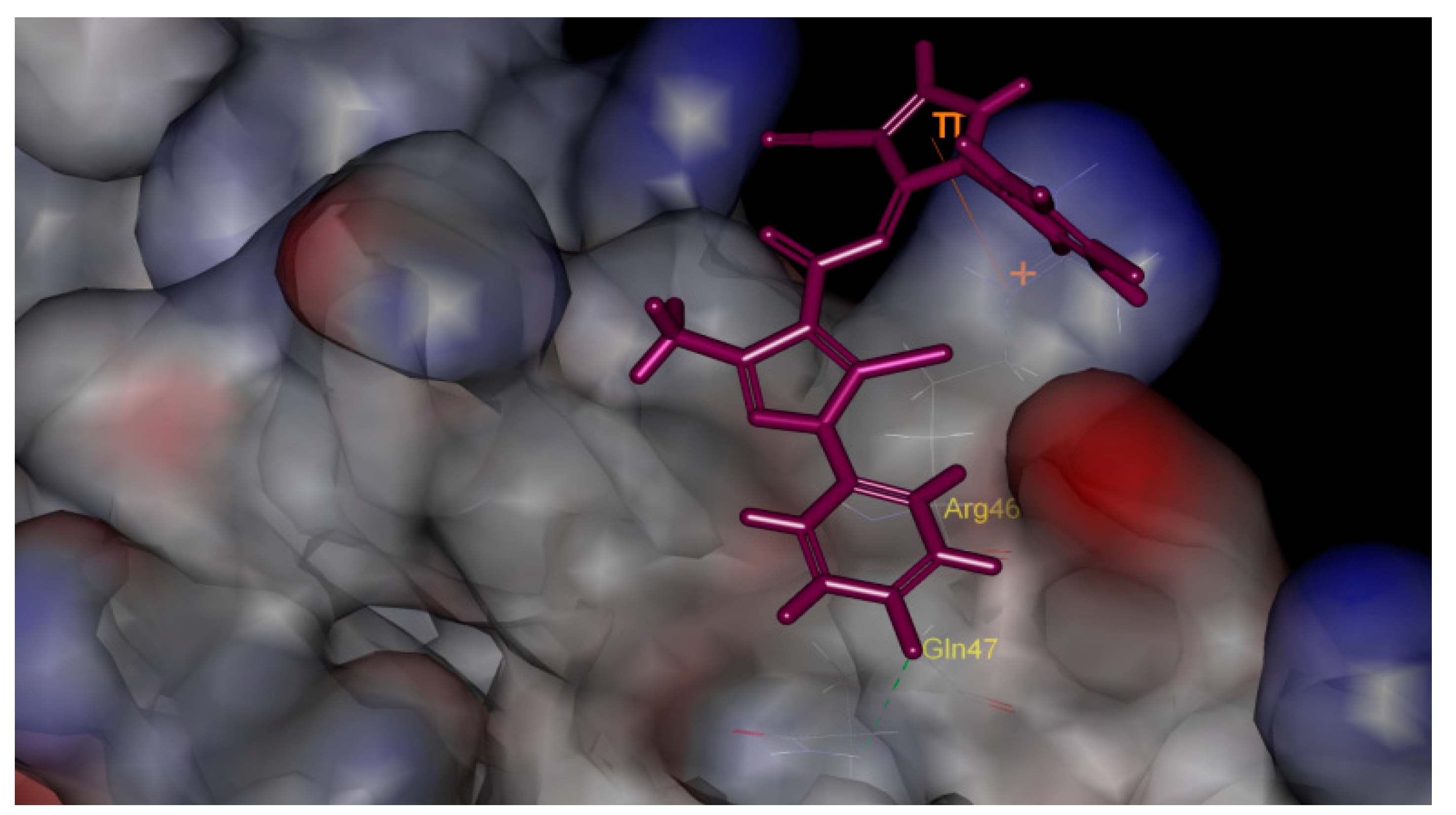
2.3. Molecular Docking
2.4. 3D-QSAR
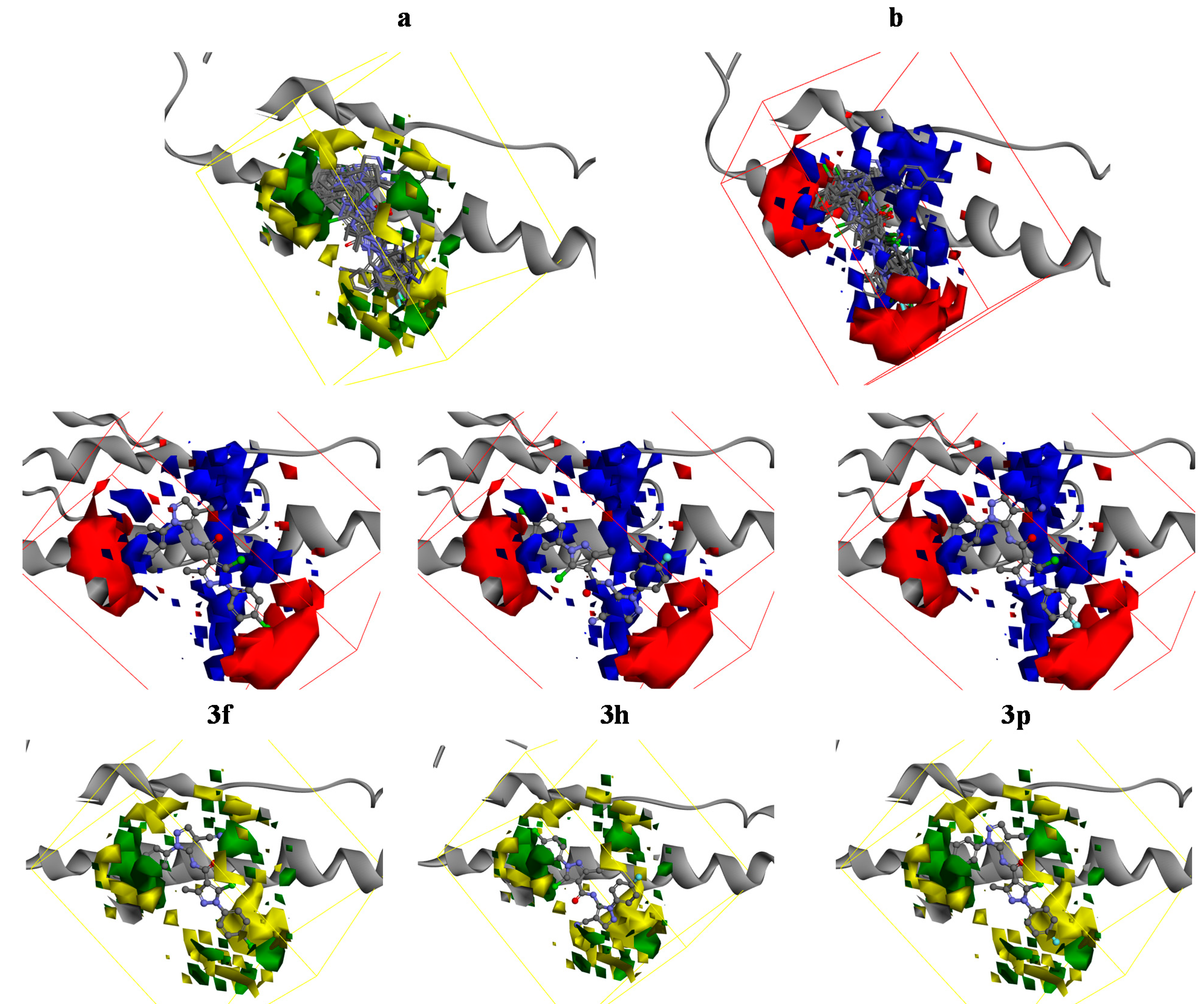
3. Experimental Section
3.1. General Information
3.2. General Procedure for Synthesis of 5-Chloro-1-aryl-3-methyl-1H-pyrazole-4-carboxylic Acids 1a–1d
3.3. General Procedure for Synthesis of 5-Amino-1-aryl-1H-pyrazole-4-carbonitriles 2a–2d
3.4. General Procedure for Synthesis of 5-Chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide Derivatives 3a–3p
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ren, Y.P.; Wong, S.M.; Lim, L.Y. Application of plant viruses as nano drug delivery systems. Pharm. Res. 2010, 27, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Ritzenthaler, C. Resistance to plant viruses: Old issue, news answers? Curr. Opin. Biotechnol. 2005, 16, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, P.; Wang, L.; Wang, Q. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, P.; Xizhi, X.; Liu, Y.; Wang, L.; Wang, Q. Design, synthesis, and antiviral activity evaluation of phenanthrene-based antofine derivatives. J. Agric. Food Chem. 2012, 60, 8544–8551. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.; Muldoon, J.; Lin, Y.C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.G.; Sharpless, K.B.; Elder, J.H.; et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 2006, 45, 1435–1439. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chapsal, B.D.; Weber, I.T.; Mitsuya, H. Design of HIV protease inhibitors targeting protein backbone: An effective strategy for combating drug resistance. Acc. Chem. Res. 2008, 41, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Bendahmane, M.; Chen, I.; Asurmendi, S.; Bazzini, A.A.; Szecsi, J.; Beachy, R.N. Coat protein-mediated resistance to TMV infection of Nicotiana tabacum involves multiple modes of interference by coat protein. Virology 2007, 366, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R. Plant Virology; Elsevier: Amsterdam, The Netherlands, 1991; p. 864. [Google Scholar]
- Ko, H.C.; Wei, B.L.; Chiou, W.F. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J. Ethnopharmacol. 2006, 107, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Jin, L.; Yang, S.; Bhadury, P.S. Environment-Friendly Antiviral Agents for Plants, 1st ed.; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Gao, S.; Zhang, R.Y.; Yu, Z.H.; Xi, Z. Antofine analogues can inhibit tobacco mosaic virus assembly through small-molecule-RNA interactions. Chembiochem 2012, 13, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Hergenrother, P.J. Targeting RNA with small molecules. Chem. Rev. 2008, 108, 1171–1224. [Google Scholar] [CrossRef] [PubMed]
- Graillot, V.; Tomasetig, F.; Cravedi, J.-P.; Audebert, M. Evidence of the in vitro genotoxicity of methyl-pyrazole pesticides in human cells. Mutat. Res. 2012, 748, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.-P.; Leroux, F.R. Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluorine Chem. 2013, 152, 2–11. [Google Scholar] [CrossRef]
- Xue, N.; Zhou, Y.; Wang, G.; Miao, W.; Qu, J. Syntheses and herbicidal activity of pyrazolyl benzoxazole derivatives. ChemInform 2010, 41, 15–21. [Google Scholar]
- Mali, J.R.; Pratap, U.R.; Jawale, D.V.; Mane, R.A. Water-mediated one-pot synthetic route for pyrazolo[3,4-b]quinolines. Tetrahedron Lett. 2010, 51, 3980–3982. [Google Scholar] [CrossRef]
- Al-Masoudi, I.A.; Al-Souda, Y.A.; Al-Salihi, N.J.; Al-Masoudi, N.A. 1,2,4-Triazoles: Synthetic approaches and pharmacological importance. Chem. Heterocycl. Comp. 2006, 42, 1377–1403. [Google Scholar] [CrossRef]
- Kumari, S.; Paliwal, S.; Chauhan, R. Synthesis of pyrazole derivatives possessing anticancer activity: Current status. Synth. Commun. 2014, 44, 1521–1578. [Google Scholar] [CrossRef]
- Rida, S.M.; Ashour, F.A.; El-Hawash, S.A.M.; Elsemary, M.M.; Badr, M.H.; Shalaby, M.A. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Siddall, T.L.; Ouse, D.G.; Benko, Z.L.; Garvin, G.M.; Jackson, J.L.; McQuiston, J.M.; Ricks, M.J.; Thibault, T.D.; Turner, J.A.; Vanheertum, J.C.; et al. Synthesis and herbicidal activity of phenyl-substituted benzoylpyrazoles. Pest Manag. Sci. 2002, 58, 1175–86. [Google Scholar] [CrossRef] [PubMed]
- Meazza, G.; Bettarini, F.; La Porta, P.; Piccardi, P.; Signorini, E.; Portoso, D.; Fornara, L. Synthesis and herbicidal activity of novel heterocyclic protoporphyrinogen oxidase inhibitors. Pest Manag. Sci. 2004, 60, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Shi, D.Q. Synthesis and herbicidal activity of 2-aroxy-propanamides containing pyrimidine and 1,3,4-thiadiazole rings. J. Heterocycl. Chem. 2014, 51, 432–435. [Google Scholar] [CrossRef]
- Satasia, S.P.; Kalaria, P.N.; Raval, D.K. Catalytic regioselective synthesis of pyrazole based pyrido[2,3-d]pyrimidine-diones and their biological evaluation. Org. Biomol. Chem. 2014, 12, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, A.; Romdhane, A.; Ben Said, A.; Majouli, K.; Ben Jannet, H. Synthesis of new pyrazole and antibacterial pyrazolopyrimidine derivatives. Turk. J. Chem. 2014, 38, 210–221. [Google Scholar] [CrossRef]
- Darwish, E.S.; Fattah, A.M.A.; Attaby, F.A.; Al-Shayea, O.N. Synthesis and antimicrobial evaluation of some novel thiazole, pyridone, pyrazole, chromene, hydrazone derivatives bearing a biologically active sulfonamide moiety. Int. J. Mol. Sci. 2014, 15, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C. Pyrazole chemistry in crop protection. Heterocycles 2007, 71, 1467–1502. [Google Scholar] [CrossRef]
- Jiang, D.X.; Zheng, X.H.; Shao, G.; Ling, Z.; Xu, H.H. Discovery of a novel series of phenyl pyrazole inner salts based on fipronil as potential dual-target insecticides. J. Agric. Food Chem. 2014, 62, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.Q.; Liu, J.; Yang, X.P.; Liu, Z.J. Stereoselective synthesis and antifungal activities of (E)-alpha-(methoxyimino)benzeneacetate derivatives containing 1,3,5-substituted pyrazole ring. J. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorganic Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef]
- Reid, T.S.; Beese, L.S. Crystal structures of the anticancer clinical candidates R1 15777 (Tipifarnib) and BMS-214662 complexed with protein farnesyltransferase suggest a mechanism of FTI selectivity. Biochemistry 2004, 43, 6877–6884. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.T.; Ding, C.Z.; Batorsky, R.; Bednarz, M.; Bhide, R.; Cho, Y.; Chong, S.; Chao, S.; Gullo-Brown, J.; Guo, P.; et al. Discovery of (R)-7-cyano-2,3,4,5-tetrahydro-1-(1H-imidazol-4-ylmethyl)-3-(phenylmethyl)-4-(2-thienylsulfonyl)-1H-1,4-benzodiazepine (BMS-214662), a farnesyltransferase inhibitor with potent preclinical antitumor activity. J. Med. Chem. 2000, 43, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Clark, A.D.; Lewi, P.J.; Heeres, J.; de Jonge, M.R.; Koymans, L.M.H.; Vinkers, H.M.; Daeyaert, F.; Ludovici, D.W.; Kukla, M.J.; et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 2004, 47, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.F.; Yao, L.H.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-containing pharmaceuticals: Efficacious roles of the nitrile pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, Q.; Chen, Z.; He, M.; Jin, L.H.; Hu, D.Y. Synthesis and bioactivity of pyrazole acyl thiourea derivatives. Molecules 2012, 17, 5139–5150. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.N.; Pil’o, S.G.; Kozachenko, A.P.; Prokopenko, V.M.; Rusanov, E.B.; Brovarets, V.S. Reaction of 2-aryl-4-cyano-1,3-oxazole-5-sulfonyl chlorides with 5-amino-1H-pyrazoles and 5-amino-1H-1,2,4-triazole. Chem. Heterocycl. Comp. 2014, 50, 76–86. [Google Scholar] [CrossRef]
- Thorson, J.S.; Hosted, T.J.; Jiang, J.; Biggins, J.B.; Ahlert, J. Natures carbohydrate chemists the enzymatic glycosylation of bioactive bacterial metabolites. Curr. Org. Chem. 2001, 5, 139–167. [Google Scholar] [CrossRef]
- Wu, G.; Robertson, D.H.; Brooks, C.L.; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER—A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the title compounds 3a–3p are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.-J.; Liao, M.; Chu, M.-J.; Ren, Z.-L.; Zhang, X.; Lv, X.-H.; Cao, H.-Q. Design, Synthesis and Anti-Tobacco Mosaic Virus (TMV) Activity of 5-Chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide Derivatives. Molecules 2015, 20, 807-821. https://doi.org/10.3390/molecules20010807
Xiao J-J, Liao M, Chu M-J, Ren Z-L, Zhang X, Lv X-H, Cao H-Q. Design, Synthesis and Anti-Tobacco Mosaic Virus (TMV) Activity of 5-Chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide Derivatives. Molecules. 2015; 20(1):807-821. https://doi.org/10.3390/molecules20010807
Chicago/Turabian StyleXiao, Jin-Jing, Min Liao, Ming-Jie Chu, Zi-Li Ren, Xin Zhang, Xian-Hai Lv, and Hai-Qun Cao. 2015. "Design, Synthesis and Anti-Tobacco Mosaic Virus (TMV) Activity of 5-Chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide Derivatives" Molecules 20, no. 1: 807-821. https://doi.org/10.3390/molecules20010807
APA StyleXiao, J.-J., Liao, M., Chu, M.-J., Ren, Z.-L., Zhang, X., Lv, X.-H., & Cao, H.-Q. (2015). Design, Synthesis and Anti-Tobacco Mosaic Virus (TMV) Activity of 5-Chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide Derivatives. Molecules, 20(1), 807-821. https://doi.org/10.3390/molecules20010807







