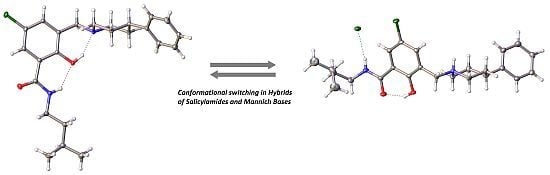
Hybrids of Salicylalkylamides and Mannich Bases: Control of the Amide Conformation by Hydrogen Bonding in Solution and in the Solid State
Abstract
:1. Introduction
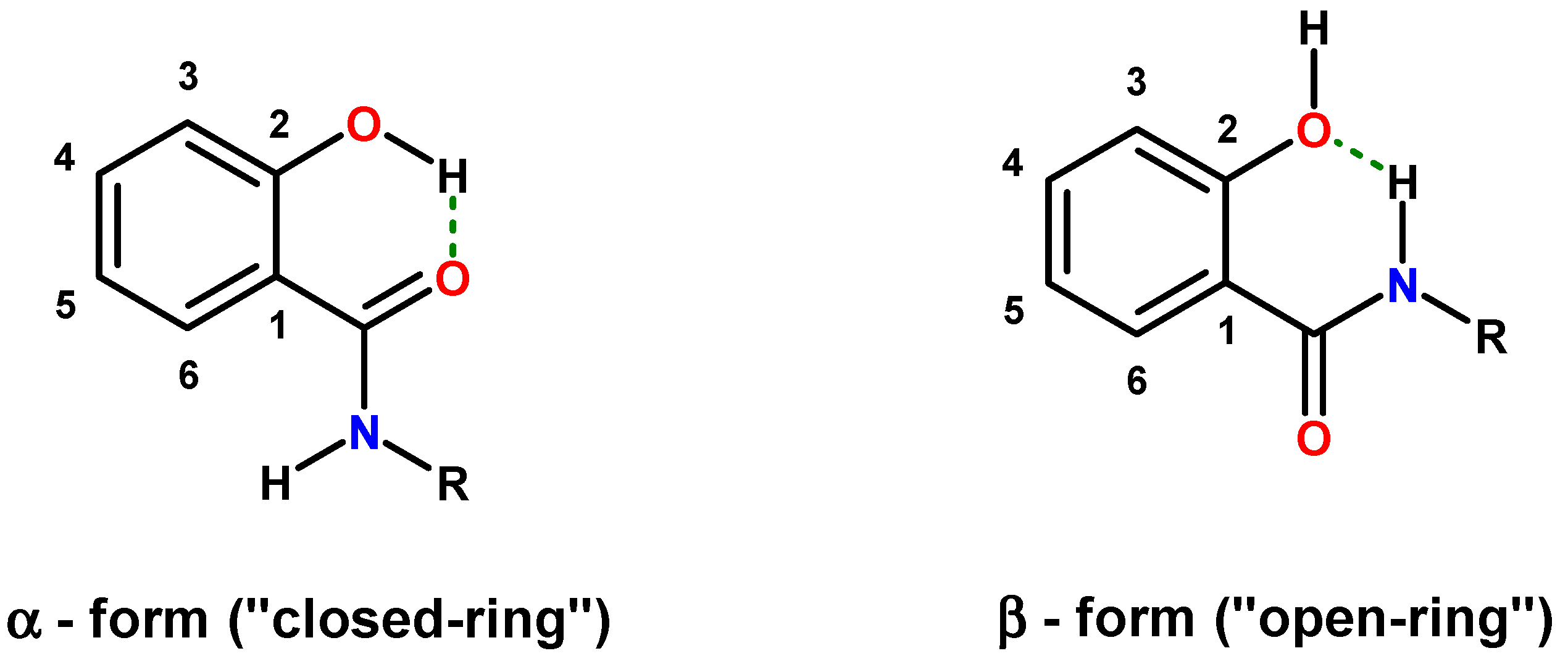
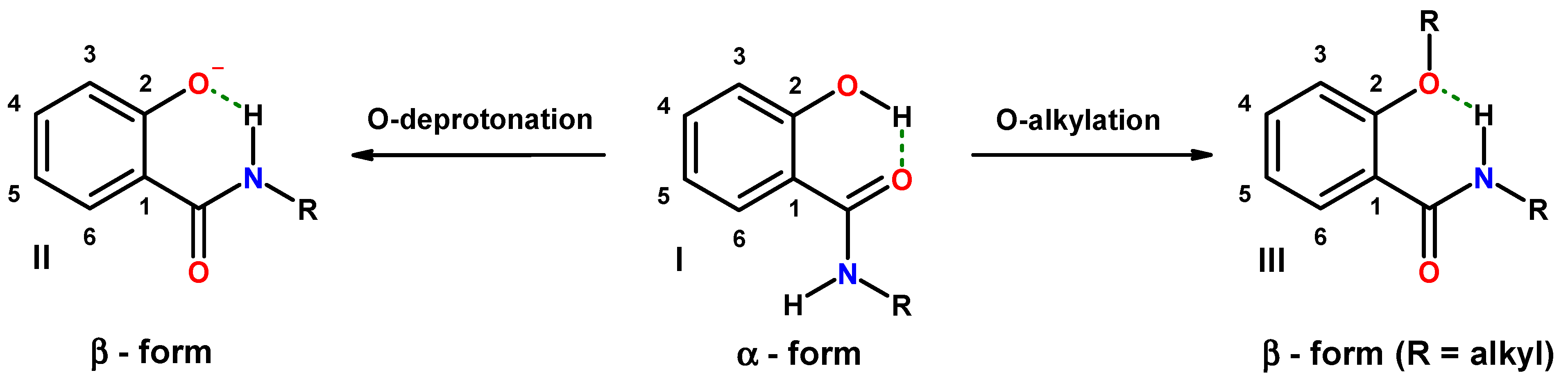

2. Results and Discussion
2.1. Hybrids of Salicylalkylamides and Mannich Bases
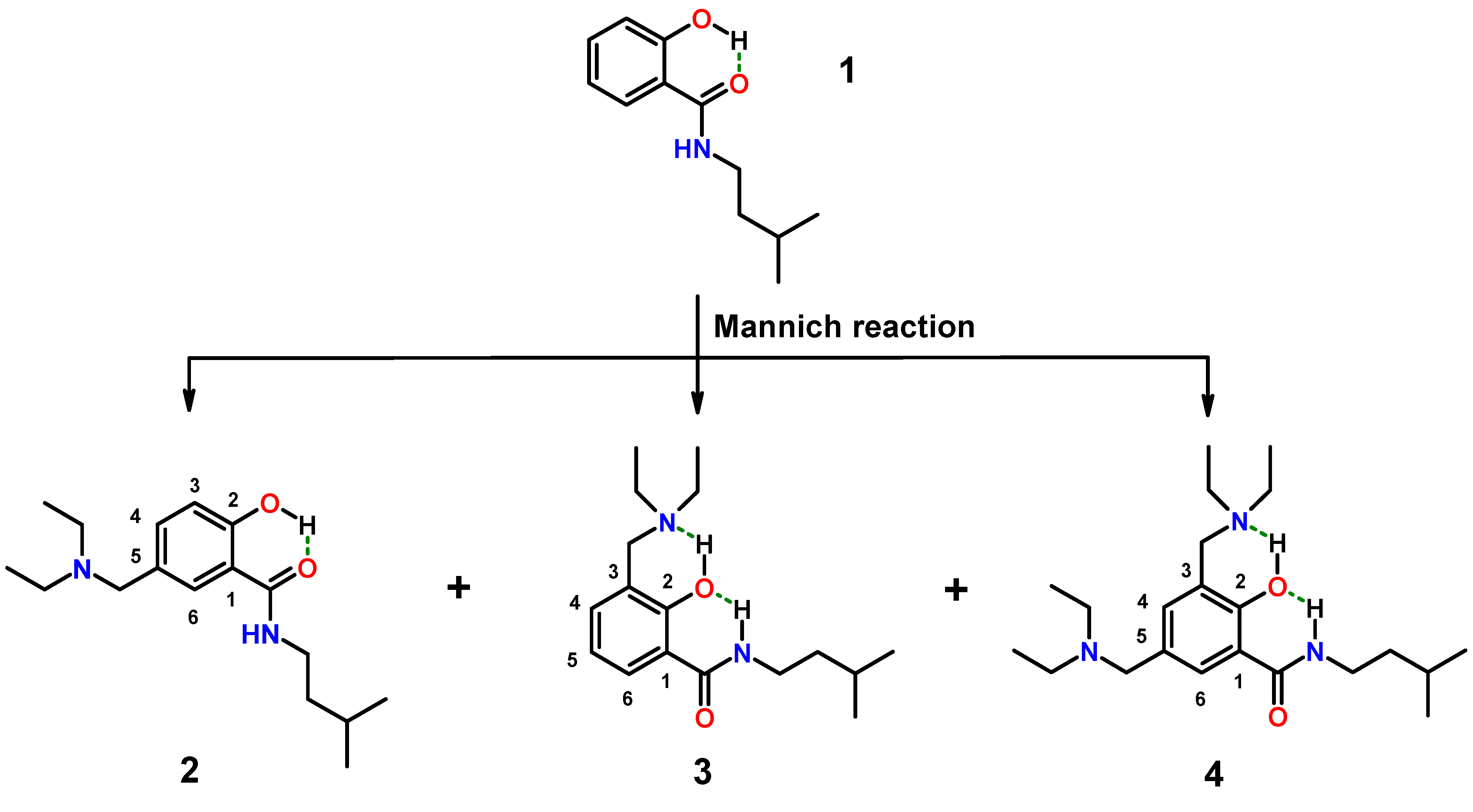
2.1.1. Assessment of the Conformation of Salicylalkylamides in Chloroform-d1 by NMR Spectroscopy
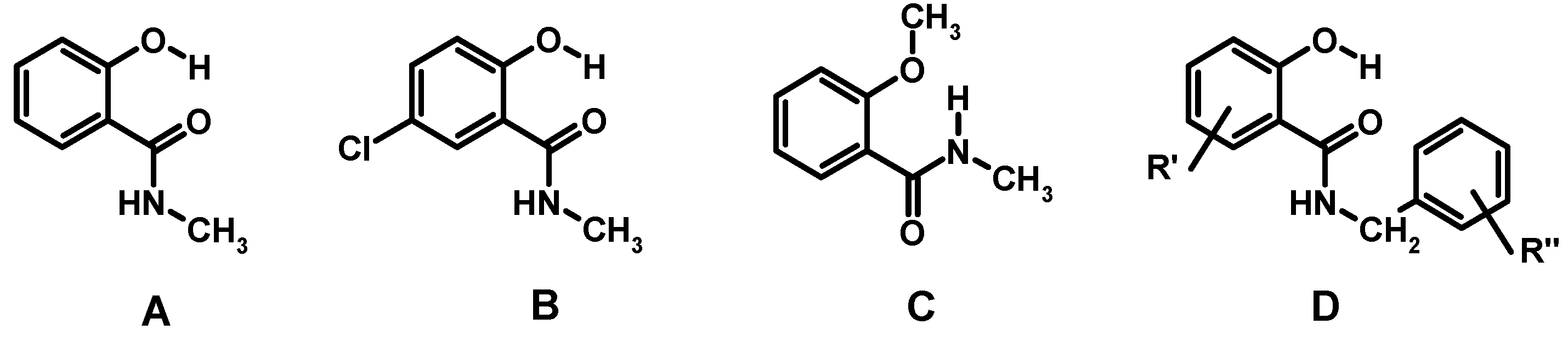
| 13C{1H} NMR (CDCl3) | 1H-NMR (CDCl3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C=O | H-6 | NH | 2-OH | Ref. |
| A | 114.6 | 161.8 | 118.8 | 134.5 | 119.0 | 125.7 | 171.1 | 7.4 | 6.8 | 12.4 | [63,64] |
| B | 115.3 | 160.1 | 120.2 | 134.0 | 123.3 | 124.9 | 169.5 | 7.3 | 6.2 b | 12.1 s | [63] |
| C | 121.1 | 157.3 | 111.1 | 132.5 | 121.4 | 132.1 | 165.9 | 8.2 | 7.8 | _ | [63,66] |
| D | * | * | * | * | * | * | * | 6.8–7.5 | <7 | 11.5–12.6 | [34] |
2.1.2. Assessment of the Conformation of Hybrids 2–4 in Chloroform-d1 by NMR Spectroscopy
| 13C{1H} NMR (400 MHz, CDCl3) | 1H-NMR (400 MHz, CDCl3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C=O | H-6 | NH | 2-OH |
| 1 | 114.5 | 161.6 | 118.6 | 134.2 | 118.7 | 125.4 | 170.0 | 7.4 | 6.4 | 12.4 s |
| 2 | 114.4 | 160.5 | 118.2 | 135.0 | 129.6 | 125.8 | 170.0 | 7.4 | 6.5 | nd |
| 3 | 119.9 | 158.2 | 122.0 | 131.3 | 118.7 | 130.8 | 166.1 | 8.1 | 8.6 | 7.5–9.5 |
| 4 | 119.0 | 156.9 | 122.1 | 132.0 | 129.5 | 131.0 | 166.1 | 7.9 | 8.5 | 10.5–11.5 |
2.1.3. Synthesis of a Small Library of Hybrids of Mannich Bases and Salicylalkylamide 5
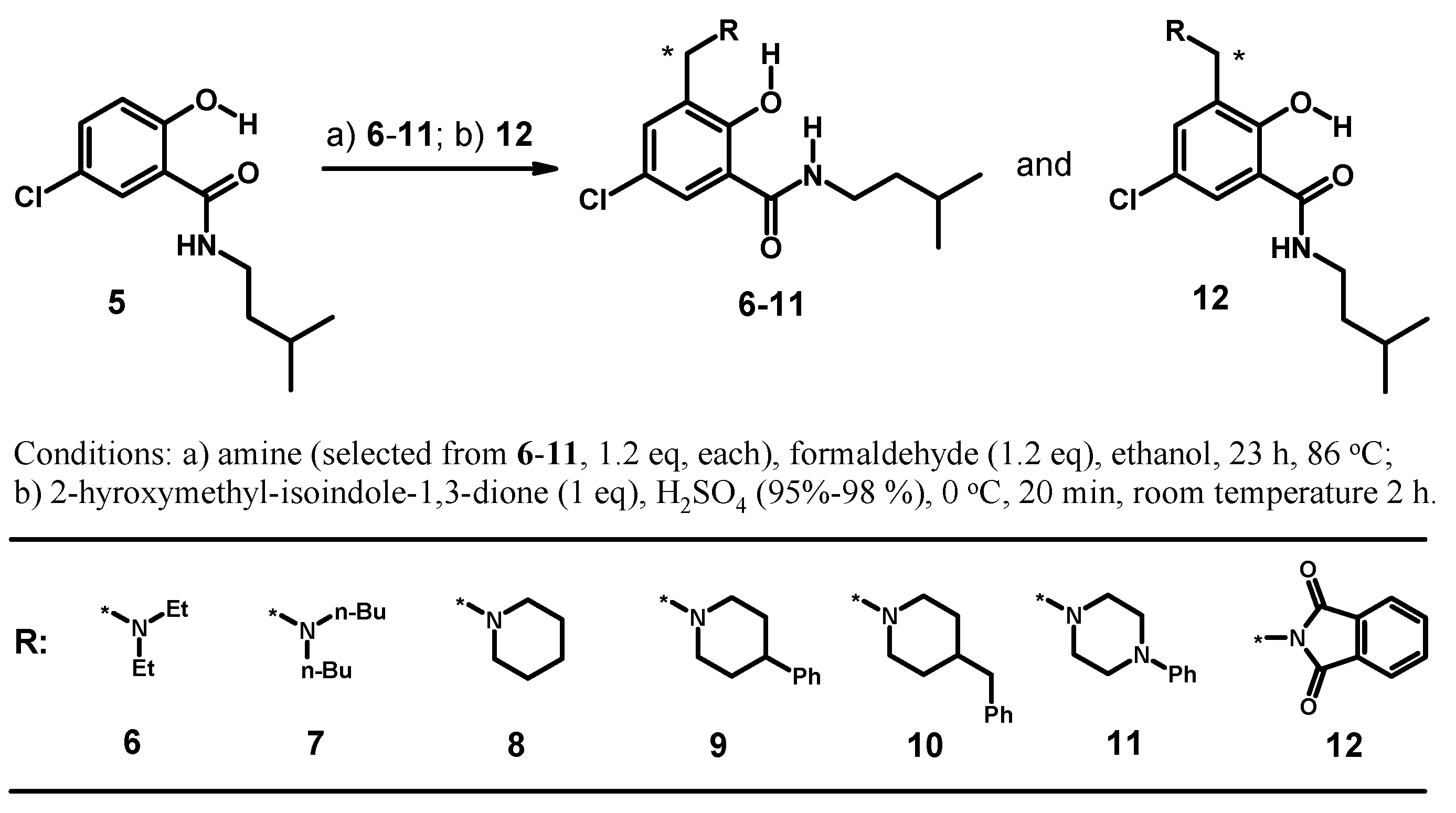
2.1.4. Assessment of the Conformation of Hybrids 6–12 in Chloroform-d1 by NMR Spectroscopy
| 13C{1H} NMR (400 MHz, CDCl3) | 1H-NMR (400 MHz, CDCl3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C=O | H-6 | NH | 2-OH |
| 5 | 115.5 | 160.2 | 120.3 | 134.1 | 123.4 | 125.0 | 169.0 | 7.2 | 6.2 | 12.3 sharp |
| 6 | 121.3 | 157.1 | 123.6 | 130.8 | 123.4 | 130.2 | 164.8 | 8.0 | 8.6 | 10.8–11.8 |
| 7 | 121.3 | 157.0 | 123.7 | 130.8 | 123.4 | 130.2 | 164.8 | 8.0 | 8.6 | 10.5–11.5 |
| 8 | 121.2 | 156.6 | 123.2 | 131.0 | 123.7 | 130.3 | 164.8 | 8.0 | 8.5 | 9.7–10.7 |
| 9 | 121.3 | 156.2 | 123.4 | 131.1 | 123.9 | 130.3 | 164.7 | 8.1 | 8.4 | 11.0–12.0 |
| 10 | 121.2 | 156.4 | 123.3 | 130.9 | 123.7 | 130.2 | 164.7 | 8.1 | 8.5 | 11.2–12.2 |
| 11 | 121.1 | 155.6 | 123.3 | 131.4 | 124.3 | 130.2 | 164.6 | 8.1 | 8.3 | 10.8–11.8 |
| 12 | 115.5 | 157.8 | 126.8 | 132.4 | 123.0 | 124.8 | 168.7 | 7.2 | 6.6 | 12.7 sharp |
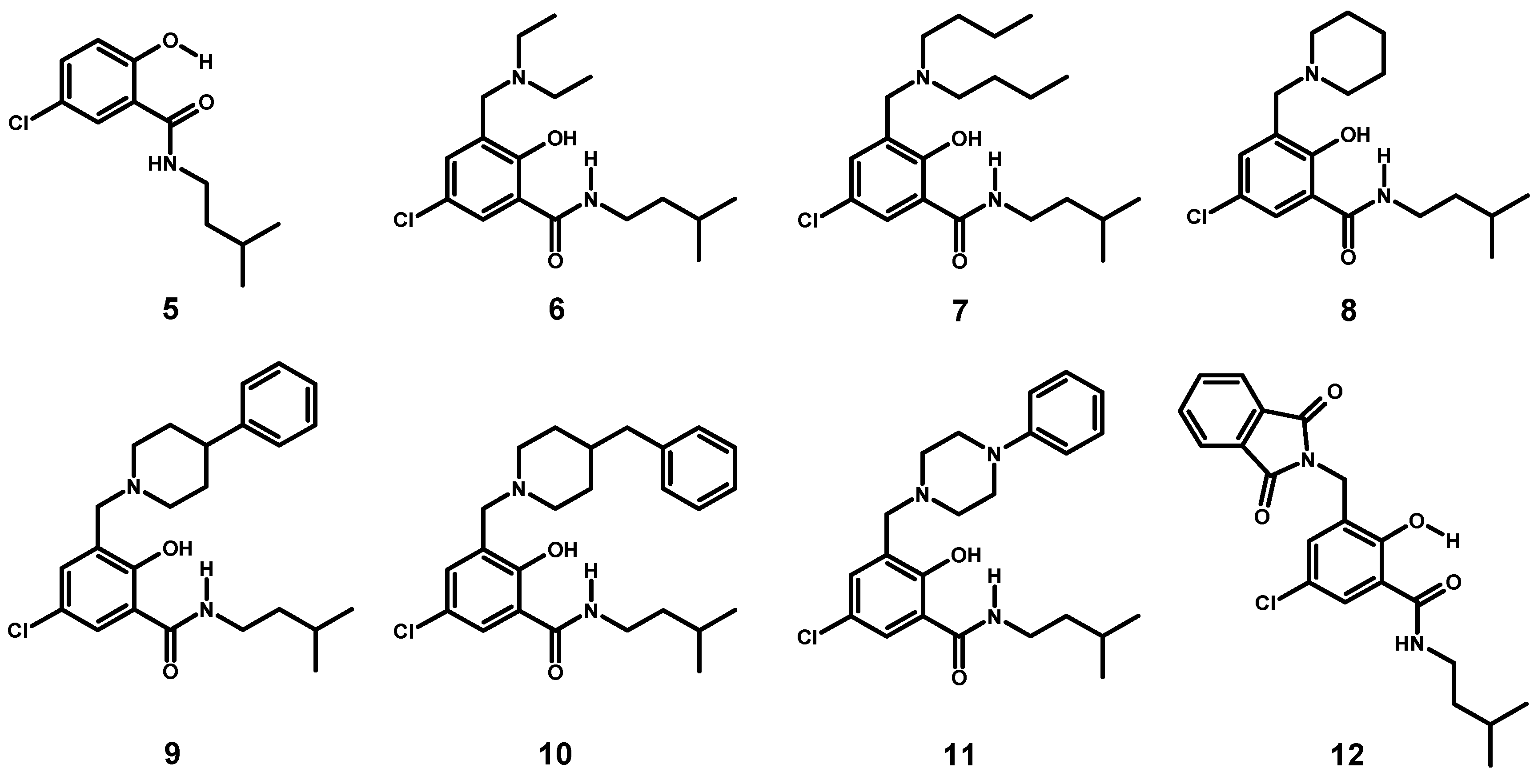
2.1.5. Reversal of the β-Conformation of Salicylalkylamide-Mannich Base Hybrids
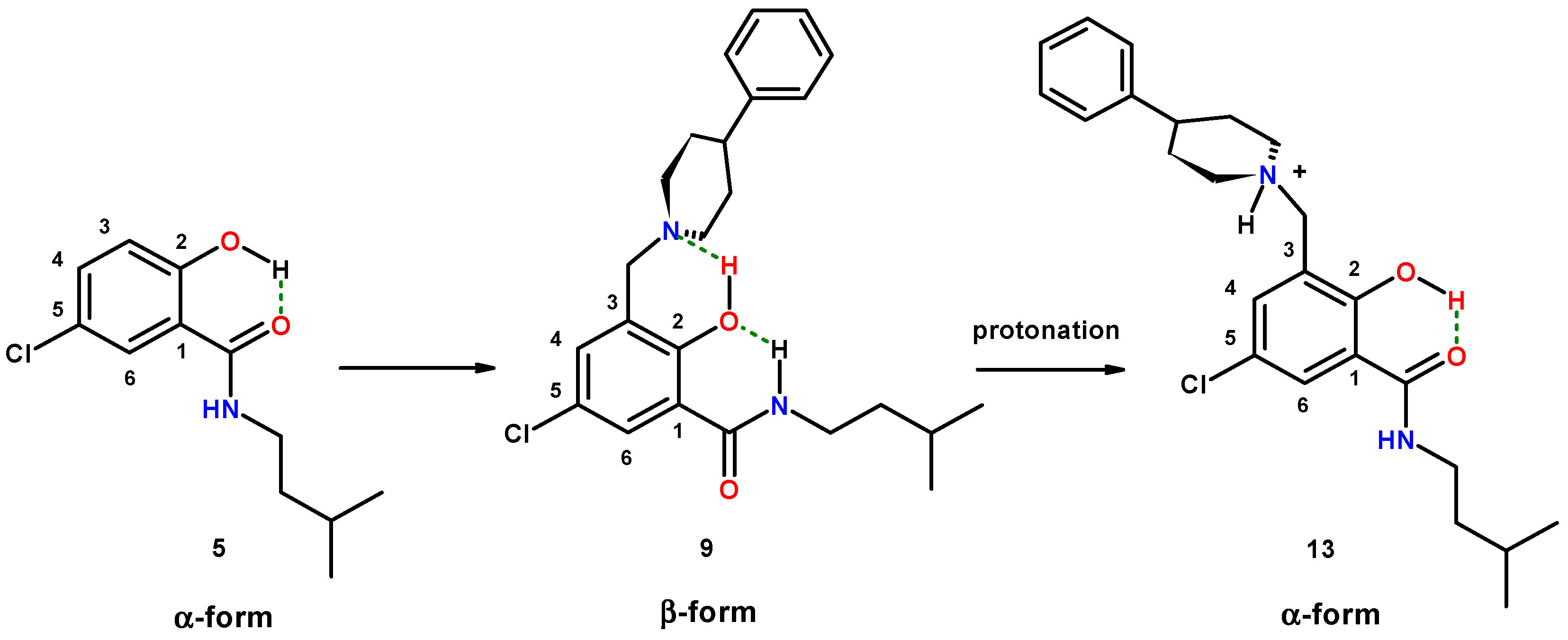
2.2. Crystallographic Structure Determination
2.2.1. 5-Chloro-2-hydroxy-N-(3-methyl-butyl)-3-(4-phenyl-piperidin-1-ylmethyl)-benzamide (9)
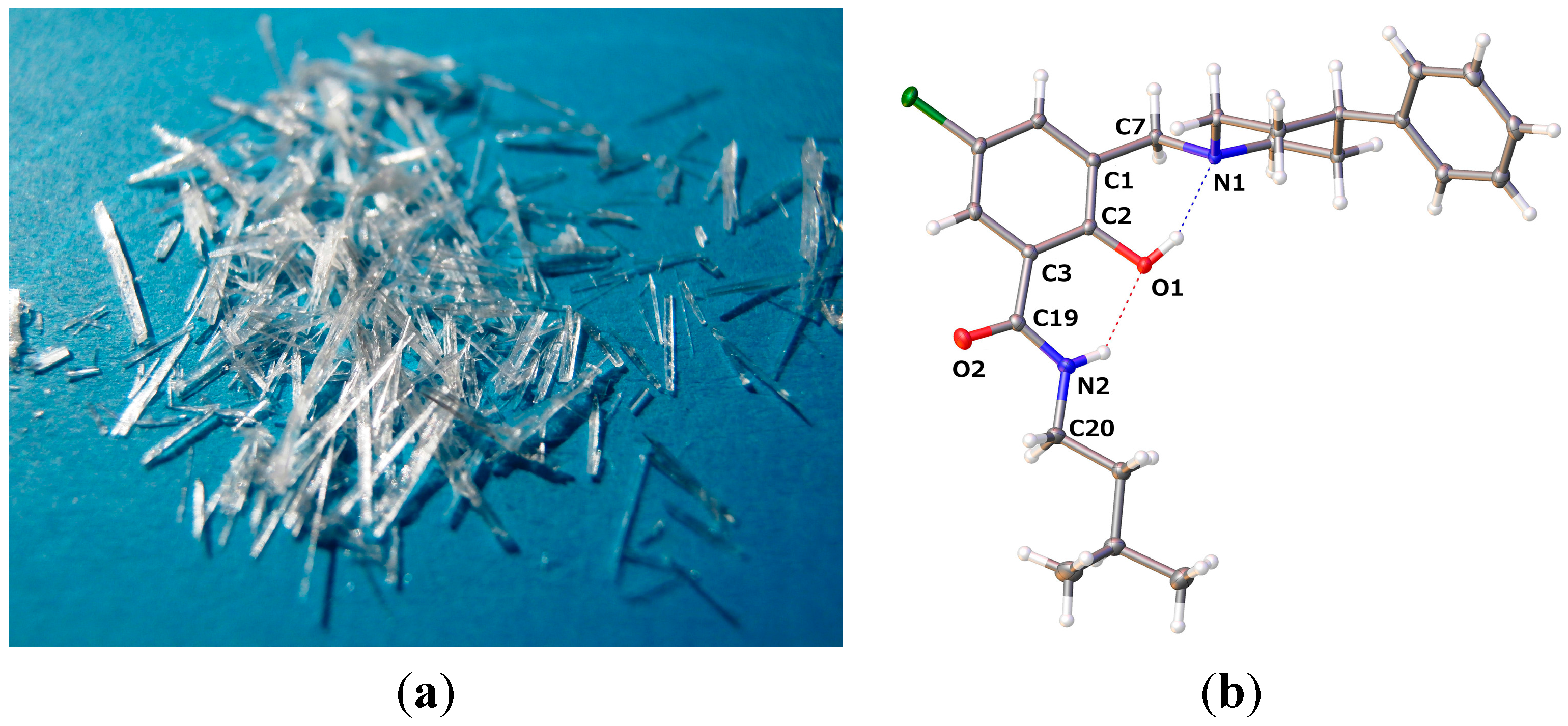
| 9 | 13 | 14 | |
|---|---|---|---|
| O2–C19 | 1.2329(12) | 1.2546(15) | 1.2576(12) |
| N2–C19 | 1.3444(13) | 1.3289(17) | 1.3288(13) |
| C3–C19 | 1.5009(13) | 1.4952(17) | 1.4885(13) |
| N2–C20 | 1.4577(13) | 1.4630(17) | 1.4635(12) |
| O1–C2 | 1.3633(11) | 1.3469(14) | 1.3461(11) |
| O1–C2–C3–C19 | −3.30(15) | 4.54(18) | 0.49(14) |
| C2–C1–C7–N1 | −48.24(12) | 100.91(13) | 107.51(10) |

| D–H···A | D–H | H···A | D···A | D–H···A |
| O1–H1···N1 | 0.84 | 1.897 | 2.6561(11) | 149.7 |
| N2–H2···O1 | 0.88 | 2.015 | 2.7120(11) | 135.3 |
2.2.2. [5-Chloro-2-hydroxy-3-(3-methyl-butylcarbamoyl)-benzyl]-4-phenylpiperidinium Chloride (13)
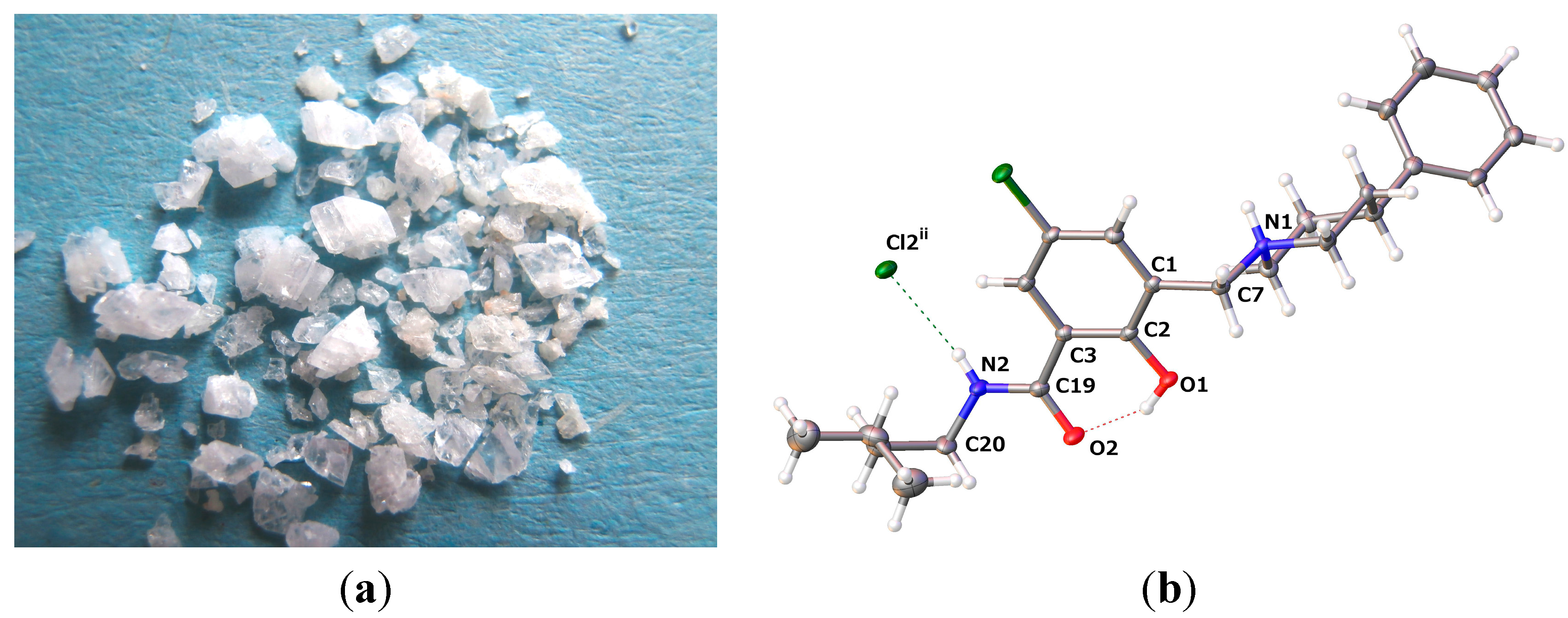
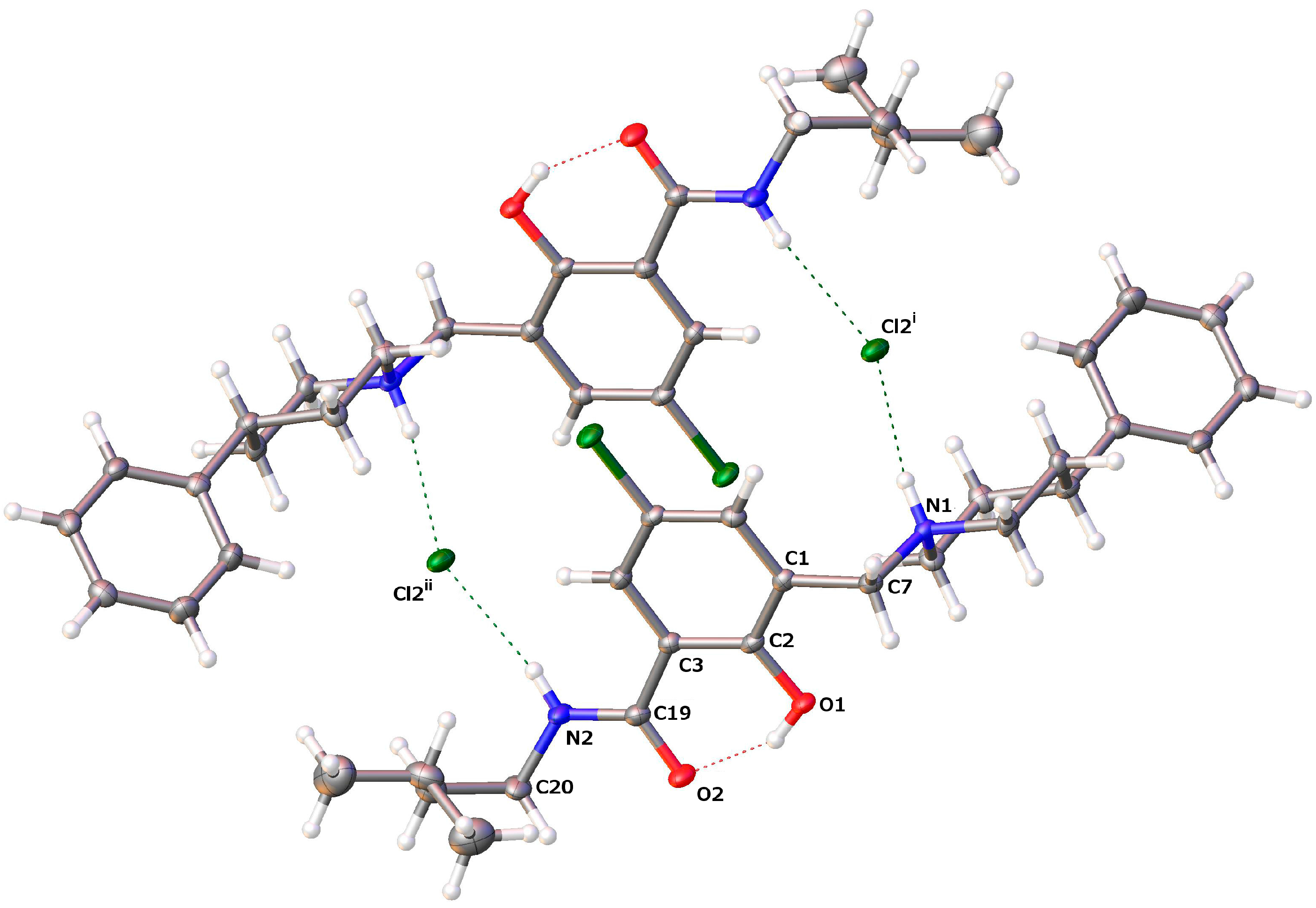
| D–H···A | D–H | H···A | D···A | D–H···A |
| O1–H1···O2 | 0.84 | 1.771 | 2.5203(13) | 147.4 |
| N1–H1A···Cl2i | 0.93 | 2.145 | 3.0617(11) | 168.4 |
| N2–H2···Cl2ii | 0.88 | 2.360 | 3.2191(12) | 165.4 |
2.2.3. Diethyl-[2-hydroxy-3-(3-methyl-butylcarbamoyl)-benzyl]-ammonium Chloride (14)

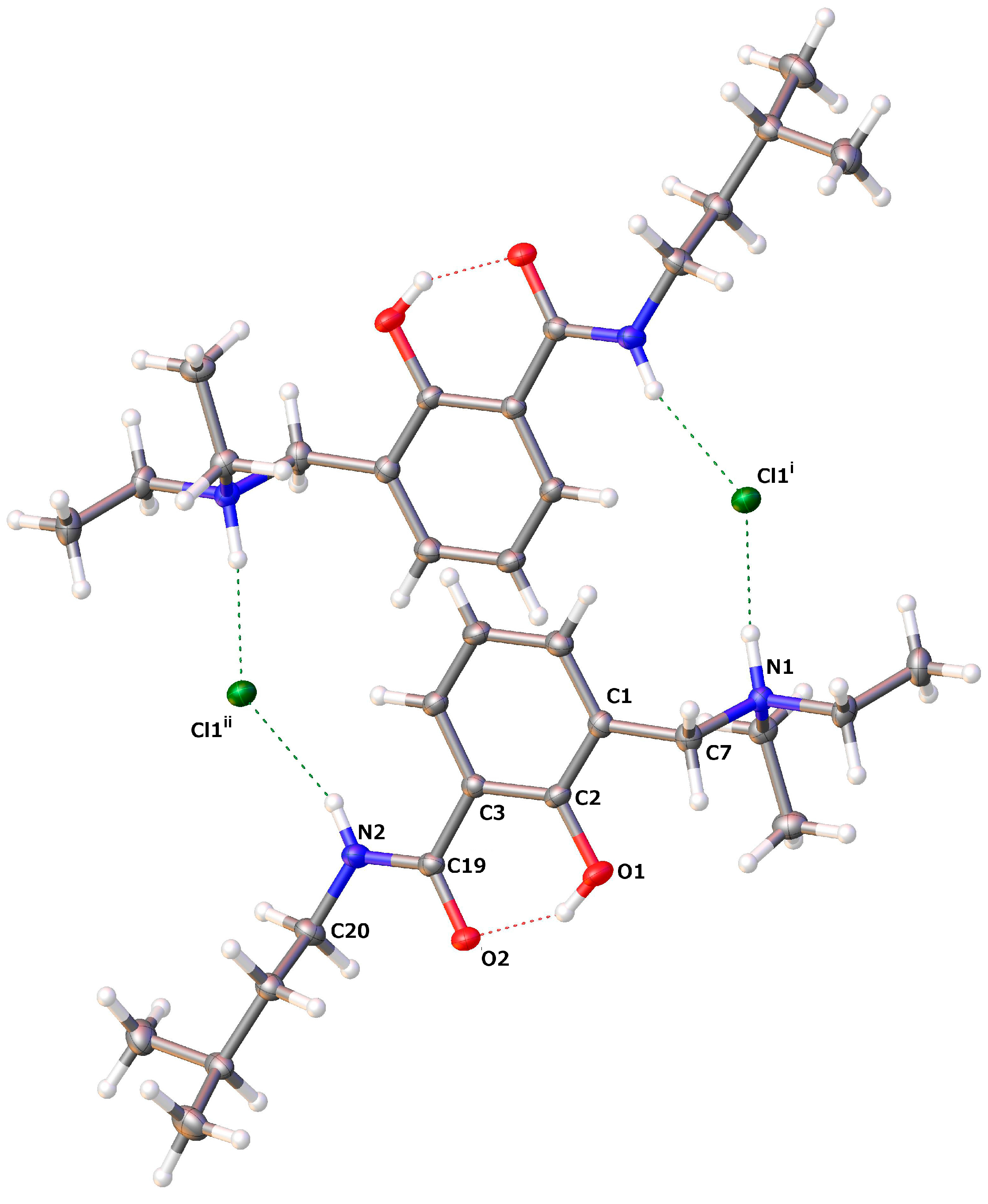
| D–H···A | D–H | H···A | D···A | D–H···A |
| O1–H1···O2 | 0.84 | 1.735 | 2.4905(11) | 148.6 |
| N1–H1A···Cl1i | 0.93 | 2.158 | 3.075(9) | 168.8 |
| N2–H2···Cl1ii | 0.88 | 2.399 | 3.2243(9) | 156.4 |
3. Experimental Section
3.1. Materials and Methods
3.2. Reaction Monitoring and Purification of Compounds
3.3. Analytical Characterization (mp, NMR)
3.4. Synthetic Procedures
3.4.1. Syntheses of Starting Materials 1 and 5
3.4.2. Aminomethylation of 1: Separation of Isomers 2–4
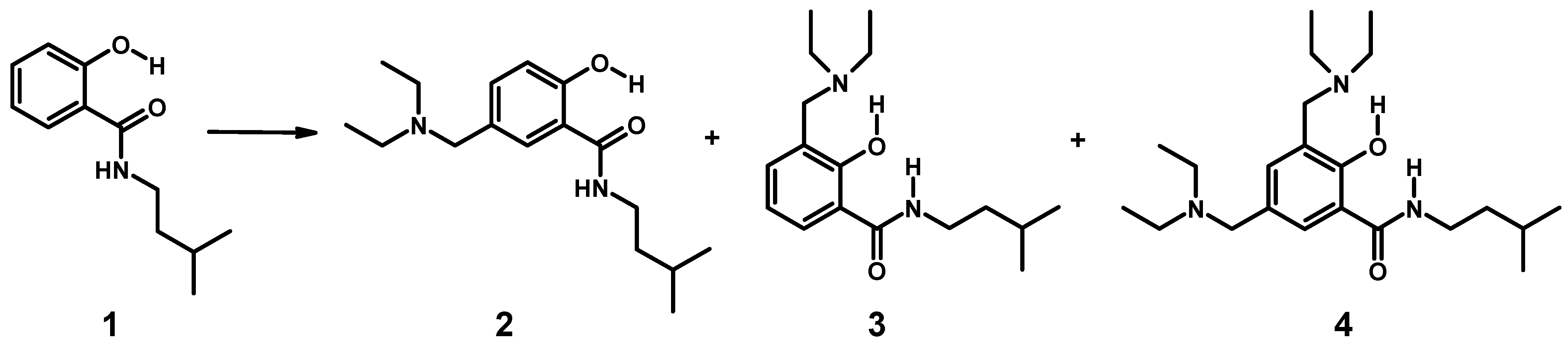
3.4.3. Aminomethylation of 5-Chloro-2-hydroxy-(3-methyl-butyl)-benzamide (5)
3.4.4. Synthesis of 5-Chloro-3-(1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-2-hydroxy-benzoic Acid (12)
3.4.5. Syntheses of Hydrochlorides 13–15 and Single Crystal Growth (Scheme 4)
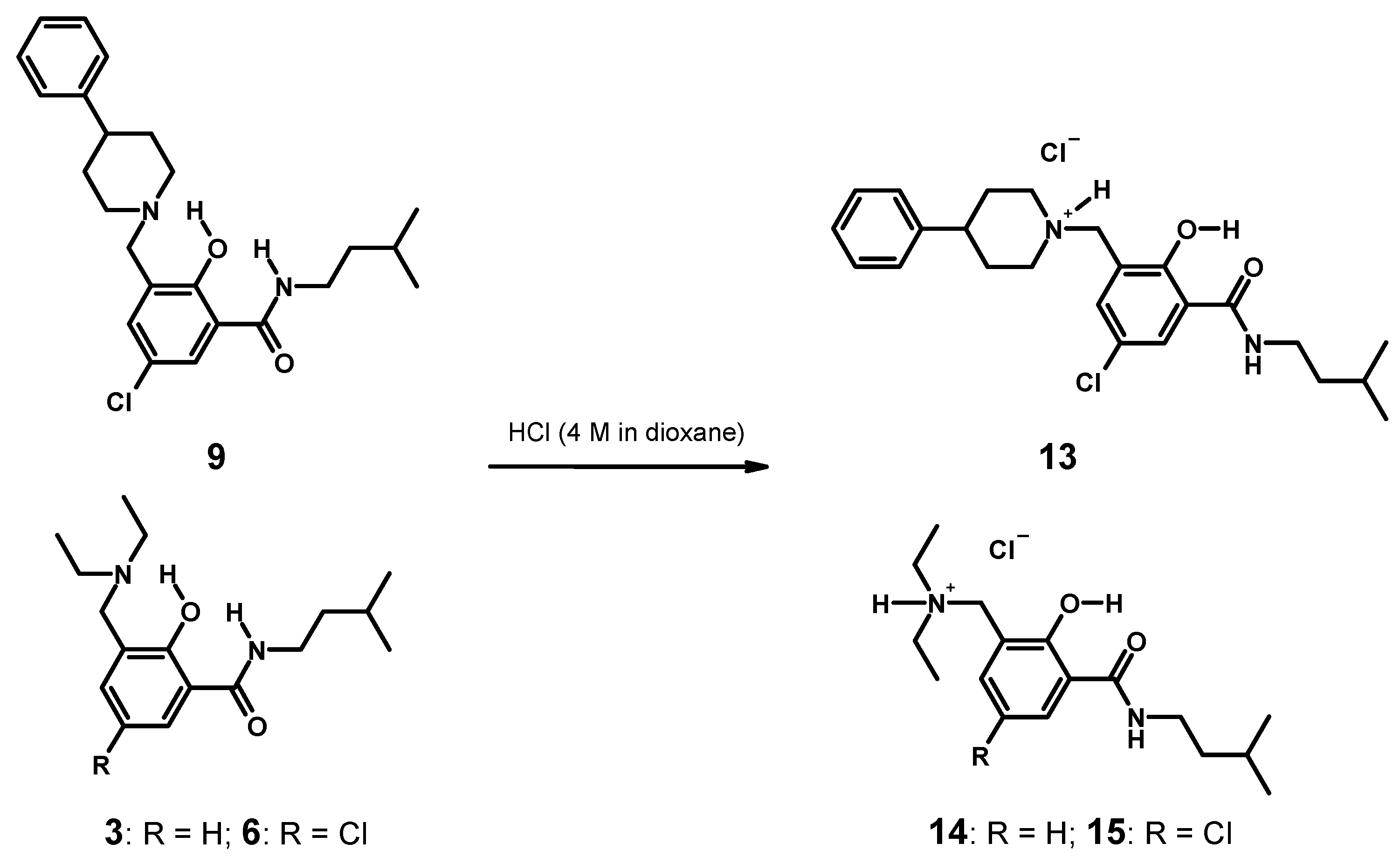
3.5. Crystallographic Structure Determination
| 9 | 13 | 14 | |
|---|---|---|---|
| CCDC deposition number | 1034222 | 1034223 | 1034221 |
| Identification code | hugs157 | hugs1572 | hugs014 |
| Empirical formula | C24H31ClN2O2 | C24H32Cl2N2O2 | C17H29ClN2O2 |
| Formula weight | 414.96 | 451.42 | 328.87 |
| Temperature | 100(2) K | 100(2) K | 100(2) K |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Crystal system | monoclinic | triclinic | monoclinic |
| Space group | P21/n | P-1 | C2/c |
| Unit cell dimensions | |||
| a (Å) | 5.8621(3) | 9.3714(4) | 19.8617(17) |
| b (Å) | 14.2609(7) | 11.9179(5) | 8.5447(6) |
| c (Å) | 25.7776(12) | 12.0435(5) | 22.7687(19) |
| α (°) | 108.9212(14) | ||
| β (°) | 92.9431(15) | 100.3377(15) | 110.078(5) |
| γ (°) | 103.9072(14) | ||
| Volume | 2,152.13(18) Å3 | 1185.61(9) Å3 | 3629.3(5) Å3 |
| Z | 4 | 2 | 8 |
| Density calculated | 1.281 g/cm3 | 1.264 g/cm3 | 1.204 g/cm3 |
| Absorption coefficient | 0.200 mm−1 | 0.296 mm−1 | 0.220 mm−1 |
| F(000) | 888 | 480 | 1424 |
| Crystal size (mm) | 0.23 × 0.18 × 0.08 | 0.21 × 0.21 × 0.13 | 0.30 × 0.14 × 0.06 |
| θ range for data collection | 2.13–30.04° | 1.86–30.12° | 1.90–30.03° |
| Index ranges | −8 ≤ h ≤ 8 | −13 ≤ h ≤ 13 | −27 ≤ h ≤ 27 |
| −20 ≤ k ≤ 20 | −16 ≤ k ≤ 16 | −12 ≤ k ≤ 12 | |
| −36 ≤ l ≤ 36 | −16 ≤ l ≤ 16 | −31 ≤ l ≤ 32 | |
| Reflections collected | 60,472 | 54,620 | 84,947 |
| Reflections independent | 6292 [Rint] = 0.0290 | 6974 [Rint] = 0.0433 | 5299 [Rint] = 0.0377 |
| Completeness to 2θ = 30.04 | 100% | 99.9% | 99.9% |
| Transmission Tmax/Tmin | 0.9847/0.9561 | 0.9617/0.9399 | 0.9870/0.9371 |
| Refinement method | Full-matrix least-squares on F2 | ||
| Data/restraints/parameters | 6292/0/265 | 6974/0/274 | 5299/0/204 |
| Goodness-of-fit on F2 | 1.012 | 1.006 | 1.027 |
| Final R indices (I > 2σ(I)) | R1 (obs. data) = 0.0349 | R1 (obs. data) = 0.0387 | R1 (obs. data) = 0.0351 |
| R indices (all data) | wR2 = 0.0957 | wR2 = 0.1084 | wR2 = 0.0967 |
| Largest diff. Peak and hole | 0.455 and −0.201 e.Å3 | 1.035 and −0.295 e.Å3 | 0.456 and −0.264 e.Å3 |
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Imramovský, A.; Pauk, K.; Pejchal, V.; Hanusek, J. Salicylanilides and Their Derivates as Perspective Anti-tuberculosis Drugs: Synthetic Routes and Biological Evaluations. Mini Rev. Org. Chem. 2011, 8, 211–220. [Google Scholar] [CrossRef]
- Krátký, M.; Vinsová, J.; Buchta, V.; Kata Horvati, K.; Bösze, S.; Jirina Stolaríková, J. New amino acid esters of salicylanilides active against MDR-TB and other microbes. Eur. J. Med. Chem. 2010, 45, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russell, R.K.; Sui, Z.; Weidner-Wells, M.A.; Werblood, H.; et al. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar]
- Hlasta, D.J.; Demers, J.P.; Foleno, B.D.; Fraga-Spano, S.A.; Guan, J.; Hilliard, J.J.; Macielag, M.J.; Ohemeng, K.A.; Sheppard, C.M.; Sui, Z.; et al. Novel inhibitors of bacterial two component systems with gram positive antibacterial activity: Pharmacophore identification based on the screening hit closantel. Bioorg. Med. Chem. Lett. 1998, 8, 1923–928. [Google Scholar]
- Hillard, J.J.; Goldschmidt, R.M.; Licata, L.; Baum, E.Z.; Bush, K. Multiple mechanism of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 1999, 43, 1693–1699. [Google Scholar] [PubMed]
- Waisser, K.; Hladuvkova, J.; Kunes, J.; Kubicova, L.; Klimesova, V.; Karajannis, P.; Kaustova, J. Synthesis and antimycobacterial activity of salicylanilides substituted in position 5. Chem. Pap. 2001, 55, 121–129. [Google Scholar]
- Imramovský, A.; Vinšová, J.; Férriz, J.M.; Kuneš, J.; Pour, M.; Doležal, M. Salicylanilide esterification: Unexpected formation of novel seven-membered rings. Tetrahedron Lett. 2006, 29, 5007–5011. [Google Scholar] [CrossRef]
- Vinšová, J.; Imramovský, A.; Buchta, V.; Čečková, M.; Doležal, M.; Štaud, F.; Jampílek, J.; Kaustová, J. Salicylanilide Acetates: Synthesis and Antibacterial Evaluation. Molecules 2007, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Vinšová, J.; Férriz, J.M.; Doležal, R.; Jampílek, J.; Kaustová, J.; Kunc, F. New Antituberculotics Originated from Salicylanilides with Promising In vitro Activity against Atypical Mycobacterial Strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Vinšová, J.; Férriz, J.M.; Buchta, V.; Jampílek, J. Salicylanilide esters of N-protected amino acids as novel antimicrobial agents. Bioorg. Med. Chem. Lett. 2009, 19, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Férriz, J.M.; Vávrová, K.; Kunc, F.; Imramovský, A.; Stolaříková, J.; Vavříková, E.; Vinšová, J. Salicylanilide carbamates: Antitubercular agents active against multidrug-resistant Mycobacterium tuberculosis strains. Bioorg. Med. Chem. 2010, 18, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Yamamoto, K.; Hamada, Y.; Ito, T. On the antimicrobial activity and syntheses of carbanilide and salicylanilide derivatives. Yakugaku Zasshi 1982, 102, 1023–1030. [Google Scholar]
- Krátký, M.; Vinšová, J. Antiviral Activity of Substituted Salicylanilides—A Review. Mini Rev. Med. Chem. 2011, 11, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Donner, P.L.; Pratt, J.K.; Jiang, W.W.; Ng, T.; Gracias, V.; Baumeister, S.; Wiedeman, P.E.; Traphagen, L.; Warrior, U.; et al. Identification of halosalicylamide derivatives as a novel class of allosteric inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2008, 18, 3173–3177. [Google Scholar]
- Jurgeit, A.; McDowell, R.; Moese, S.; Meldrum, E.; Schwendener, R.; Greber, U.F. Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects. PLoS Pathog. 2012, 8, e1002976. [Google Scholar] [CrossRef] [PubMed]
- Combrink, K.D.; Gulgeze, H.B.; Yu, K.-L.; Pearce, B.C.; Trehan, A.K.; Wei, J.; Deshpande, M.; Krystal, M.; Torri, A.; Luo, G.; et al. Salicylamide inhibitors of influenza virus fusion. Bioorg. Med. Chem. Lett. 2000, 10, 1649–1652. [Google Scholar] [CrossRef]
- Yu, K.-L.; Ruediger, E.; Luo, G.; Cianci, C.; Danetz, S.; Tiley, L.; Trehan, A.K.; Monkovic, I.; Pearce, B.; Martel, A.; et al. Novel quinolizidine salicylamide influenza fusion inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 2177–2180. [Google Scholar]
- Singh, H.; Singh, A.K.; Sharma, S.; Iyer, R.N. Synthesis of 5-chloro-3'-nitro-4-substituted salicylanilides, a new series of anthelmintic and antimicrobial agents. J. Org. Chem. 1977, 20, 826–829. [Google Scholar]
- Sjogren, E.B.; Rider, M.A.; Nelson, P.H.; Bingham, S.; Poulton, A.L.; Emanuel, M.A.; Komuniecki, R. Synthesis and biological activity of a series of diaryl susbtituted alpha-cyano-beta hydroxypenamides, a new class of anthelmintic agents. J. Med. Chem. 1991, 34, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Gamo, F.J.; Sanz, L.M.; Vidal, J.; de Cozar, C.; Alvarez, E.; Lavandera, J.L.; Vanderwall, D.E.; Green, D.V.S.; Kumar, V.; Hasan, S.; et al. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar]
- Gstach, H.; Chiba, P.; Mastalir, M. Preparation of Amidophenoxy-Propanol-Amines as Anitimalarial Agents. WO 2013186153 A2; 19 December 2013. [Google Scholar]
- Gabriel Navarrete-Vazquez, G.; Chavez-Silva, F.; Argotte-Ramos, R.; Rodriguez-Gutierrez, M.C.; Chan-Bacab, M.J.; Roberto Cedillo-Rivera, R.; Rosa Moo-Puc, R.; Hernandez-Nunez, E. Synthesis of benzologues of Nitazoxanide and Tizoxanide: A comparative study of their in vitro broad-spectrum antiprotozoal activity. Bioorg. Med. Chem. Lett. 2011, 21, 3168–3171. [Google Scholar] [CrossRef] [PubMed]
- Stec, J.; Huang, Q.; Pieroni, M.; Kaiser, M.; Fomovska, A.; Mui, E.; Witola, W.H.; Bettis, S.; McLeod, R.; Brun, R.; et al. Synthesis, Biological Evaluation, and Structure-Activity Relationships of N-Benzoyl-2-hydroxybenzamides as Agents Active against P. falciparum (K1 strain), Trypanosomes, and Leishmania. J. Med. Chem. 2012, 55, 3088–3100. [Google Scholar]
- Waisser, K.; Peřina, M.; Holý, P.; Pour, M.; Bureš, O.; Kuneš, J.; Klimešová, V.; Buchta, V.; Kubanová, P.; Kaustová, J. Anti mycobacterial and antifungal isosters of salicylamides. Arch. Pharm. Med. Chem. 2003, 336, 322–335. [Google Scholar] [CrossRef]
- Nawwar, G.A.M. Salicylanilides containg amino-acid or pyran moieties with molluscicidal activity. Arch. Pharm. (Weinheim) 1994, 327, 201–205. [Google Scholar] [CrossRef]
- Shoeb, H.A. Synthesis and molluscicidal activity of some anilides. Egypt. J. Chem. 1980, 22, 245–254. [Google Scholar]
- Högberg, T.; Norinder, U.; Rämsby, S.; Stensland, B. Crystallographic, theoretical and molecular modelling studies on the conformations of the salicylamide, raclopride, a selective dopamine-D2 antagonist. J. Pharm. Pharmacol. 1987, 39, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, T.; Rice, J.; Hodge, C.N.; Basarab, G.S.; Pierce, J.; Lindqvist, Y. Crystal structure of scytalone dehydratase—A disease determinant of the rice pathogen, magnaporthe grisea. Structure 1994, 2, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Fitzner, J.N.; Stevens, T.; Chin, W.; Wright, C.D.; Boyce, J.P. Salicylanilides: Selective inhibitors of interleukin-12p40 production. Bioorg. Med. Chem. 2008, 16, 8760–8764. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.P.; Brown, M.E.; Chin, W.; Fitzner, J.N.; Paxton, R.J.; Shen, M.; Stevens, T.; Wolfson, M.F.; Wright, C.D. Identification of 14–3-3z by Chemical Affinity with Salicylanilide Inhibitors of Interleukin-12p40 Production. Bioconjugate Chem. 2008, 19, 1775–1784. [Google Scholar] [CrossRef]
- Deng, W.; Guo, Z.; Guo, Y.; Feng, Z.; Juany, Y.; Chu, F. Acryloylamino-salicylanilides as EGFR PTK inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Steffen, J.D.; Coyle, D.L.; Damodaran, K.; Beroza, P.; Jacobson, M.K. Discovery and Structure Activity Relationships of Modified Salicylanilides as Cell Permeable Inhibitors of Poly(ADP-ribose) Glycohydrolase (PARG). J. Med. Chem. 2011, 54, 5403–5413. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.R.; Hamel, E.; Lee, D.J.; Pryor, D.E.; Cushman, M. Synthesis, anticancer activity, and inhibition of tubulin polymerization by conformationally restricted analogues of lavendustin A. J. Med. Chem. 2003, 46, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Waisser, K.; Perina, M.; Klimesova, V.; Kaustova, J. On the relationship between the structure and antimycobacterial activity of substituted N-benzylsalicylamides. Collect. Czechoslov. Chem. Commun. 2003, 68, 1275–1294. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Q.-L.; Jiang, Q.-Q.; Jiang, Q.-J.; Jiang, Y.-B. Anion-Triggered Substituent-Dependent Conformational Switching of Salicylanilides. New Hints for Understanding the Inhibitory Mechanism of Salicylanilides. J. Org. Chem. 2007, 72, 9947–9953. [Google Scholar]
- Suezawa, H.; Hirota, M.; Yuzuri, T.; Hamada, Y.; Takeuchi, I.; Sugiura, M. Studies on the conformations of antimicrobial salicylanilide derivatives by spectroscopy. Bull. Chem. Soc. Jpn. 2000, 73, 2335–2339. [Google Scholar] [CrossRef]
- Bilton, C.; Allen, F.H.; Shields, G.P.; Howard, J.A.K. Intramolecular hydrogen bonds: Common motifs, probabilities of formation and implications for supramolecular organization. Acta Crystallogr. Sect. B 2000, 56, 849–856. [Google Scholar] [CrossRef]
- Kuhn, B.; Mohr, P.; Stahl, M. Intramolecular hydrogen bonding in medicinal chemistry. J. Med. Chem. 2010, 53, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Hodge, C.N.; Pierce, J. A diazine heterocycle replaces a six-membered hydrogen-bonded array in the active site of scytalone dehydratase. Bioorg. Med. Chem. Lett. 1993, 3, 1605–1608. [Google Scholar] [CrossRef]
- Liechti, C.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Block, M.H.; Kenny, P.W.; Thomson, D.S.; Yu, M.T. The aminoquinazoline group as a replacement for the salicylamide group: The design and synthesis of a novel highly selective β1 adrenoceptor partial agonist. Drug Des. Discov. 1992, 9, 167–176. [Google Scholar] [PubMed]
- Furet, P.; Bold, G.; Hofmann, F.; Manley, P.; Meyer, T.; Altmann, K.-H. Identification of a new chemical class of potent angiogenesis inhibitors based on conformational considerations and database searching. Bioorg. Med. Chem. Lett. 2003, 13, 2967–2971. [Google Scholar] [CrossRef] [PubMed]
- Marimganti, S.; Cheemala, M.N.; Ahn, J.-M. Novel Amphiphilic α-Helix Mimetics. Org. Lett. 2009, 11, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Bai, S.; Yap, G.P.A.; Tarwade, V.; Fox, J.M. Abiotic metallofoldamers as electrochemically responsive molecules. J. Am. Chem. Soc. 2005, 127, 10590–10599. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Du, G.; Liu, K.; Jiang, L.; Ling, J.; Shen, Z. Chiroptical Inversion Induced by Rotation of a Carbon–Carbon Single Bond: An Experimental and Theoretical Study. J. Phys. Chem. A 2014, 118, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Velcheva, E.A.; Stamboliyska, B.A. Structural changes caused by the conversion of 2-hydroxybenzamide (salicylamide) into the oxyanion. J. Mol. Struct. 2008, 875, 264–271. [Google Scholar] [CrossRef]
- Kanamori, D.; Okamura, T.; Yamamoto, H.; Shimizu, S.; Tsujimoto, Y.; Ueyama, N. Structures of the small-molecule bcl-2 inhibitor (BH3I-2) and its related simple model in protonated and deprotonated forms. Bull. Chem. Soc. Jpn. 2004, 77, 2057–2064. [Google Scholar] [CrossRef]
- Kanamori, D.; Okamura, T.; Yamamoto, H.; Ueyama, N. Linear-to-Turn Conformational Switching Induced by Deprotonation of Unsymmetrically Linked Phenolic Oligoamides. Angew. Chem. Int. Ed. 2005, 44, 969–972. [Google Scholar] [CrossRef]
- Cignitti, M.; Ramusino, M.C.; Farina, A.; Rajevic, M. Conformational properties of o-alkoxy-benzamides in different solvents. J. Mol. Struct. 1996, 384, 9–16. [Google Scholar] [CrossRef]
- Koll, A.; Karpfen, A.; Wolschann, P. Structural and energetic consequences of the formation of intramolecular hydrogen bonds. J. Mol. Struct. 2006, 790, 55–64. [Google Scholar] [CrossRef]
- Koll, A.; Parasuk, V.; Parasuk, W.; Karpfen, A.; Wolschann, P. Theoretical study on the intramolecular hydrogen bond in chloro-substituted N,N-dimethylaminomethylphenols. I. Structural effects. J. Mol. Struct. 2004, 700, 81–90. [Google Scholar] [CrossRef]
- Koll, A.; Melikova, S.M.; Karpfen, A.; Wolschann, P. Spectroscopic and structural consequences of intramolecular hydrogen bond formation in ortho-dimethylaminomethylphenol. J. Mol. Struct. 2001, 559, 127–145. [Google Scholar] [CrossRef]
- Koll, A.; Wolschann, P. Mannich bases as model compounds for intramolecular hydrogen bonding. Part 2. Structure and properties in solution. Monatsh. Chem. 1999, 130, 983–1001. [Google Scholar]
- Koll, A.; Wolschann, P. Mannich bases as model compounds for intramolecular hydrogen bonding I. Solid state structures and molecular calculations. Monatsh. Chem. 1996, 127, 475–486. [Google Scholar]
- Ansari, S.; Robien, W.; Schlederer, M.; Wolschann, P. 1H-NMR investigations of the conformation of aryl-(hydroxynaphthyl)-methylpiperidines. Intramolecular interactions, IV. Monatsh. Chem. 1989, 120, 1003–1014. [Google Scholar]
- Haslinger, E.; Wolschann, P. Intramolecular interactions I. The use of some Mannich bases of naphthols as model compounds for intramolecular hydrogen bonding. Monatsh. Chem. 1980, 111, 563–574. [Google Scholar]
- Arend, M.; Westermann, B.; Risch, N. Modern variants of the mannich reaction. Angew. Chem. Int. Ed. 1998, 37, 1045–1070. [Google Scholar] [CrossRef]
- Bundgaard, H.; Klixbiill, U.; Falch, E. Prodrugs as drug delivery systems. o-Acyloxymethyl salicylamide N-Mannich bases as double prodrug forms for amines. Int. J. Pharm. 1986, 29, 19–28. [Google Scholar]
- Johansen, M.; Bundgaard, H. Pro-drugs as drug delivery systems xiii. Kinetics of decomposition of n-mannich bases of salicylamide and assessment of their suitability as possible pro-drugs for amines. Int. J. Pharm. 1980, 7, 119–127. [Google Scholar]
- Brandes, R.; Roth, H.J. Configuration of the mannich bases of amides and methyl esters of β-resorcylic, gentisic, and 2-hydroxy-2-naphthoic acids. Arch. Pharm. (Weinheim) 1967, 300, 992–1000. [Google Scholar] [CrossRef]
- Soliman, F.M.; Said, M.M.; Maigali, S.S. Chemistry of phosphorus ylides. Part 22. Effect of newly synthesized niclosamide mannich bases, phosphopyranones, phosphoranylidenes, and oxaphosphinin on some metabolic aspects of biomphalaria alexandrina. Monatsh. Chem. 2005, 136, 241–251. [Google Scholar]
- Stavrovskaya, V.I.; Drusvyatskaya, S.K. Acid amides in the mannich reaction. II. Aminomethylation of salicyl- and 5-chlorosalicylamides. 1967, 1, 164–168. [Google Scholar]
- Kondo, M. Spectroscopic studies of solvent effects on intramolecular hydrogen bonding in N-substituted salicylamides. Bull. Chem. Soc. Jpn. 1979, 52, 521–523. [Google Scholar] [CrossRef]
- Kondo, M. The nuclear magnetic resonance study of several o-Disubstituted Benzenes. Bull. Chem. Soc. Jpn. 1972, 45, 2790–2793. [Google Scholar] [CrossRef]
- Steinwender, E.; Mikenda, W. O-H...O(S) hydrogen bonds in 2-hydroxy(thio)benzamides. Survey of spectroscopic and structural data. Monatsh. Chem. 1990, 121, 809–820. [Google Scholar]
- Jo, Y.; Ju, J.; Choe, J.; Song, K.H.; Lee, S. The scope and limitation of nickel-catalyzed aminocarbonylation of aryl bromides from formamide derivatives. J. Org. Chem. 2009, 74, 6358–6361. [Google Scholar] [CrossRef] [PubMed]
- Galan, J.F.; Brown, J.; Wildin, J.L.; Liu, Z.; Liu, D.; Moyna, G.; Pophristic, V. Intramolecular Hydrogen Bonding in ortho-Substituted Arylamide Oligomers: A Computational and Experimental Study of ortho-Fluoro- and ortho-Chloro-N-methylbenzamides. J. Phys. Chem. B 2009, 113, 12809–12815. [Google Scholar] [CrossRef] [PubMed]
- Galan, F.J.; Tang, C.N.; Chakrabarty, S.; Liu, Z.; Moynaab, G.; Pophristic, V. Conformational preferences of furan- and thiophene-based arylamides: A combined computational and experimental study. Phys. Chem. Chem. Phys. 2013, 15, 11883–11892. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Yan, Y.; Zeng, H.; Skrzypczak-Jankunn, E.; Kim, Y.W.; Zhu, J.; Ickes, H. A New Approach for the Design of Supramolecular Recognition Units: Hydrogen-Bonded Molecular Duplexes. J. Am. Chem. Soc. 1999, 121, 5607–5608. [Google Scholar] [CrossRef]
- Liu, Z.; Remsing, R.C.; Liu, D.; Moyna, G.; Pophristic, V. Hydrogen Bonding in ortho-Substituted Arylamides: The Influence of Protic Solvents. J. Phys. Chem. 2009, 113, 7041–7044. [Google Scholar] [CrossRef]
- McConnell, H.M. Theory of nuclear magnetic shielding in molecules. I. Long-range dipolar shielding of protons. J. Chem. Phys. 1957, 27, 226–229. [Google Scholar]
- Paulsen, H.; Todt, K. Magnetic Anisotropy of the Amide Group. Angew. Chem. Int. Ed. 1966, 5, 899–900. [Google Scholar] [CrossRef]
- Brown, R.F.C.; Radom, L.; Sternhell, S.; Rae, I.D. Proton magnetic resonance spectra of some aromatic amines and derived amides. Can. J. Chem. 1968, 46, 2577–2587. [Google Scholar] [CrossRef]
- Anker, L.; Van De Waterbeemd, H.; Testa, B.; Lauterwein, J. NMR Conformational Study of Aminoalkylbenzamides, Aminoalkyl-o-Anisamides, and Metoclopramide, a Dopamine Receptor Antagonist. Helv. Chim. Acta 1984, 67, 706–716. [Google Scholar] [CrossRef]
- ApSimon, J.W.; Demarco, P.V.; Mathieson, D.W.; Craig, W.G.; Karim, A.; Saunders, L.; Whalley, W.B. NMR—The chemical shift—IV: The anisotropies of the carbonyl group. Tetrahedron 1970, 26, 119–146. [Google Scholar] [CrossRef]
- Stewart, W.E.; Siddall, T.H., III. Nuclear magnetic resonance studies of amides. Chem. Rev. 1970, 70, 517–551. [Google Scholar]
- Buděšínský, M.; Kulhánek, J.; Böhm, S.; Cigler, P.; Exner, O. 13C and 1H nuclear magnetic resonance of methyl-substituted acetophenones and methyl benzoates: Steric hindrance and inhibited conjugation. Magn. Res. Chem. 2004, 42, 844–851. [Google Scholar] [CrossRef]
- Baranac-Stojanovic, M. New insight into the anisotropic effects in solution-state NMR spectroscopy. RSC Adv. 2014, 4, 308–321. [Google Scholar] [CrossRef]
- Sobczyk, L.; Grabowski, S.J.; Krygowski, T.M. Interrelation between H-bond and Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3513–3560. [Google Scholar] [CrossRef] [PubMed]
- Kawski, P.; Kochel, A.; Perevozkina, M.G.; Filarowski, A. The intramolecular hydrogen bond in 2-hydroxy-benzamides. J. Mol. Struct. 2006, 790, 65–73. [Google Scholar] [CrossRef]
- Olah, G.A.; Wang, Q.; Sandford, G.; Oxyzoglou, A.B.; Surya Prakash, G.K. Superelectrophilic Tscherniac Amidomethylation of Aromatics with N-Hydroxymethylphthalimide in Trifluoromethanesulfonic Acid. Synthesis 1993, 11, 1077–1079. [Google Scholar] [CrossRef]
- Zaugg, H.E.; DeNet, R.W.; Fraser, J.E.; Kotre, A.M. Tscherniac-Einhorn reaction. II. Kinetics and mechanism. J. Org. Chem. 1969, 34, 14–18. [Google Scholar]
- Sahu, S.K.; Azam, M.A.; Banerjee, M.; Choudhury, P.; Sutradhar, S.; Panda, P.K.; Misro, P.K. Synthesis and biological evaluation of 3-(phthalimidomethyl)- 4-(5-substituted isoxazoline and pyrazoline) substituted benzanilides. J. Indian Chem. Soc. 2007, 84, 1011–1015. [Google Scholar]
- Sasada, Y.; Takano, T.; Kakudo, M. Crystal structure of salicylamide. Bull. Chem. Soc. Jpn. 1964, 37, 940–946. [Google Scholar] [CrossRef]
- Pertlik, F. Crystal Structures and Hydrogen Bonding Schemes in Four Benzamide Derivatives (2-Hydroxy-benzamide, 2-Hydroxy-thiobenzamide, 2-Hydroxy-N,N-dimethyl-benzamide, and 2-Hydroxy-N,N-dimethyl-thiobenzamide. Monatsh. Chem. 1990, 121, 129–139. [Google Scholar] [CrossRef]
- Pertlik, F. Crystal structure of 2-hydroxy-N-methylbenzamide and 2-hydroxy-N-methylthiobenzamide. Z. Kristallogr. 1992, 202, 17–23. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pauk, K.; Padelkova, Z.; Hanusek, J. Crystal Structure of the 5-Chloro Salicylamides: Three Different Types of the H-bonding Influenced Linear Chain Formation in the Solid State. Crystalls 2012, 2, 349–361. [Google Scholar] [CrossRef]
- Zhang, Q.-X.; Zhang, B.-S. N-Benzyl-2-hydroxybenzamide. Acta Crystallogr. Sect. E 2008, 64, o884. [Google Scholar] [CrossRef]
- Etter, M.C.; Urbanczyk-Lipkowska, Z.; Ameli, T.M.; Panunto, T.W. Intra- versus intermolecular hydrogen bonds in salicylamide derivatives. J. Chem. Cryst. 1988, 18, 491–507. [Google Scholar]
- Aarset, K.; Page, E.M.; Rice, D.A. Hydrogen Bonding in the Gas-Phase: The Molecular Structures of 2-Hydroxybenzamide (C7H7NO2) and 2-Methoxybenzamide (C8H9NO2), Obtained by Gas-Phase Electron Diffraction and Theoretical Calculations. J. Phys. Chem. 2013, 117, 3034–3040. [Google Scholar] [CrossRef]
- Song, X.-Q. Tetrakis[2-(benzylaminocarbonyl)phenoxymethyl]-methane. Acta Crystallogr. Sect. E 2008, 64, o1911. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Geffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Koshy, K.T. Comparative stability of benzamide, salicylamide, and some N-substituted derivatives. J. Pharm. Sci. 1969, 58, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, Z.-P. 2-(hydroxymethyl)isoindoline-1,3-dione. Acta Crystallogr. Sect. E 2006, 62, o4276–o4277. [Google Scholar] [CrossRef]
- Pressprich, M.R.; Chambers, J. SAINT-Plus, version 7.06a and APEX2; Bruker-Nonius AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–12 are available from the corresponding author.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dank, C.; Kirchknopf, B.; Mastalir, M.; Kählig, H.; Felsinger, S.; Roller, A.; Arion, V.B.; Gstach, H. Hybrids of Salicylalkylamides and Mannich Bases: Control of the Amide Conformation by Hydrogen Bonding in Solution and in the Solid State. Molecules 2015, 20, 1686-1711. https://doi.org/10.3390/molecules20011686
Dank C, Kirchknopf B, Mastalir M, Kählig H, Felsinger S, Roller A, Arion VB, Gstach H. Hybrids of Salicylalkylamides and Mannich Bases: Control of the Amide Conformation by Hydrogen Bonding in Solution and in the Solid State. Molecules. 2015; 20(1):1686-1711. https://doi.org/10.3390/molecules20011686
Chicago/Turabian StyleDank, Christian, Barbara Kirchknopf, Matthias Mastalir, Hanspeter Kählig, Susanne Felsinger, Alexander Roller, Vladimir B. Arion, and Hubert Gstach. 2015. "Hybrids of Salicylalkylamides and Mannich Bases: Control of the Amide Conformation by Hydrogen Bonding in Solution and in the Solid State" Molecules 20, no. 1: 1686-1711. https://doi.org/10.3390/molecules20011686
APA StyleDank, C., Kirchknopf, B., Mastalir, M., Kählig, H., Felsinger, S., Roller, A., Arion, V. B., & Gstach, H. (2015). Hybrids of Salicylalkylamides and Mannich Bases: Control of the Amide Conformation by Hydrogen Bonding in Solution and in the Solid State. Molecules, 20(1), 1686-1711. https://doi.org/10.3390/molecules20011686