Synthesis and Evaluation of Paeonol Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
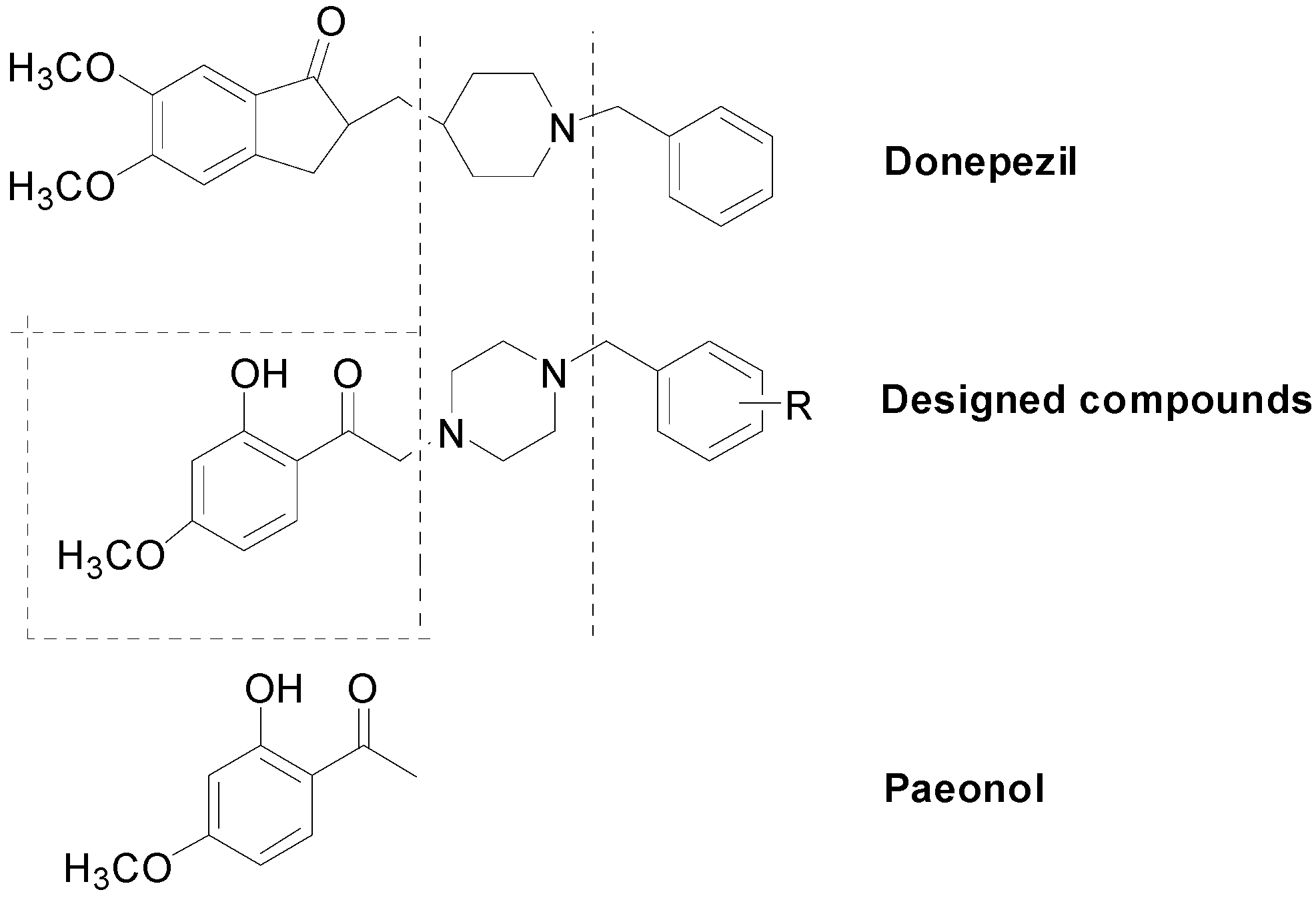
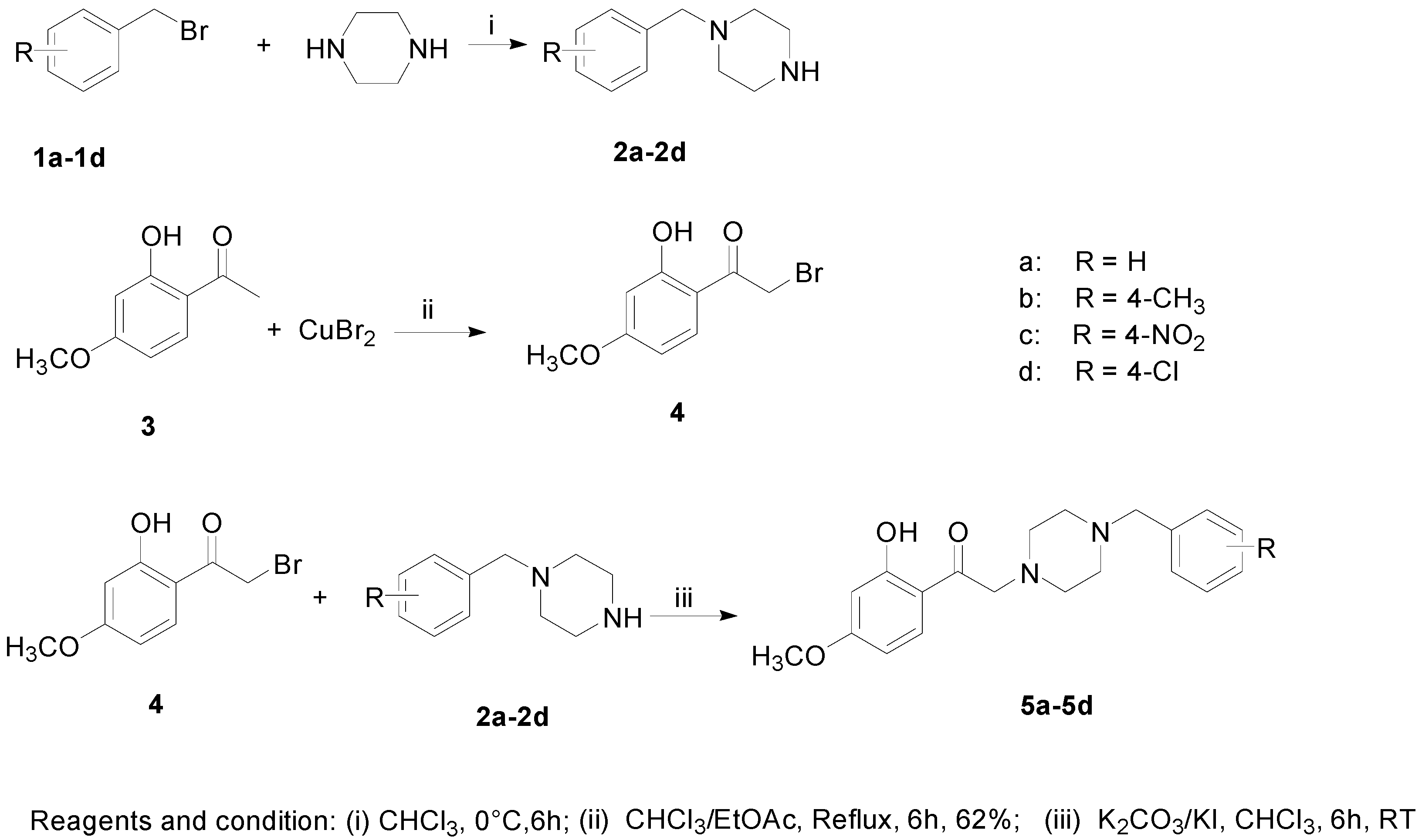
2.2. Biological Evaluation
2.2.1. Free Radical Scavenging Activity in Vitro (DPPH Assay)
| Compounds | Molecular Weight | DPPH Scavenging (a IC50, μM) | AChE Inhibition (b IC50, μM) |
|---|---|---|---|
| 5a | 340.1 | 142.79 ± 8.83 | 1.59 ± 0.011 |
| 5b | 354.2 | 157.85 ± 6.88 | 0.61 ± 0.009 |
| 5c | 385.4 | 191.64 ± 8.06 | 7.04 ± 0.032 |
| 5d | 374.1 | 187.32 ± 13.73 | 2.63 ± 0.153 |
| Paeonol | 166.2 | 309.67 ± 5.41 | nt |
| Donepezil | 379.4 | nt | 0.053 ± 0.003 |
2.2.2. Inhibitory Effect on PC12 Cell Death Induced by Oxidative Stress
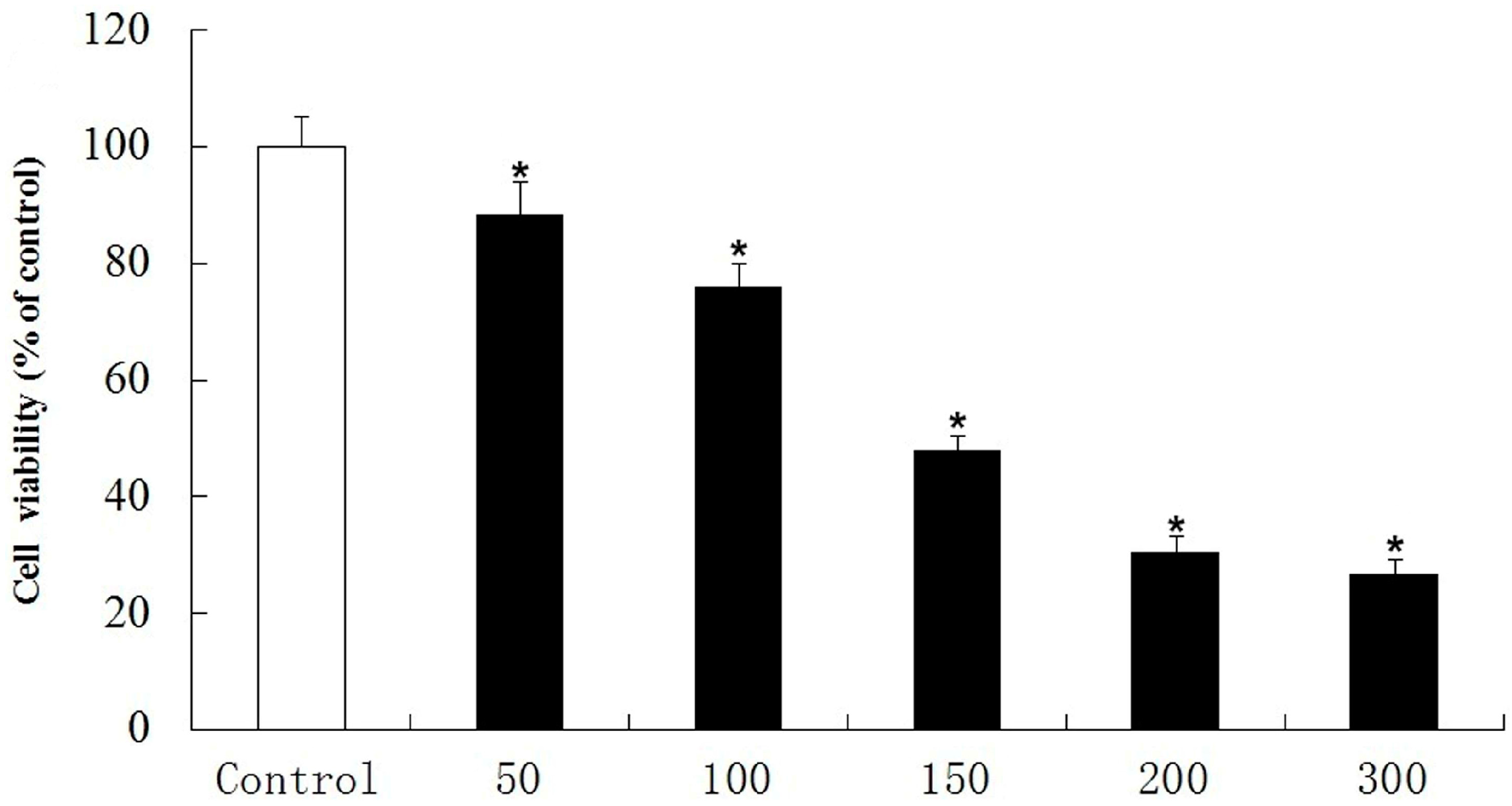
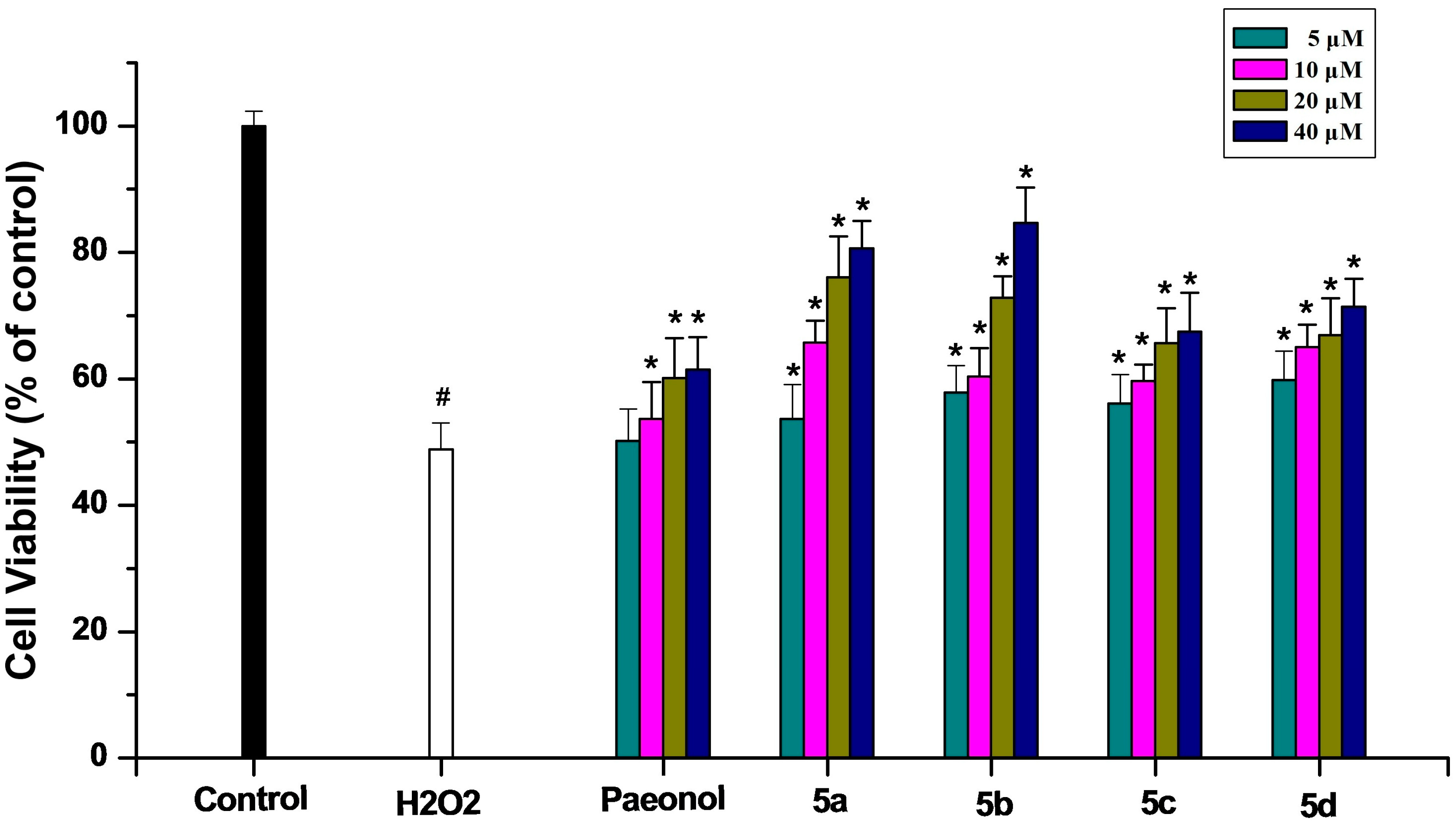
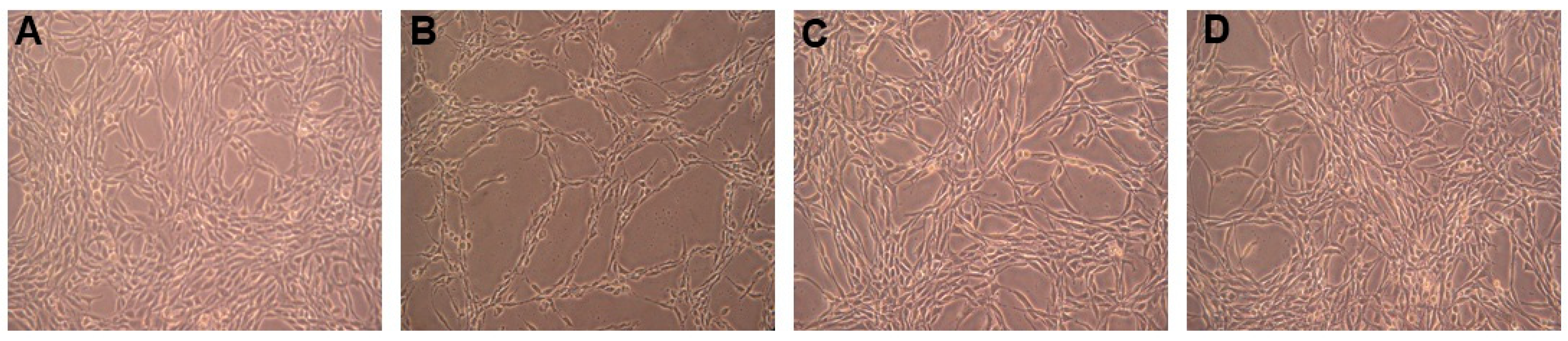
2.2.3. Anticholinesterase Activity
2.2.4. Molecular Docking
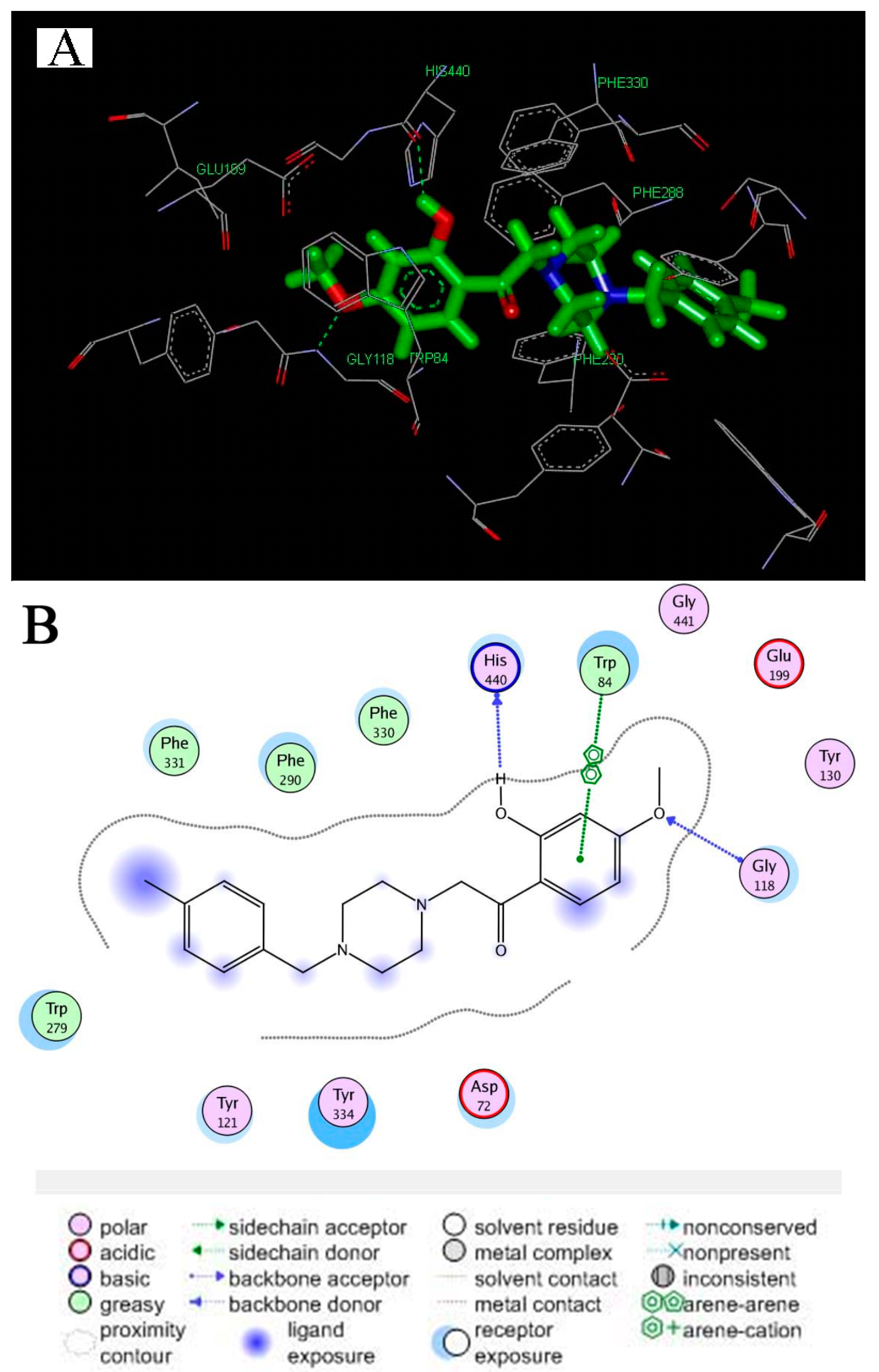
2.2.5. Metal-Chelating Study
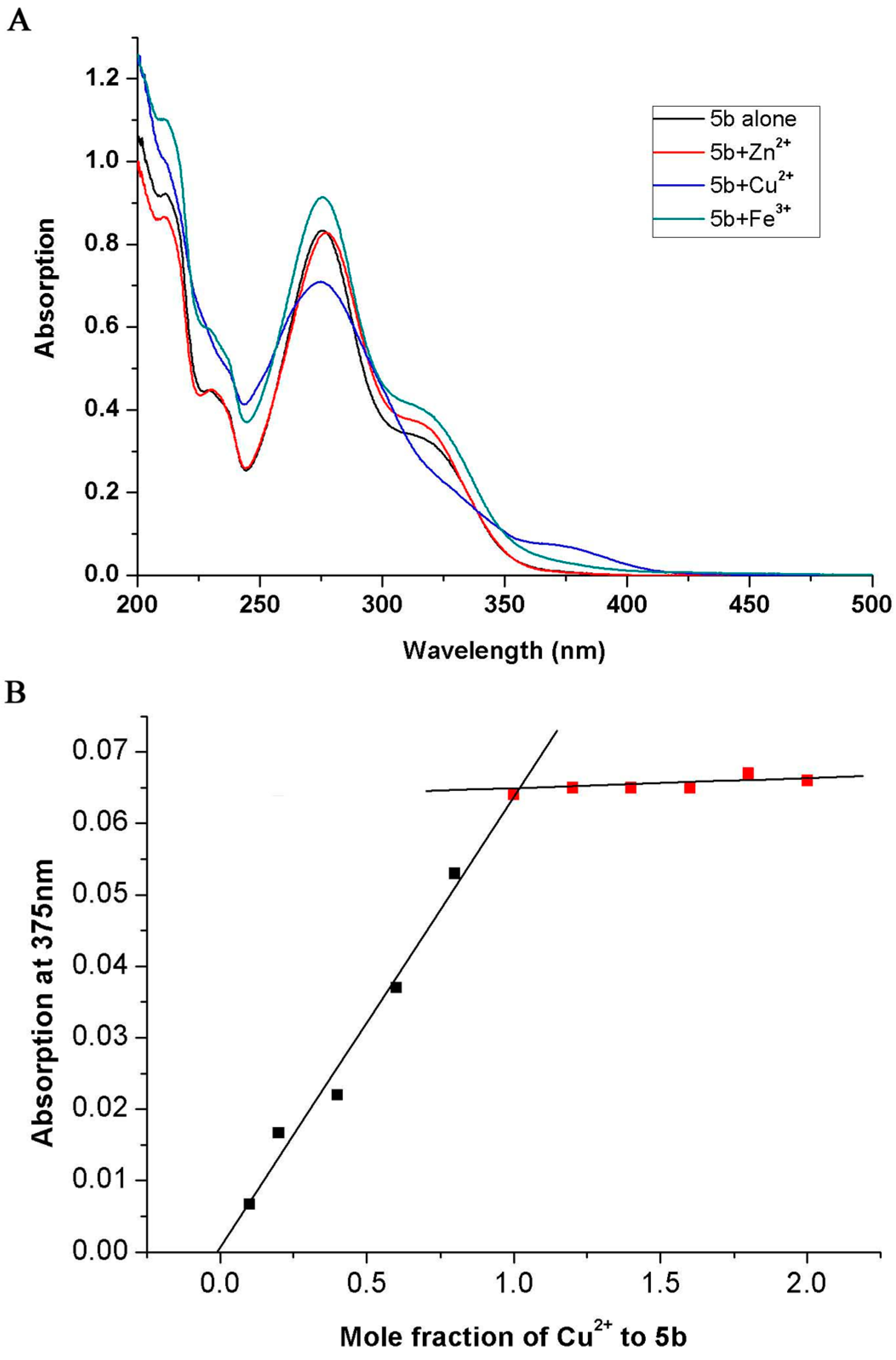
3. Experimental Section
3.1. Chemistry
3.2. Synthesis
3.3. DPPH Radical Scavenging Activity
3.4. Protection of PC12 Cells against Hydrogen Peroxide-Induced Damage
3.4.1. Cell Culture
3.4.2. Cell Viability Assay
3.5. In Vitro AChE Inhibition Assay
3.6. Molecular Docking
3.7. Metal-Chelating Studies
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palmer, A.M. Neuroprotective therapeutics for Alzheimer’s disease: Progress and prospects. Trends Pharmacol. Sci. 2011, 32, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.; Downton, C. Alzheimer disease: Progress or profit? Nat. Med. 2006, 12, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.D.; Mucke, L. 100 Years and counting: Prospects for defeating Alzheimer’s disease. Science 2006, 314, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors andbeyond. Curr. Alzheimer Res. 2009, 6, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, R.; Wirth, Y.; Janetzky, W.; Hartmann, S. Efficacy of memantine in delaying clinical worsening in Alzheimer’s disease (AD): Responder analyses of nine clinical trials with patients with moderate to severe AD. Int. J. Geriatr. Psychiatry 2012, 27, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Podlisny, M.B. Deciphering the genetic basis of Alzheimer’s disease. Annu. Rev. Genomics Hum. 2002, 3, 67–99. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Schelterns, P.; Feldman, H. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 2004, 44, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Mayer, R.T.; Himel, C.M. Conformers ofacetylcholinesterase: A mechanism of allosteric control. Mol. Pharmacol. 1994, 45, 74–83. [Google Scholar] [PubMed]
- Carreiras, M.C.; Mendes, E.; Perry, M.J.; Francisco, A.P.; Marco-Contelles, J. The multifactorial nature of Alzheimer’s disease for developing potential therapeutics. Curr. Top. Med. Chem. 2013, 13, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Sainz, R.; Mayo, J.C.; Alvares, F.L.; Reiter, R.J. Antioxidant strategies in protection against neurodegenerative disorders. Expert Opin. Ther. Pat. 2003, 13, 1513–1543. [Google Scholar] [CrossRef]
- Minarini, A.; Milelli, A.; Simoni, E.; Rosini, M.; Laura Bolognesi, M.; Marchetti, C.; Tumiatti, V. Multifunctional tacrine derivatives in Alzheimer’s disease. Curr. Top. Med. Chem. 2013, 13, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Salga, S.M.; Ali, H.M.; Abdullah, M.A.; Abdelwahab, S.I.; Wai, L.K.; Buckle, M.J.; Hadi, A.H.A. Synthesis, characterization, acetylcholinesterase inhibition, molecular modeling and antioxidant activities of some novel schiff bases derived from 1-(2-ketoiminoethyl) piperazines. Molecules 2011, 16, 9316–9330. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.Z.; Ge, Q.H.; Qu, R.; Li, Q.; Ma, S.P. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J. Neurol. Sci. 2009, 277, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Hsu, Y.Y.; Shih, Y.T.; Lo, Y.C. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in rat primary microglia and cortical neurons. Shock 2012, 37, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, L.; Hou, D.; Tang, J.; Sun, J.; Bondy, S.C. Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer’s disease. Brain Res. 2011, 1388, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, G.; Yang, S.; Wang, X.; Cheng, H.; Wang, F.; Li, Q. Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via down regulation of ERK activation and inhibition of apoptosis. Planta Med. 2011, 77, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Lahiri, D.K. Metal and inflammatory targets for Alzheimer’s disease. Curr. Drug Targets 2004, 5, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Tan, C.Y.; Zu, X.Y.; Zhai, X.; Liu, F.; Chu, B.Z.; Ma, X.H.; Chen, Y.Z.; Gong, P.; Jiang, Y.Y. Exploration of (S)-3-aminopyrrolidine as a potentially interesting scaffold for discovery of novel Ab1 and PI3K dual inhibitors. Eur. J. Med. Chem. 2011, 46, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Huang, L.; Luo, Z.; Liu, A.; Lu, C.; Xie, Z.; Li, X. O-Hydroxyl- or o-amino benzylamine-tacrine hybrids: Multifunctional biometalschelators, antioxidants, and inhibitors of cholinesterase activity and amyloid-β aggregation. Bioorganic Chem. Lett. 2012, 20, 5884–5892. [Google Scholar] [CrossRef]
- Lu, C.; Guo, Y.; Yan, J.; Luo, Z.; Luo, H.B.; Yan, M.; Ling, H.; Li, X. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer’s disease. J. Med. Chem. 2013, 56, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Sang, Z.; Yuan, W.; Li, Y.; Liu, Q.; Bai, P.; Shi, Y.; Ang, W.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 76, 314–331. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, P.F.; Chen, S.B.; Huang, Z.H.; Huang, S.L.; Tan, J.H.; Li, D.; Gu, L.Q.; Huang, Z.S. Synthesis and evaluation of 7,8-dehydrorutaecarpine derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 63, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and Evaluation of Paeonol Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Molecules 2015, 20, 1304-1318. https://doi.org/10.3390/molecules20011304
Zhou A, Wu H, Pan J, Wang X, Li J, Wu Z, Hui A. Synthesis and Evaluation of Paeonol Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Molecules. 2015; 20(1):1304-1318. https://doi.org/10.3390/molecules20011304
Chicago/Turabian StyleZhou, An, Hongfei Wu, Jian Pan, Xuncui Wang, Jiaming Li, Zeyu Wu, and Ailing Hui. 2015. "Synthesis and Evaluation of Paeonol Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease" Molecules 20, no. 1: 1304-1318. https://doi.org/10.3390/molecules20011304
APA StyleZhou, A., Wu, H., Pan, J., Wang, X., Li, J., Wu, Z., & Hui, A. (2015). Synthesis and Evaluation of Paeonol Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Molecules, 20(1), 1304-1318. https://doi.org/10.3390/molecules20011304





