Ginsenoside Rc Promotes Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Down-Regulating C/EBPα and PPARγ
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ginsenoside Rc Inhibit 3T3-L1 Preadipocyte Proliferation
2.2. Ginsenoside Rc Was Effective in Inhibiting Adipocyte Differentiation
| Treatment Concentration | Optical Density (λ = 595 nm) | |||
|---|---|---|---|---|
| 24 h | 48 h | 96 h | 144 h | |
| Control | 0.634 ± 0.017 aD | 0.808 ± 0.021 aC | 1.210 ± 0.025 aB | 1.503 ± 0.031 aA |
| 6.25 µM | 0.599 ± 0.019 bD | 0.775 ± 0.019 bC | 1.125 ± 0.056 bB | 1.257 ± 0.065 bA |
| 12.5 µM | 0.574 ± 0.021 bcD | 0.755 ± 0.018 bcC | 1.095 ± 0.043 bB | 1.211 ± 0.060 bcA |
| 25 µM | 0.550 ± 0.011 cD | 0.725 ± 0.012 cdC | 1.055 ± 0.047 bcB | 1.157 ± 0.058 bcdA |
| 50 µM | 0.553 ± 0.018 cD | 0.713 ± 0.012 deC | 1.007 ± 0.013 cdB | 1.124 ± 0.080 cdA |
| 100 µM | 0.513 ± 0.010 dD | 0.698 ± 0.017 deC | 0.950 ± 0.053 dB | 1.072 ± 0.062 dA |
| 200 µM | 0.460 ± 0.012 eC | 0.494 ± 0.020 eC | 0.564 ± 0.051 eB | 0.619 ± 0.019 eA |
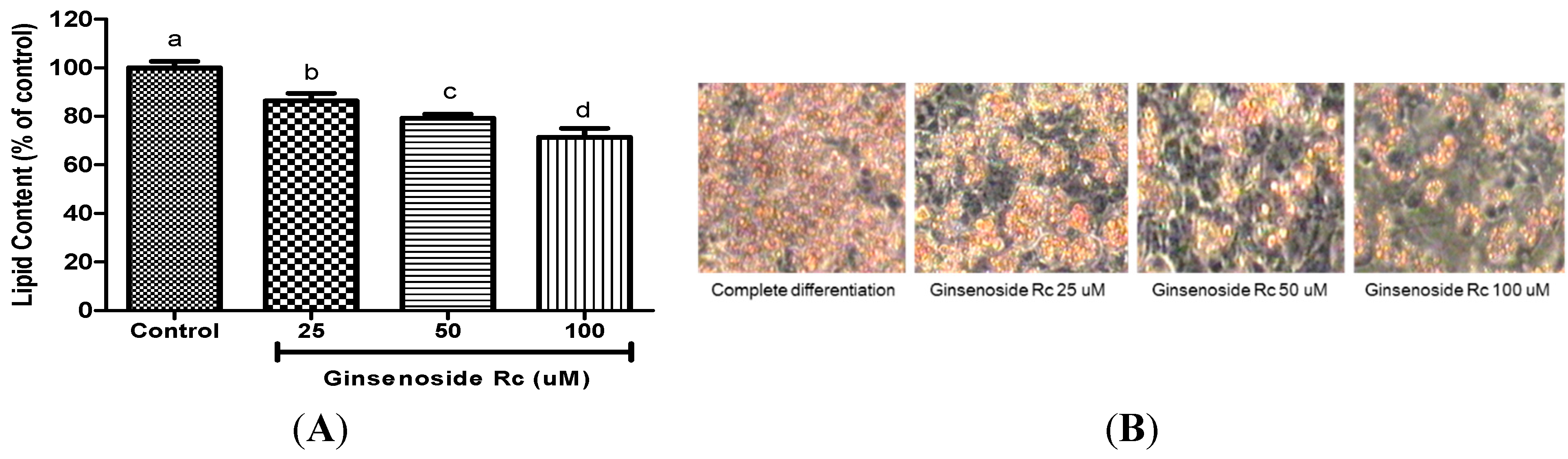
2.3. Inhibition of Intracellular Triglyceride Content in 3T3-L1
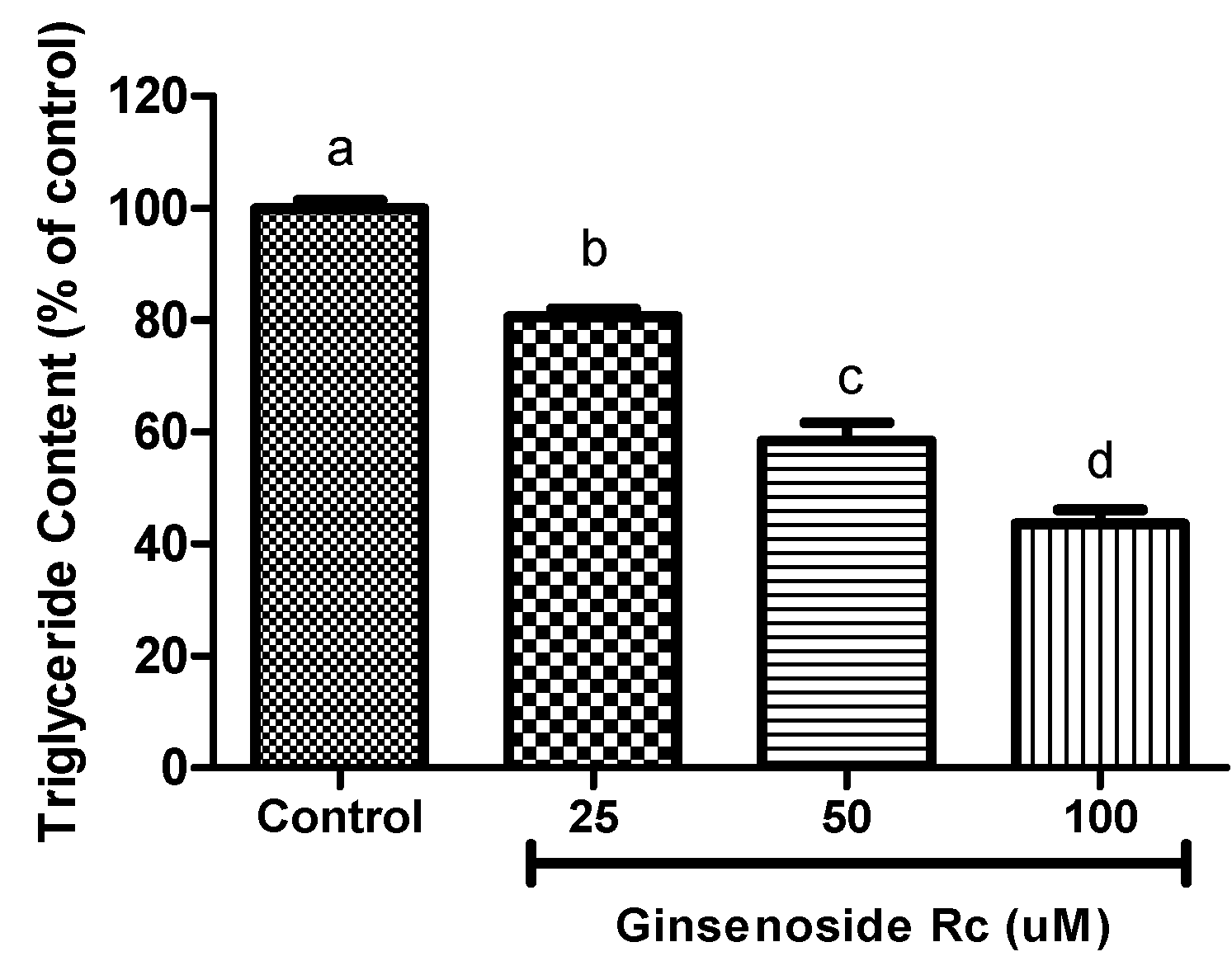
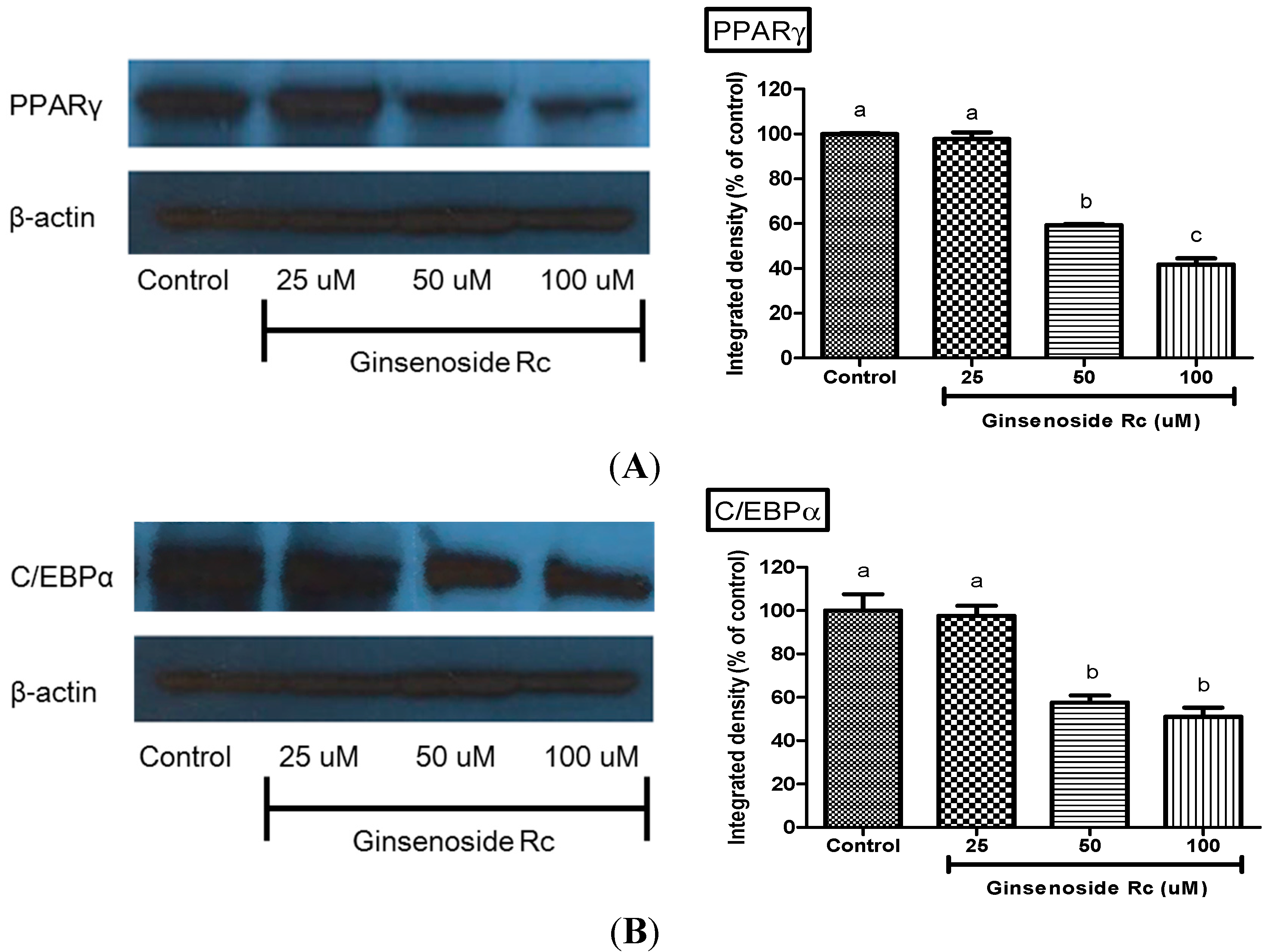
2.4. Modulation of Adipocyte Differentiation-Related Protein by Rc
2.5. Discussion
3. Experimental Section
3.1. 3T3-L1 Cell Culture and Adipocyte Differentiation
3.2. MTT Assay
3.3. Determination of Lipid Accumulation by Oil Red O Staining
3.4. Measurement of Triglyceride Content
3.5. Western Blot Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Fuzzati, N. Analysis methods of ginsenosides. J. Chromatogr. B 2004, 812, 119–133. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, J.T. New achievements in ginseng research and its future prospects. Chin. J. Integr. Med. 2009, 15, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Q.; Xu, Q.; Liang, X.M. Analysis of ginsenosides in ginseng by liquid chromatography-atmospheric pressure chemical-ionization mass-spectrometry. Chin. J. Chromatogr. 2005, 23, 389–393. (In Chinese) [Google Scholar]
- Sanada, S.; Kondo, N.; Shoji, J.; Tanaka, O.; Shibata, S. Studies on the saponins of ginseng. I. Structures of ginsenoside-Ro, -Rb1, -Rb2, -Rc and -Rd. Chem. Pharm. Bull. 1974, 22, 421–428. [Google Scholar] [CrossRef]
- Sanada, S.; Kondo, N.; Shoji, J.; Tanaka, O.; Shibata, S. Studies on the saponins of ginseng. II. Structures of ginsenoside-Re, -Rf, -Rg2. Chem. Pharm. Bull. 1974, 22, 2407–2411. [Google Scholar] [CrossRef]
- Sanada, S.; Shoji, J. Studies on the saponins of ginseng. III. Structures of ginsenoside Rb3, and 20-glucoginsenoside-Rf. Chem. Pharm. Bull. 1978, 26, 1694–1697. [Google Scholar] [CrossRef]
- Kim, SI.; Park, J.H.; Ryu, J.H.; Park, J.M.; Baek, N.I. Ginsenoside Rg5, a genuine dammarane glyciside from Korean red ginseng. Arch. Pharm. Res. 1996, 19, 551–553. [Google Scholar] [CrossRef]
- Baek, N.I.; Kim, D.S.; Lee, Y.H.; Park, J.D.; Lee, C.B.; Kim, S. Ginsenoside Rh4, a genuine dammarane glyciside from Korean red ginseng. Planta Med. 1996, 62, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Park, J.H.; Kim, T.H. A dammarane glyciside, 20(E)-ginsenoside F4 from Korean red ginseng. Arch. Pharm. Res. 1996, 19, 335–337. [Google Scholar] [CrossRef]
- Baek, N.I.; Kim, J.M.; Park, J.H.; Ryu, J.H.; Kim, D.S.; Lee, Y.H.; Park, J.D.; Kim, S. Ginsenoside Rs3, a genuine dammarane glycoside from Korean red ginseng. Arch. Pharm. Res. 1997, 20, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Choi, S.; Ko, Y.S.; Park, C.S.; Oh, S.; Koh, S.R.; Oh, U.; Oh, J.W.; Rhee, M.H.; Nah, S.Y. Effects of ginsenosides on vanilloidreceptor(VR1) channels expressed in Xenopus oocytes. Mol. Cells 2001, 12, 342–346. [Google Scholar] [PubMed]
- Choi, S.S.; Han, E.J.; Han, K.J.; Lee, H.K.; Suh, H.W. Antinociceptive effects of ginsenosides injected in tracerebroventricularly orintrathecally insubstance P-induced painmodel. Planta Med. 2003, 69, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Moon, Y.S.; Jung, J.S.; Min, S.K.; Son, B.K.; Suh, H.W.; Song, D.K. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axisinmice. J. Neurosci. Lett. 2003, 343, 62–66. [Google Scholar] [CrossRef]
- Choi, S.E.; Choi, S.; Lee, J.H.; Whiting, P.J.; Lee, S.M.; Nah, S.Y. Effects of ginsenosides on GABAA receptor channels expressed in Xenopus oocytes. Arch. Pharmacal. Res. 2003, 26, 28–33. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [PubMed]
- Couillard, C.; Mauriege, P.; Imbeault, P.; Prud’homme, D.; Nadeau, A.; Tremblay, A.; Bouchard, C.; Despres, J.P. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Roncari, D.A.; Lau, D.C.; Kindler, S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism 1981, 30, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Sorisky, A.; Magun, R.; Gagnon, A.M. Adipose cell apoptosis: Death in the energy depot. Int. J. Obes. Relat. Metab. Disord. 2000, 24, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell. Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [PubMed]
- Rosen, E.D. The transcriptional basis of adipocyte development. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Choi, C.G.; Park, S.H. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch. Pharm. Res. 2001, 24, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Lee, K.S.; Sung, M.K. Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-α, PPAR-γ, and LPL mRNA expressions. Life Sci. 2005, 77, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Reaves, L.A.; Edens, N.K. Ginseng extract inhibits lipolysis in rat adipocytes in vitro by activating phosphodiesterase 4. J. Nutr. 2006, 136, 337–342. [Google Scholar] [PubMed]
- Yun, S.N.; Moon, S.J.; Ko, S.K.; Im, B.O.; Chung, S.H. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch. Pharm. Res. 2004, 27, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, K.; Okuda, H. Enhancement of adipose differentiation of mouse 3T3-L1 fibroblasts by ginsenosides. Phytother. Res. 1987, 1, 58–60. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Xu, B.; Zeng, G.; Tan, J.; He, X.; Hu, C.; Zhou, Y. Chemical constituents of Morus alba L. and their inhibitory effect on 3T3-L1 preadipocyte proliferation and differentiation. Fitoterapia 2014, 98, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Lee, M.-S.; Kim, H.-J.; Sung, M.-J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity Effect of Ginsenoside Rg3 involves the AMPK and PPAR-γ Signal Pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Haaz, S.; Fontaine, K.R.; Cutter, G.; Limdi, N.; Perumean-Chaney, S.; Allison, D.B. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: An update. Obes. Rev. 2006, 7, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Yang, Y.; Jiang, B.; Jin, H.; Zhou, L.; Liu, S.; Chen, M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci. 2007, 80, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Siraj, F.M.; Natarajan, S.; Huq, M.A.; Kim, Y.J.; Yang, D.C. Structural investigation of ginsenoside Rf with PPARγ major transcriptional factor of adipogenesis and its impact on adipocyte. J. Ginseng Res. 2014. [CrossRef]
- Gao, Y.; Yang, M.F.; Su, Y.P.; Jiang, H.M.; You, X.J.; Yang, Y.J.; Zhang, H.L. Ginsenoside Re reduces insulin resistance through activation of PPAR-γ pathway and inhibition of TNF-α production. J. Ethnopharmacol. 2013, 147, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hai-ning, Y.; Xiao-man, C.; Zun-chen, W.; Sheng-rong, S.; Das, U.N. Effect of yellow capsicum extract on proliferation and differentiation of 3T3-L1 preadipocytes. Nutrition 2014, 30, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Tzenga, T.F.; Liub, I.M. 6-Gingerol prevents adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells. Phytomedicine 2013, 20, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365, 561–575. [Google Scholar] [PubMed]
- Tang, Q.Q.; Grønborg, M.; Huang, H.; Kim, J.W.; Otto, T.C.; Pandey, A.; Lane, M.D. Sequential phosphorylation of CCAAT enhancer-binding protein β by MAPK and glycogen synthase kinase 3β is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9771. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Seale, P.; Tomaru, T.; Erdjument-Bromage, H.; Cooper, M.P.; Ruas, J.L.; Chin, S.; Tempst, P.; Lazar, M.A.; Spiegelman, B.M. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008, 22, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.O.; Paielli, D.L.; Gilbert, J.D. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994, 8, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert antiadipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of ginsenoside Rc is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-W.; Kim, S.S. Ginsenoside Rc Promotes Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Down-Regulating C/EBPα and PPARγ. Molecules 2015, 20, 1293-1303. https://doi.org/10.3390/molecules20011293
Yang J-W, Kim SS. Ginsenoside Rc Promotes Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Down-Regulating C/EBPα and PPARγ. Molecules. 2015; 20(1):1293-1303. https://doi.org/10.3390/molecules20011293
Chicago/Turabian StyleYang, Ji-Won, and Sung Soo Kim. 2015. "Ginsenoside Rc Promotes Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Down-Regulating C/EBPα and PPARγ" Molecules 20, no. 1: 1293-1303. https://doi.org/10.3390/molecules20011293
APA StyleYang, J.-W., & Kim, S. S. (2015). Ginsenoside Rc Promotes Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Down-Regulating C/EBPα and PPARγ. Molecules, 20(1), 1293-1303. https://doi.org/10.3390/molecules20011293





