(R,R)-Tartaric Acid Dimethyl Diester from X-Ray and Ab Initio Studies: Factors Influencing Its Conformation and Packing
Abstract
Introduction
Methods
X-ray diffraction
Ab-initio calculations
Results and Discussion
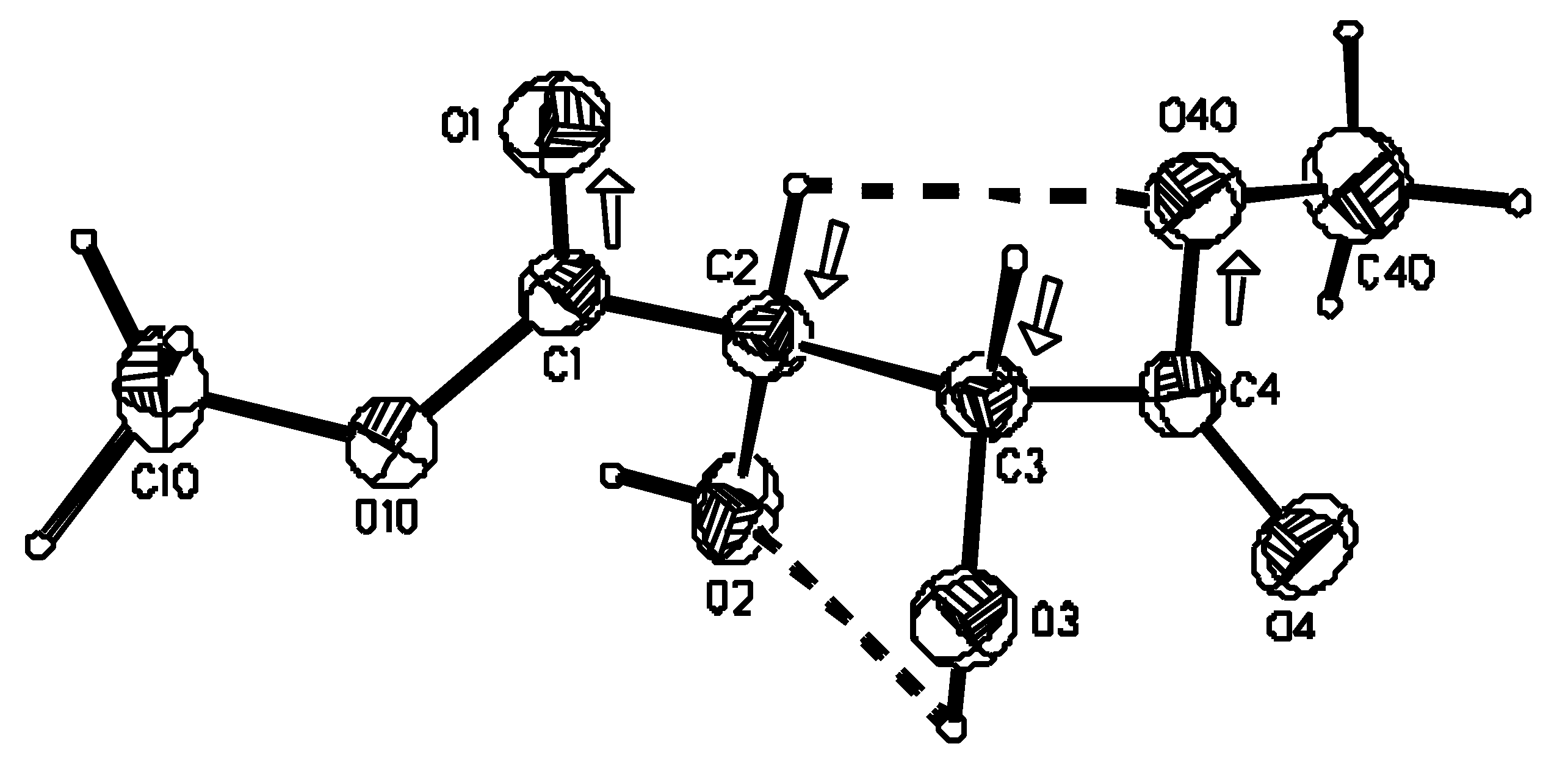
X-ray crystallography
Crystal structure
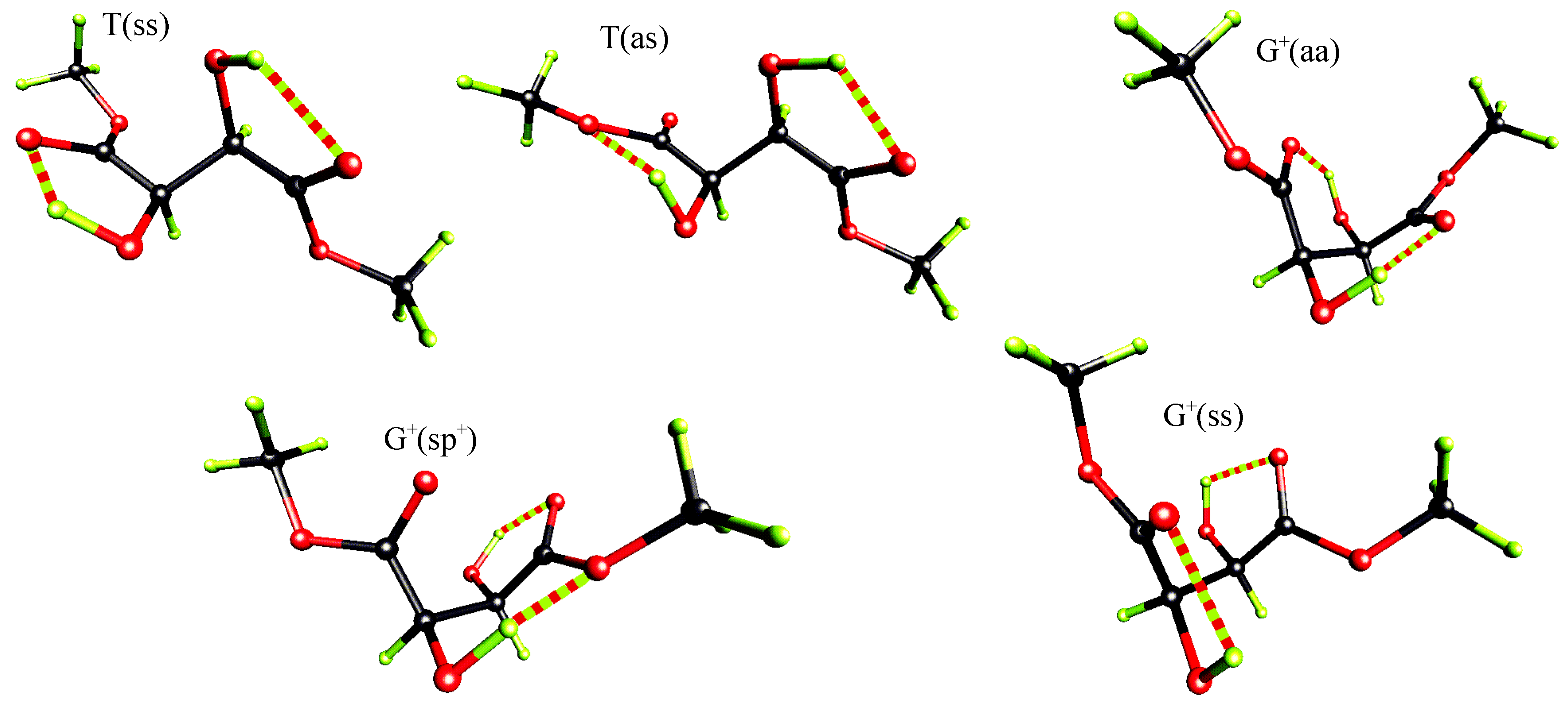
Ab initio quantum chemical studies
Conclusions
Acknowledgements
References
- Eggli, M.; Dobler, M. Helv. Chim. Acta 1989, 72, 1136.
- Pedersen, S. F.; Dewan, J. C.; Eckman, R. R.; Sharpless, K. B. J. Am. Chem. Soc. 1987, 109, 1279. [CrossRef]
- Marcott, C.; Blackburn, C. C.; Faulkner, T. R.; Moscowitz, A.; Overend, J. J. Am. Chem. Soc. 1978, 100, 5262. [CrossRef]
- Su, C. N.; Keiderling, T. A. J. Am. Chem. Soc. 1980, 102, 511. [CrossRef]
- Freedman, T. B.; Balukjian, G. A.; Nafie, L. A. J. Am. Chem. Soc. 1985, 107, 6213. [CrossRef]
- Polvarapu, P. L.; Ewig, C. S.; Chandramouly, T. J. Am. Chem. Soc. 1985, 109, 6213.
- For references for the various split-valence basis sets, explanations of basis set notation, and both the RHF and MP2 methods see: Hehre, W. J.; Radom, L.; Schleyer, P. v. R.; Pople, J. A. Ab Initio Molecular Orbital Theory; Wiley: New York, 1986. [Google Scholar]
- Ascenso, J.; Gil, V. M. S. Can. J. Chem. 1980, 58, 1376.
- Hasan, M. Org. Magn. Res. 1980, 14, 309.
- (a) Barron, L. D. Tetrahedron 1978, 34, 607. (b) Barron, L. D.; Gargano, A. R.; Hecht, L.; Polvarapu, P. L.; Sugeta, H. Spectrochim. Acta 1992, 48A, 1051.
- Hasan, M.; Rychlewski, J.; Rychlewska, U. Computational Methods in Science and Technology 1996, 2, 51.
- Gawronski, J.; Gawronska, K.; Skowronek, P.; Rychlewska, U.; Warzajtis, B.; Rychlewski, J.; Hoffmann, M.; Szarecka, A. Tetrahedron. submitted for publication.
- Lehmann, M. S.; Larsen, F. K. Acta Crystallogr. 1974, A30, 580.
- Sheldrick, G. M. SHELXS86. In Program for the solution of crystal structures; Univ. of Göttingen: Germany, 1993. [Google Scholar]
- Sheldrick, G. M. SHELXL93. In Program for the Refinement of Crystal Structure; Univ. of Göttingen: Germany, 1986. [Google Scholar]
- Gaussian 94, Revision C.3, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Gill, P. M. W.; Johnson, B. G.; Robb, M. A.; Cheeseman, J. R.; Keith, T.; Petersson, G. A.; Montgomery, J. A.; Raghavachari, K.; Al-Laham, M. A.; Zakrzewski, V. G.; Ortiz, J. V.; Foresman, J. B.; Cioslowski, J.; Stefanov, B. B.; Nanayakkara, A.; Challacombe, M.; Peng, C. Y.; Ayala, P. Y.; Chen, W.; Wong, M. W.; Andres, J. L.; Replogle, E. S.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Binkley, J. S.; Defrees, D. J.; Baker, J.; Stewart, J. P.; Head-Gordon, M.; Gonzalez, C.; Pople, J. A. Gaussian Inc.: Pittsburgh, PA, 1995.
- Stereochemical Workstation Operation Manual, Release 3.4, Siemens Analytical X-Ray Instruments, Inc., Madison, Wisconsin, USA, 1989.
- Szczepanska, B.; Rychlewska, U. Correlations, Transformations and Interactions in Organic Chemistry; pp. 233–244. Jones, D.W., Katrusiak, A., Eds.; Oxford University Press, 1994. [Google Scholar]
- Allen, F. H.; Kennard, O. 3D Search and Research using the Cambridge Structural Database. Chemical Design Automation News, 1993; 8, 131. [Google Scholar]
- Szarecka, A.; Hoffmann, M.; Rychlewski, J.; Rychlewska, U. J. Mol. Struct. 1996, 374, 363.
- (+)-Tartaric acid: (a) Stern, F.; Beevers, C. A. Acta Crystallogr. 1950, 3, 341. (b) Okaya, Y.; Stemple, N. R.; Kay, M. I. ibid. 1996, 21, 237. (c) Albertsson, J.; Oskarsson, A.; Stahl, K. J. Appl. Crystallogr. 1979, 12, 537.
- For explanation of graph set notation see: (a) Etter, M. C. J. Am. Chem. Soc. 1982, 104, 1095. (b) Etter, M. C.; MacDonald, J. C.; Bernstein, J. Acta Crystallogr. 1990, B46, 256. (c) Etter, M. C. Acc. Chem. Res. 1990, 23, 120. (d) Bernstein, J.; Etter, M. C.; MacDonald, J. C. J. Chem. Soc. Perkin Trans. 2. 1990, 695. (e) Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N. L. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555.
- Cramer, C. J.; Truhlar, D. G. J. Am. Chem. Soc. 1994, 99, 3892.
- Sample Availability: not available
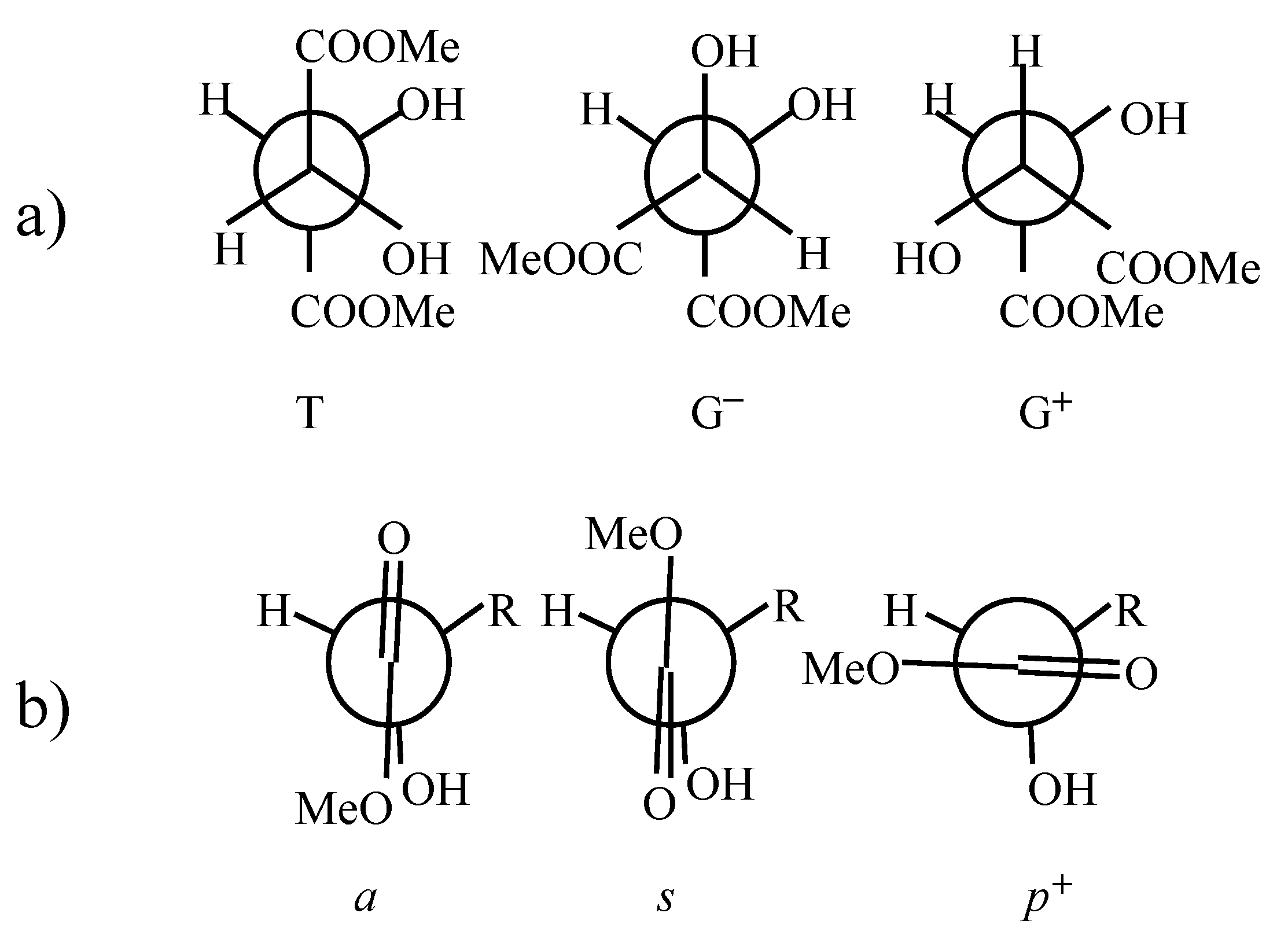

| Empirical formula | C6H10O6 |
| Formula weight | 178.14 |
| m.p. | 57 - 60 °C |
| Temperature | 293 K |
| Wavelength | Cu Kα (1.54178 Å) |
| Crystal system | monoclinic |
| Space group | P21 |
| Unit cell dimensions | a = 5.654(2) Å |
| b = 8.450(1) Å | |
| c = 8.437(2) Å | |
| β = 91.36(2)° | |
| Volume | 403.0(2) Å3 |
| Z | 2 |
| Density (calculated) | 1.468 g cm−3 |
| Absorption coefficient | 1.176 mm−1 |
| Crystal size | 0.3 x 0.3 x 0.5 mm |
| 2θmax for data collection | 115° |
| Index ranges | −6 ≤ h ≤6, -9≤ k ≤9, −9≤ l ≤0 |
| All data | 1047 |
| Observed reflections | |
| [I > 2σ(I)] | 1033 |
| Extinction parameter | 1.56(7) x 10−4 |
| Weighting scheme | w = 1/[σ2(Fo2) + (0.0324P)2 + 0.0664P] where P= (Fo2+2Fc2) / 3 |
| Goodness-of-fit on F2 | 1.163 |
| Final R indices [I>2σ(I)] | R1 = 0.0241, wR2 = 0.0624 |
| R indices (all data) | R1 = 0.0243, wR2 = 0.0625 |
| Largest diff. peak and hole | 0.17 and −0.12Å−3 |
| x | y | z | U(eq) | |
|---|---|---|---|---|
| C(1) | 8169(3) | 3011(3) | 10732(2) | 34(1) |
| C(2) | 7240(3) | 3257(3) | 9045(2) | 35(1) |
| C(3) | 4687(3) | 2648(3) | 8892(2) | 34(1) |
| C(4) | 3880(3) | 2589(3) | 7178(2) | 36(1) |
| C(10) | 9915(4) | 4162(4) | 13006(2) | 54(1) |
| C(40) | 4767(5) | 1551(4) | 4664(2) | 65(1) |
| O(1) | 8411(3) | 1705(2) | 11293(2) | 47(1) |
| O(2) | 7181(3) | 4847 | 8542(2) | 47(1) |
| O(3) | 3142(2) | 3561(3) | 9809(2) | 43(1) |
| O(4) | 2147(3) | 3236(3) | 6643(2) | 53(1) |
| O(10) | 8694(2) | 4343(2) | 11486(1) | 41(1) |
| O(40) | 5325(3) | 1696(3) | 6329(2) | 53(1) |
| torsion angle | value |
| O(1) - C(1) - C(2) - O(2) | -176.8 (2) |
| O(10) - C(1) - C(2) - O(2) | 2.4 (2) |
| O(1) - C(1) - C(2) - C(3) | 63.9 (2) |
| O(10)- C(1) - C(2) - C(3) | -116.9 (2) |
| O(2) - C(2) - C(3) - O(3) | -58.4 (2) |
| C(1) - C(2) - C(3) - O(3) | 66.0 (2) |
| O(2) - C(2) - C(3) - C(4) | 66.3 (2) |
| C(1) - C(2) - C(3) - C(4) | -169.2 (1) |
| O(3) - C(3) - C(4) - O(4) | 0.2 (2) |
| C(2) - C(3) - C(4) - O(4) | -124.4 (2) |
| O(3) - C(3) - C(4) - O(40) | -178.7 (2) |
| C(2) - C(3) - C(4) - O(40) | 56.7 (2) |
| O(1) - C(1) - O(10) - C(10) | 8.1 (3) |
| C(2) - C(1) - O(10) - C(10) | -171.1 (2) |
| O(4) - C(4) - O(40) - C(40) | 1.6 (3) |
| C(3) - C(4) - O(40) - C(40) | -179.5 (2) |
| D-H…A | D…A (Å) | D-H (Å) | H…A (Å) | ∠D-H…A (°) |
| O(3)–H(3O)…O(2) | 2.766(2) | 0.97 | 2.43 | 100 |
| O(2)–H(2O)…O(1i) | 2.946(2) | 0.97 | 2.00 | 164 |
| O(3)–H(3O)…O(1ii) | 2.942(2) | 0.97 | 2.03 | 155 |
| C(2)–H(2)…O(40) | 2.836(2) | 1.10 | 2.44 | 100 |
| C(40)–H(403) …O(4iii) | 3.493(3) | 1.10 | 2.47 | 154 |
| C(3)–H(3)…O(2iv) | 3.393(2) | 1.10 | 2.47 | 141 |
| level of theory | some properties | T(ss) | T(as) | G+(aa) | G+(sp+) | G+(ss) |
|---|---|---|---|---|---|---|
| MP2/6-31G* | ΔE [kcal/mol] | 0.00 | 1.20 | 1.49 | 1.60 | 1.38 |
| D [debye] | 3.46 | 1.07 | 2.66 | 1.63 | 2.34 | |
| C-C*-C*-C | 169.7 | -177.9 | 56.4 | 55.2 | 41.6 | |
| O=C-C*-O | 5.9 | -3.9 | 155.6 | 64.7 | 21.1 | |
| O=C-C*-O | 5.9 | -170.7 | 155.6 | -4.9 | 21.1 | |
| H-O-C*-C* | 103.4 | 127.7 | 62.4 | 54.7 | 89.2 | |
| H-O-C*-C* | 103.4 | 61.7 | 62.4 | 129.2 | 89.2 | |
| RHF/6-31G* | ΔE [kcal/mol] | 0.00 | 1.18 | 2.19 | 2.90 | 3.38 |
| D [debye] | 3.39 | 0.87 | 2.45 | 1.54 | 2.17 | |
| C-C*-C*-C | 172.7 | -176.1 | 59.2 | 57.3 | 45.6 | |
| O=C-C*-O | 3.2 | -5.2 | 154.1 | 66.4 | 20.3 | |
| O=C-C*-O | 3.2 | -168.8 | 154.1 | -8.6 | 20.3 | |
| H-O-C*-C* | 107.5 | 132.2 | 63.3 | 57.4 | 94.3 | |
| H-O-C*-C* | 107.5 | 66.5 | 63.3 | 137.1 | 94.3 | |
| RHF/3-21G | ΔE [kcal/mol] | 0.00 | 2.19 | 5.98 | 2.45 | 2.48 |
| D [debye] | 3.01 | 1.25 | 4.43 | 2.36 | 2.78 | |
| C-C*-C*-C | 164.1 | 171.4 | 60.2 | 56.1 | 46.9 | |
| O=C-C*-O | 0.1 | -3.6 | -167.0 | 83.1 | -2.7 | |
| O=C-C*-O | 0.1 | -174.3 | -167.0 | -16.9 | -2.7 | |
| H-O-C*-C* | 108.4 | 119.4 | 55.0 | 45.7 | 58.5 | |
| H-O-C*-C* | 108.4 | 92.8 | 55.0 | 137.0 | 58.5 |
© 1997 MDPI. All rights reserved
Share and Cite
Rychlewska, U.; Warzajtis, B.; Hoffmann, M.; Rychlewski, J. (R,R)-Tartaric Acid Dimethyl Diester from X-Ray and Ab Initio Studies: Factors Influencing Its Conformation and Packing. Molecules 1997, 2, 106-113. https://doi.org/10.3390/20700106
Rychlewska U, Warzajtis B, Hoffmann M, Rychlewski J. (R,R)-Tartaric Acid Dimethyl Diester from X-Ray and Ab Initio Studies: Factors Influencing Its Conformation and Packing. Molecules. 1997; 2(7):106-113. https://doi.org/10.3390/20700106
Chicago/Turabian StyleRychlewska, Urszula, B. Warzajtis, M. Hoffmann, and J. Rychlewski. 1997. "(R,R)-Tartaric Acid Dimethyl Diester from X-Ray and Ab Initio Studies: Factors Influencing Its Conformation and Packing" Molecules 2, no. 7: 106-113. https://doi.org/10.3390/20700106
APA StyleRychlewska, U., Warzajtis, B., Hoffmann, M., & Rychlewski, J. (1997). (R,R)-Tartaric Acid Dimethyl Diester from X-Ray and Ab Initio Studies: Factors Influencing Its Conformation and Packing. Molecules, 2(7), 106-113. https://doi.org/10.3390/20700106




