Amberlyst 15 Catalyzed Prenylation of Phenols: One-Step Synthesis of Benzopyrans
Abstract
:Introduction
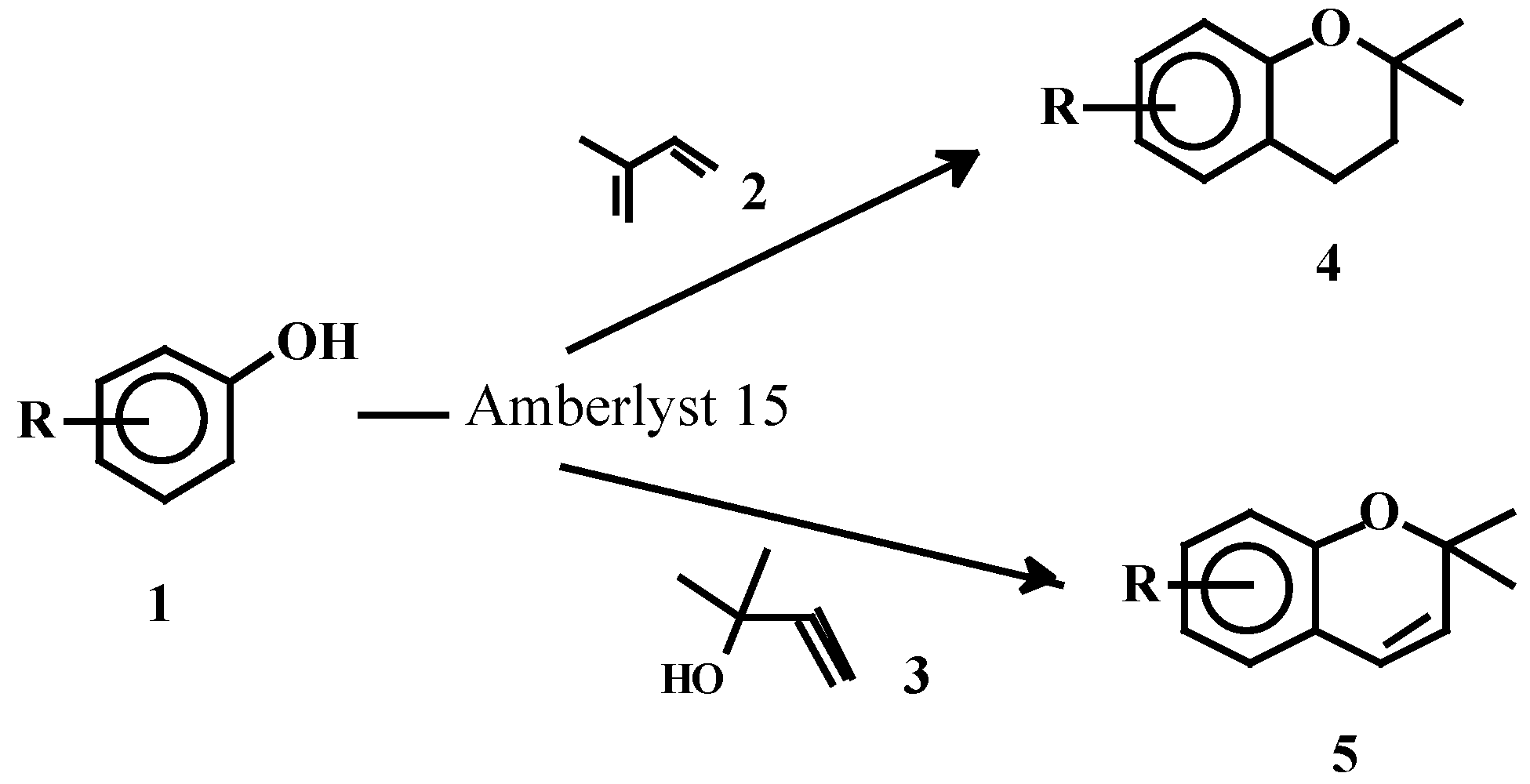
Results and Discussion
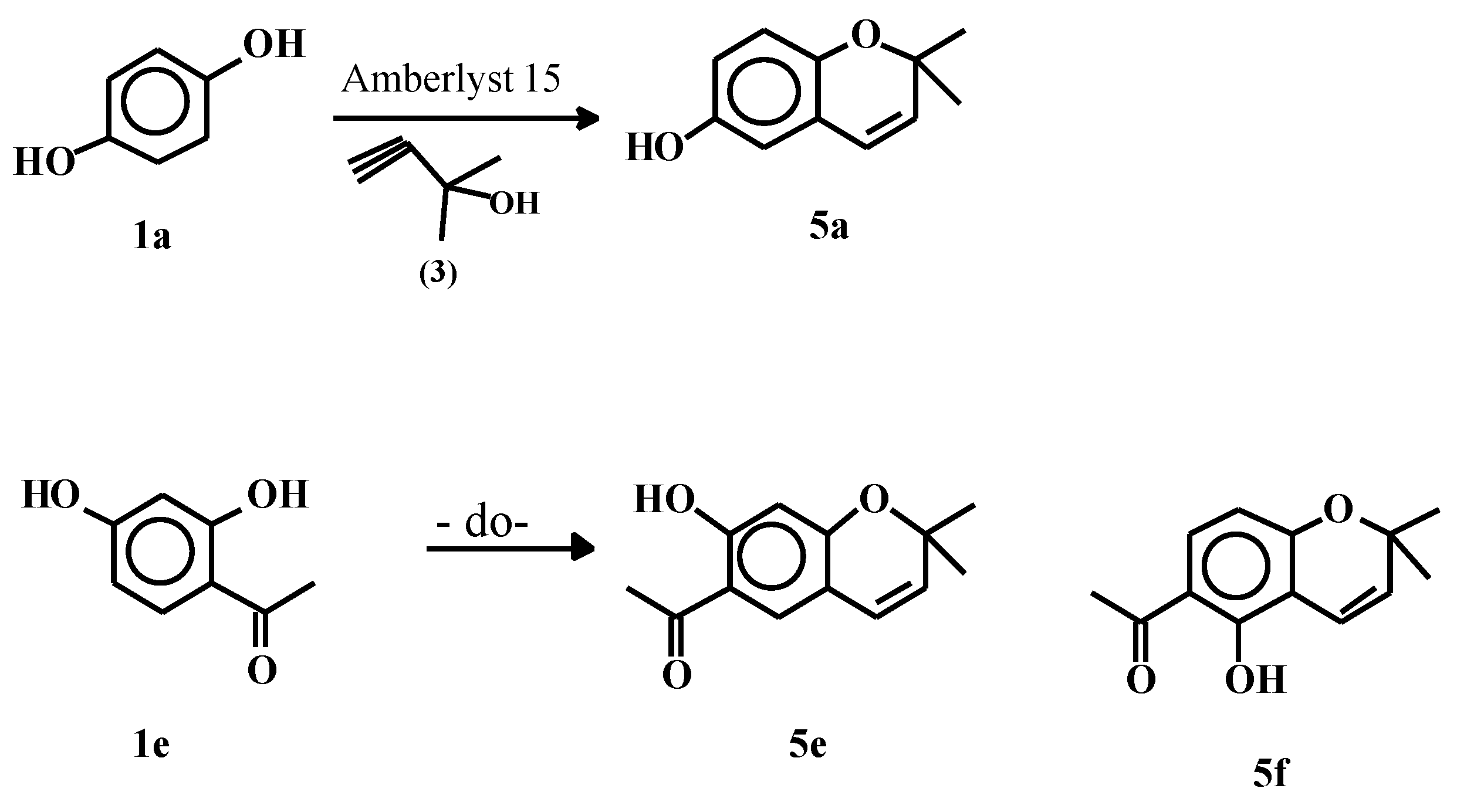
Synthesis of 2,2-dimethylchromans 4
Synthesis of 2,2-dimethylchromenes 5
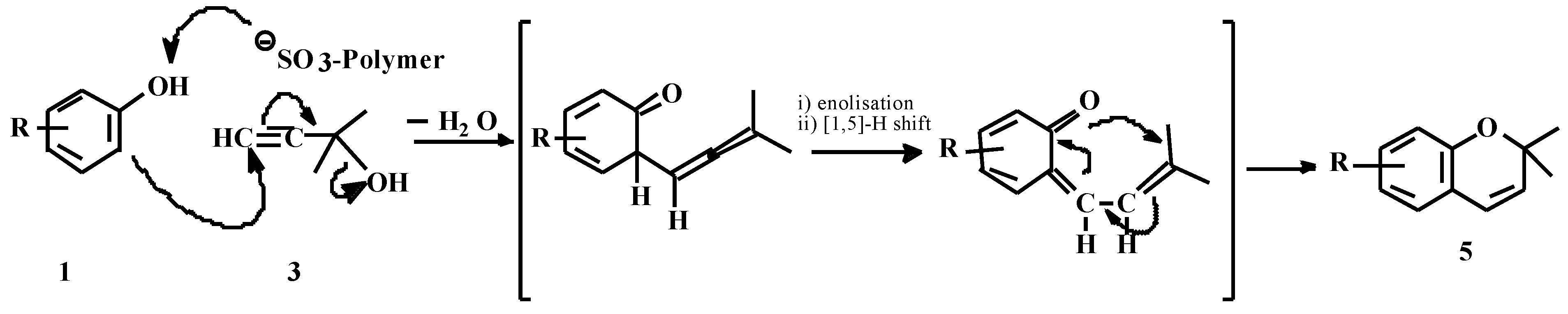
Mechanism of chromene formation
Experimental Section
General
Typical procedure for the preparation of 2,2-dimethylchromans:
Typical procedure for the preparation of 2,2-dimethylchromenes
References
- Nunata, A.; Kanbara, S.; Takahashi, C.; Fujiki, R.; Yoneda, M.; Usami, Y.; Fujita, E. Phytochem 1992, 31, 1209. Brown, P. E.; Lewis, R.; Waring, M. A. J. Chem. Soc. Perkin Trans. I 1990, 2979. Srivastava, R.; Proksch, P. Entomol. Gener 1991, 15, 265. Bergmann, R.; Gericke, R. J. Med. Chem. 1990, 33, 492. Bergmann, R.; Harting, J.; Lues, L.; Schittenhelm, C.; Gericke, R. J. Med. Chem. 1991, 34, 3074. Merrill, G. B. J. Chem. Ecology 1989, 15, 2073. [CrossRef] [PubMed]Satoh, Y.; Stanton, J. L.; Hutchison, A. J.; Libby, A. J.; Kowalski, T. J.; Lee, W. H.; White, D. H.; Kimble, E. F. J. Med. Chem. 1993, 36, 3580.
- Kashman, Y.; Gustafson, K. R.; Fuller, R. W.; Cardellina, J. H., II; McMahon, J. B.; Currens, M. J.; Buckheit, R. W., Jr.; Hughes, S. H.; Cragg, G. M.; Boyd, M. R. J. Med. Chem. 1992, 35, 2735. [PubMed]Currens, M. J.; Mariner, J. M.; McMahon, J. B.; Boyd, M. R. J. Pharmacol. Exp. Ther. 1996, 279, 652. [PubMed]Currens, M. J.; Gulakowski, R. J.; Mariner, J. M.; Moran, R. A.; Buckheit, R. W., Jr.; Gustafson, K. R.; McMahon, J. B.; Boyd, M. R. J. Pharmacol. Exp. Ther. 1996, 279, 645–651. Hizi, A.; Tal, R.; Shaharabany, M.; Currens, M. J.; Boyd, M. R.; Hughes, S. H.; McMahon, J. B. Antimicrob. Agents Chemother. 1993, 37, 1037. [CrossRef]
- Livingstone, R.; Watson, R. B. J. Chem. Soc. 1956, 3701. [CrossRef] Spath, E.; Hillel, R. Ber. Dtsch. Chem. Ges. 1939, 72, 963, 2093. Mukerjee, S.K.; Sarkar, S. C.; Seshadri, T.R. Tetrahedron 1969, 25, 1063. Banerji, A.; Goomer, N. C. Indian J. Chem. 1981, 20B, 144. Ahluwalia, V. K. J. Chem. Soc. Perkin Trans. 1 1982, 335. Bandaranayake, W. M.; Crombie, L.; Whiting, D. A. J. Chem. Soc. (C) 1970, 811. Banerji, A.; Kalena, G. P. Heterocycle 1989, 28, 711, and references therein.
- Ahluwalia, V. K.; Arora, K. K. J. Chem. Soc. Perkin Trans. 1 1982, 335.
- Ahluwalia, V. K.; Arora, K. K. Tetrahedron 1981, 37, 1437.
- Schweizer, E. E.; Meeder-Nycz, D. Chromenes, Chromanones and Chromones; Ellis, G. P., Ed.; J. Wiley and Sons: New York, 1975; p. 55. [Google Scholar]
- Ahluwalia, V. K.; Arora, K. K.; Jolly, R. S. Acta Chim. Hung. 1983, 114, 117.
- Zsindely, J.; Schmid, H. Helv. Chim. Acta 1968, 51, 1510. [CrossRef]
- Howard, B. M.; Clarkson, K.; Bernstein, R.L. Tetrahedron Lett. 1979, 4449. Banerji, A.; Goomer, N. C. Indian J. Chem. 1984, 23B, 885.
- Steelink, C.; Marshall, G. J. Org. Chem. 1979, 44, 1429. [CrossRef]
- Bohlman, F.; Buhmann, U. Chem. Ber. 1972, 105, 863.
- Mukerjee, S. K.; Sarkar, S. C.; Seshadri, T. R. Indian J. Chem. 1970, 8, 861.
- Tachibana, Y. Bull. Chem. Soc. Japan 1980, 555.
- Miller, J. A.; Wood, H. C. S. J. Chem. Soc. C 1968, 1837.
- Bajwa, B. S.; Lal, P.; Seshadri, T. R. Indian J. Chem. 1973, 11, 100.
- Nickl, J. Chem. Ber. 1958, 91, 1372.
- Bridge, W.; Croker, A. J.; Cubin, T.; Robertson, A. J. Chem. Soc. 1937, 1530.
- Backouse, T.; Robertson, A. J. Chem. Soc. 1939, 1257.
- Miranda, M. A.; Primo, J.; Tormos, R. Tetrahedron 1989, 45, 7593.
- Anthonsen, T. Acta Chem. Scand. 1969, 23, 3605. [CrossRef]
- Sample Availability: not available.

© 1997 MDPI. All rights reserved
Share and Cite
Kalena, G.P.; Jain, A.; Banerji, A. Amberlyst 15 Catalyzed Prenylation of Phenols: One-Step Synthesis of Benzopyrans. Molecules 1997, 2, 100-105. https://doi.org/10.3390/20700100
Kalena GP, Jain A, Banerji A. Amberlyst 15 Catalyzed Prenylation of Phenols: One-Step Synthesis of Benzopyrans. Molecules. 1997; 2(7):100-105. https://doi.org/10.3390/20700100
Chicago/Turabian StyleKalena, G. P., A. Jain, and A. Banerji. 1997. "Amberlyst 15 Catalyzed Prenylation of Phenols: One-Step Synthesis of Benzopyrans" Molecules 2, no. 7: 100-105. https://doi.org/10.3390/20700100
APA StyleKalena, G. P., Jain, A., & Banerji, A. (1997). Amberlyst 15 Catalyzed Prenylation of Phenols: One-Step Synthesis of Benzopyrans. Molecules, 2(7), 100-105. https://doi.org/10.3390/20700100



