Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review
Abstract
:1. Introduction
2. Results and Discussion
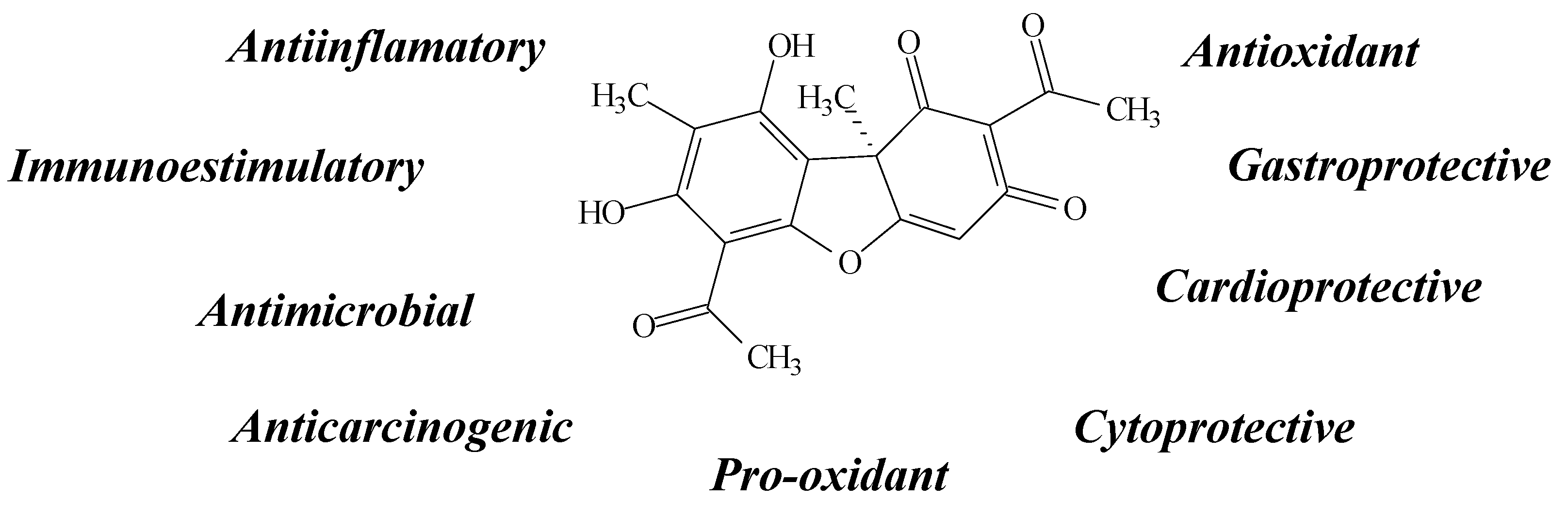
2.1. Usnic Acid
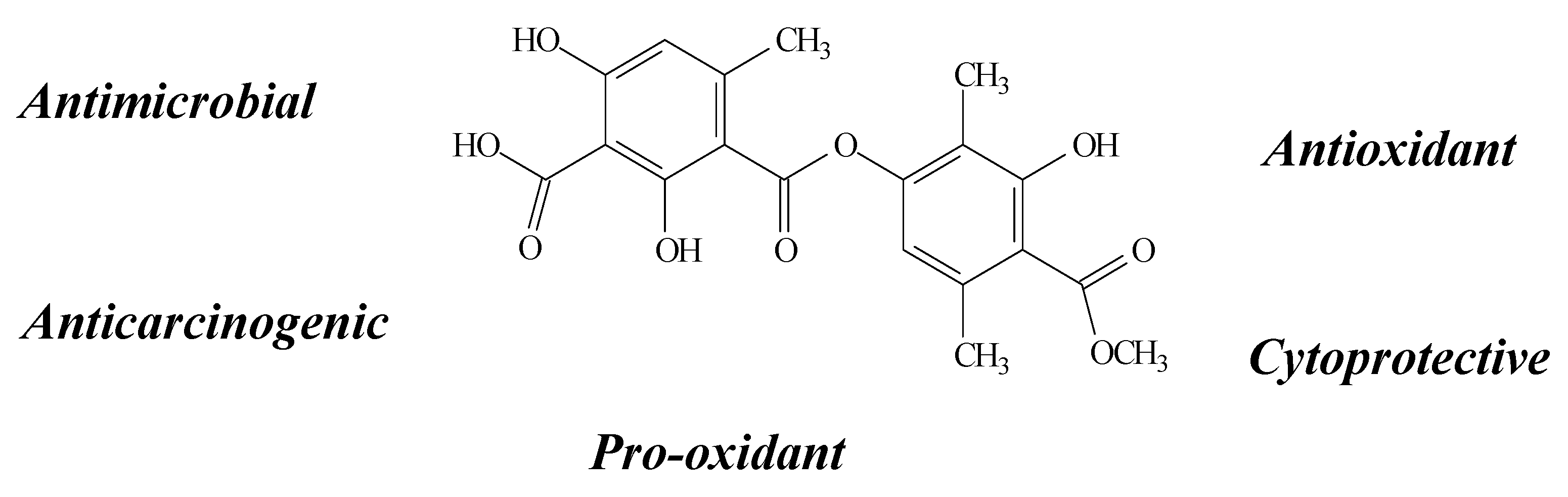
2.2. Atranorin
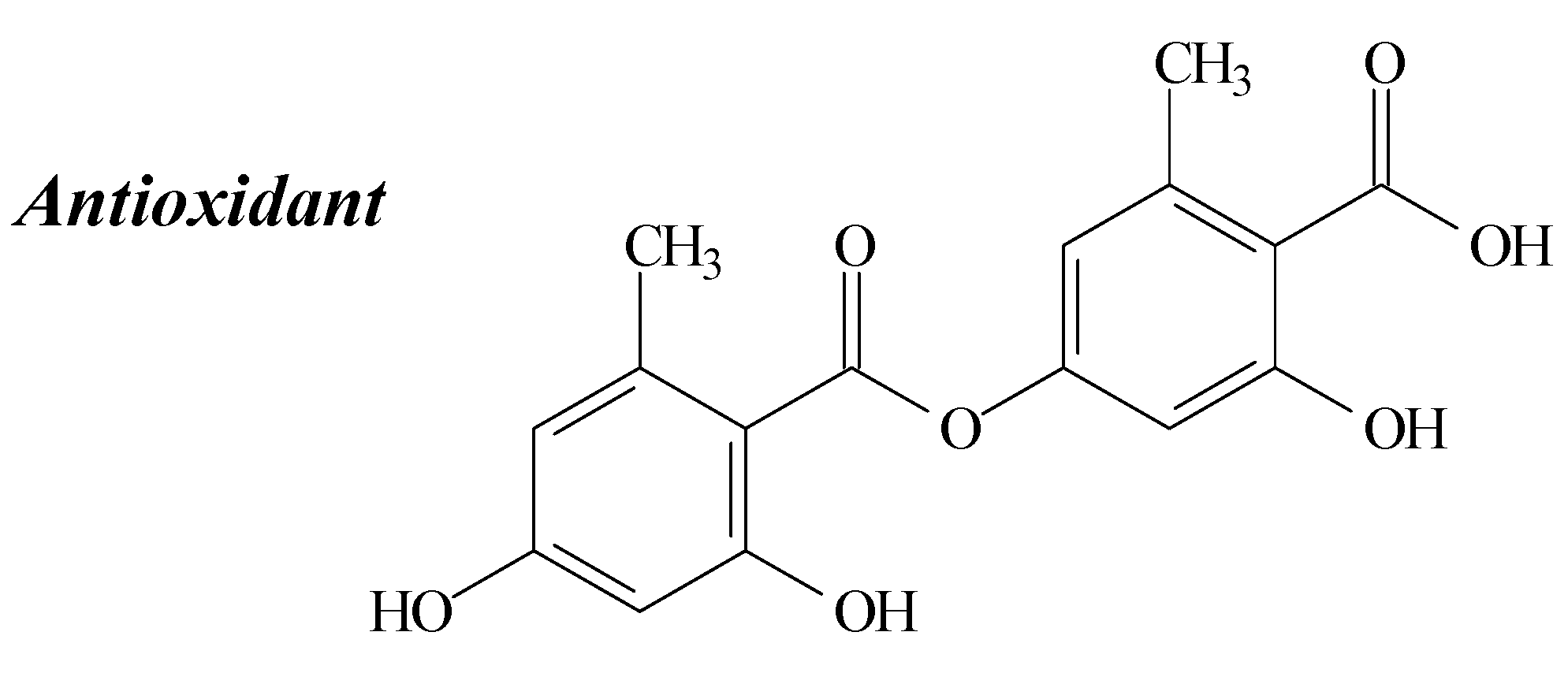
2.3. Lecanoric Acid
2.4. Diffractaic Acid
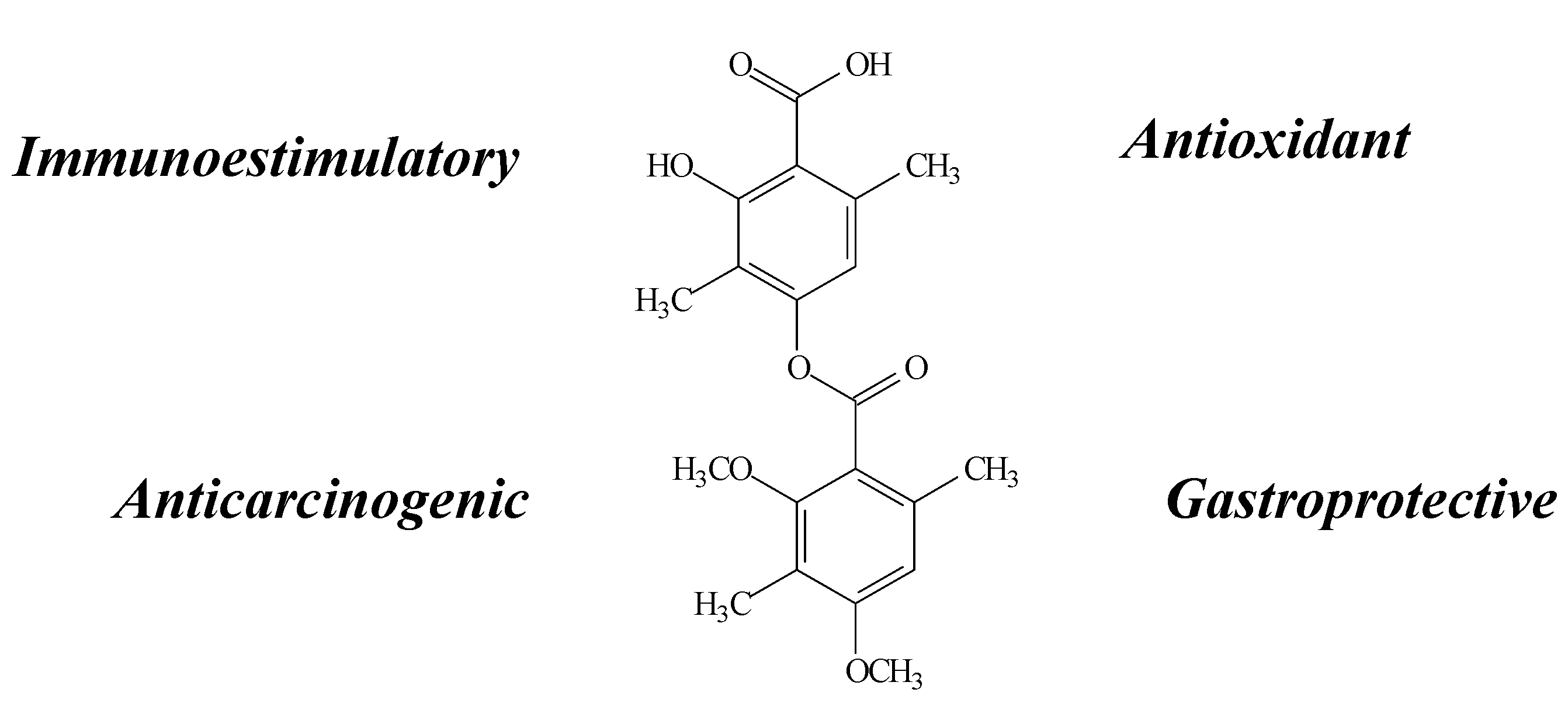
2.5. Lobaric Acid
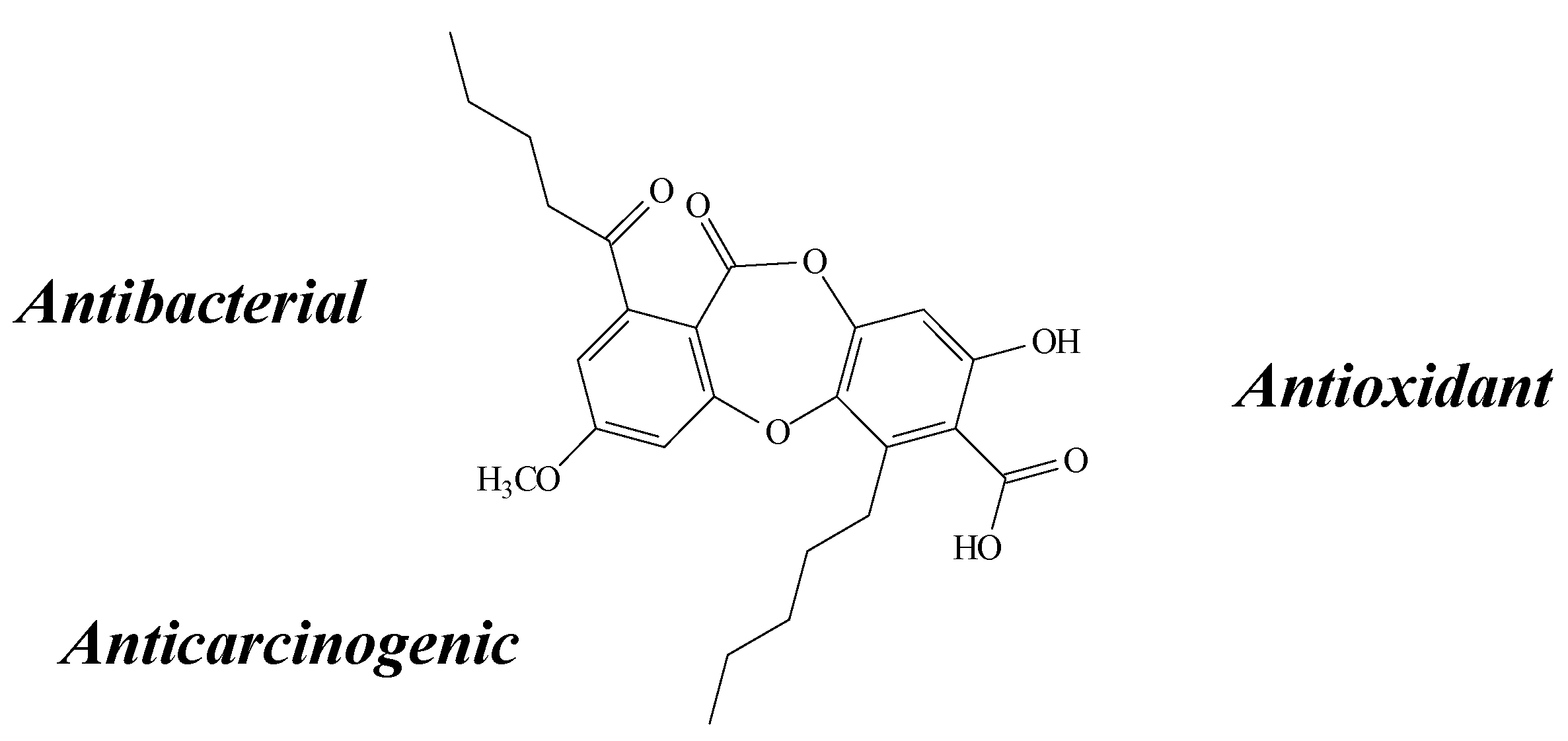
2.6. Stictic Acid
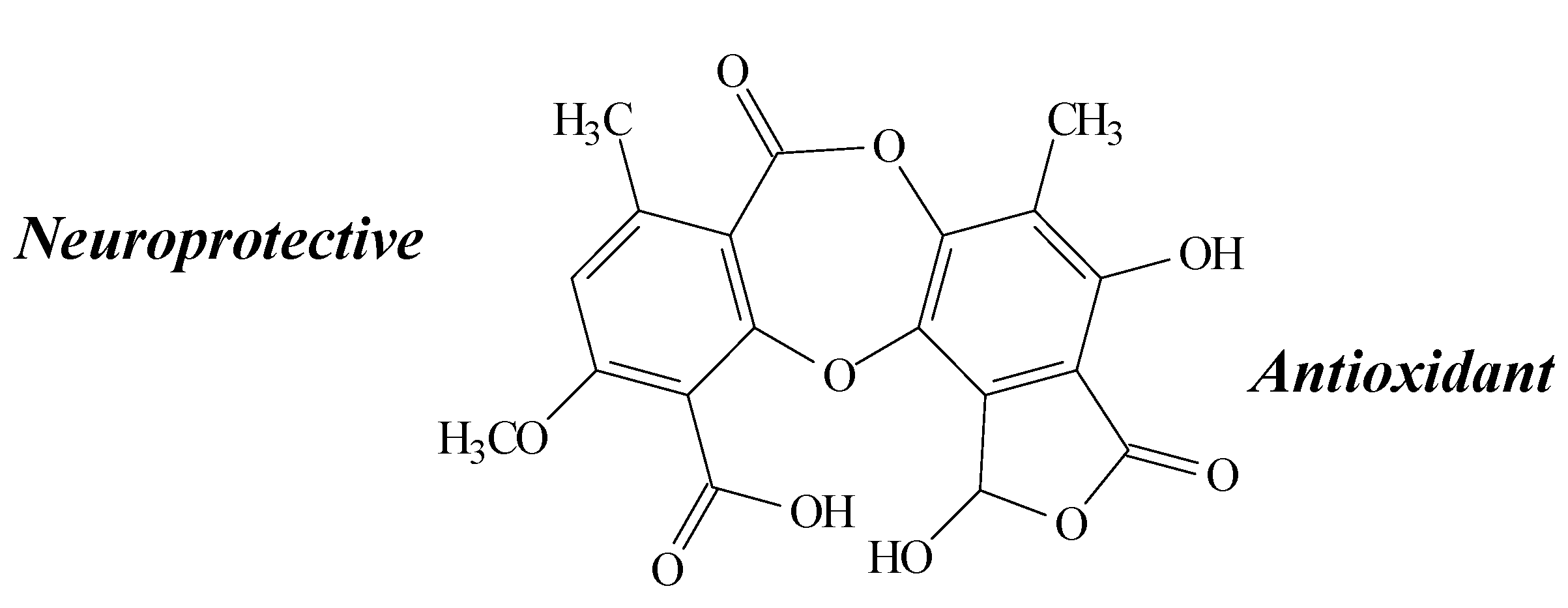
2.7. Fumarprotocetraric Acid
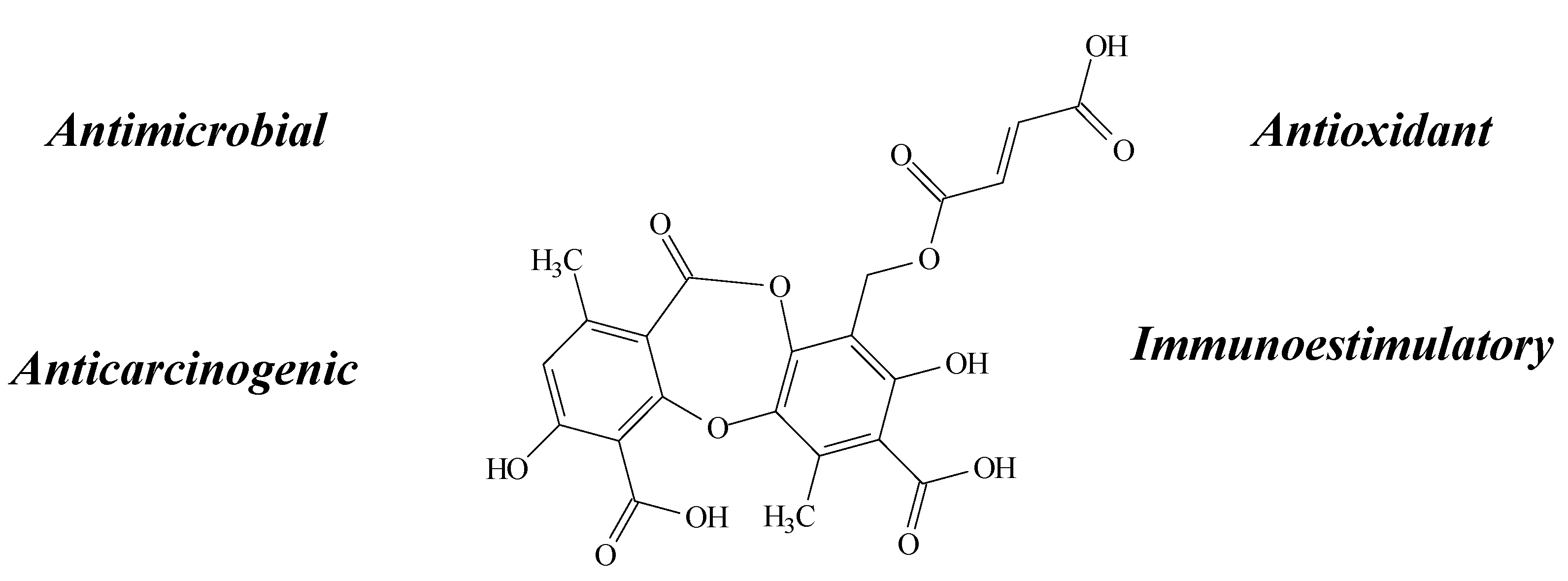
2.8. Salazinic Acid
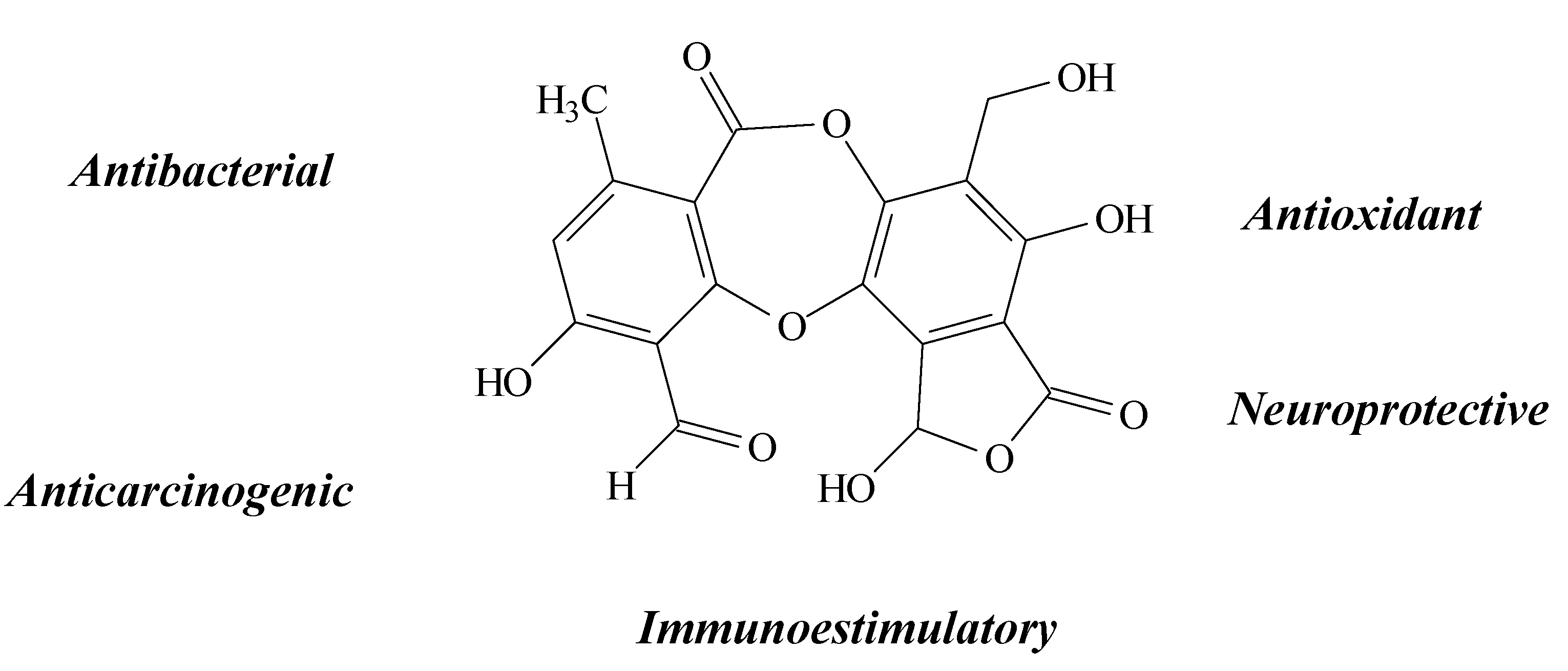
2.9. Physodic Acid
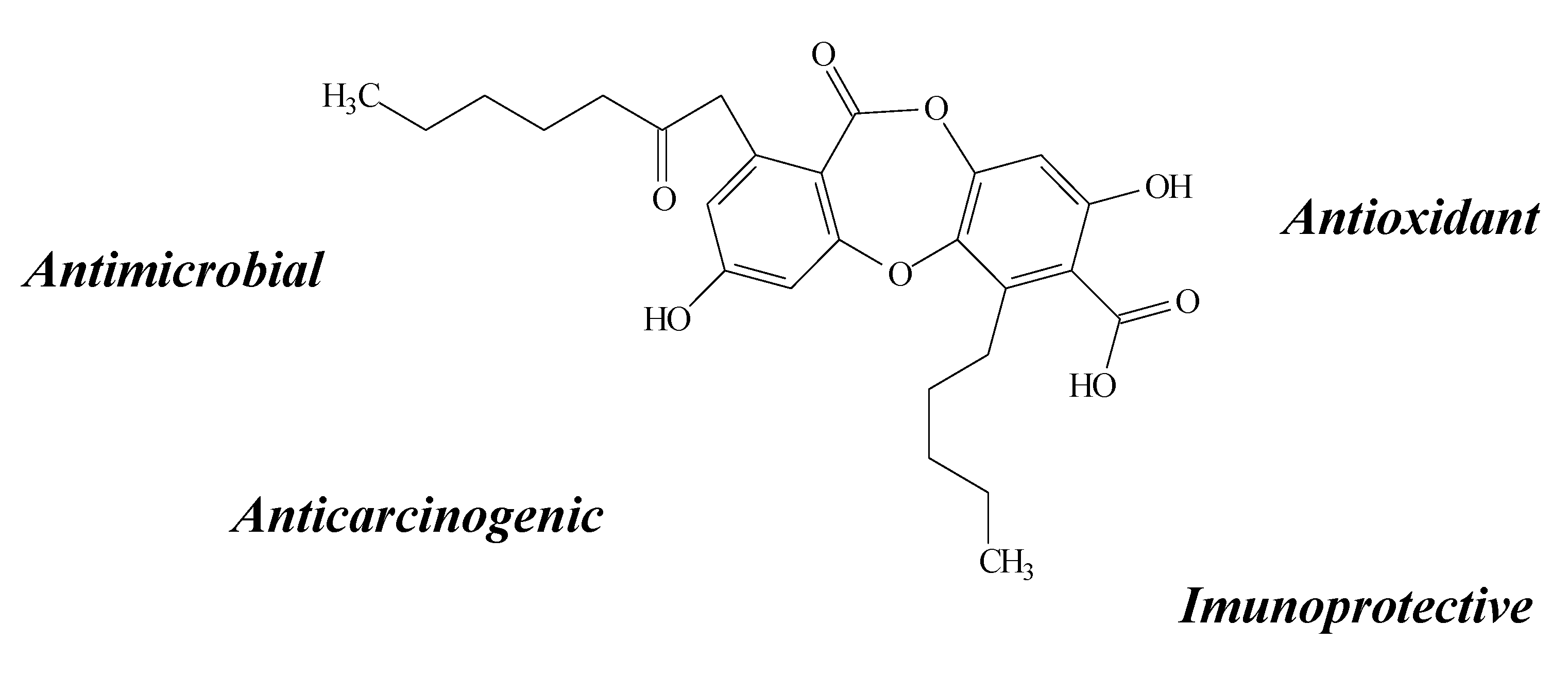
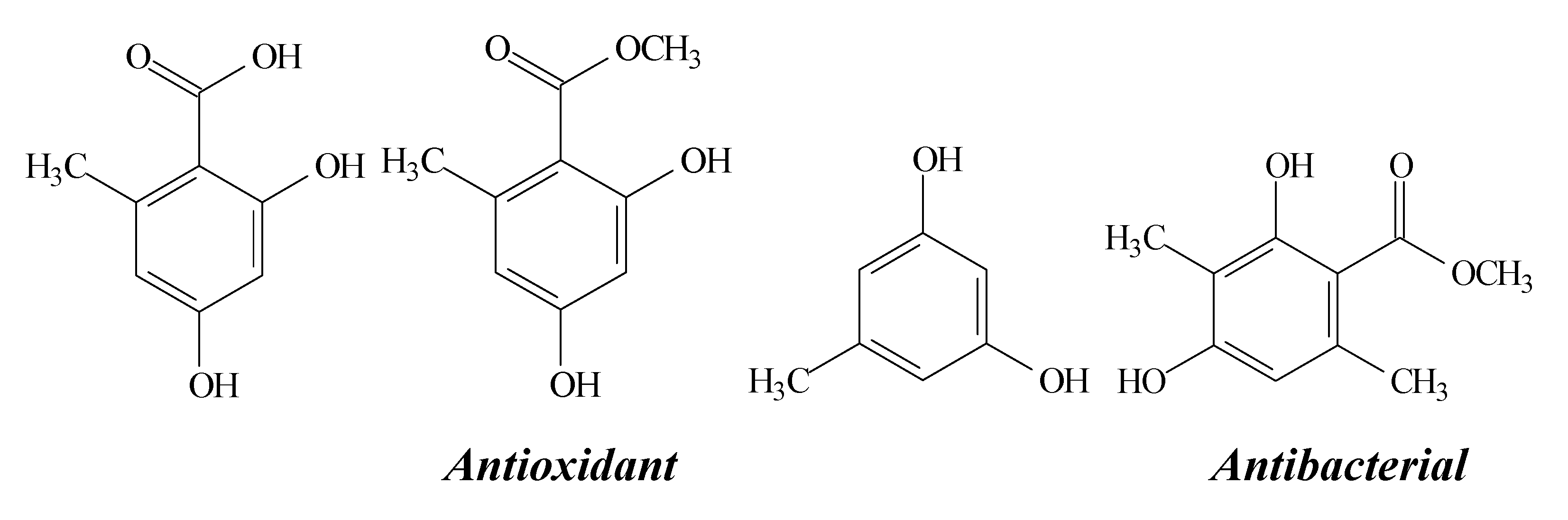
2.10. Orcinol, Orsellinic Acid and other Orsellinates
2.11. Other Compounds
2.11.1. Antioxidant
2.11.2. Antimicrobial
2.11.3. Anticarcinogenic
| Substance/Chemical Class | Authors, Year, Country | Source | Assay | Activity | Results/Mechanism of Action |
|---|---|---|---|---|---|
| Usnic acid (dibenzofuran) | Marante et al. [24], 2003, Spain | Lethariella canariensis | In vitro: MIC, MTT and LPO | Antibacterial, anticarcinogenic and antioxidant | Anti-proliferative effect against U937 and HL-60 |
| Santos et al. [18], 2004, Brazil | Usnea meridionalis Zahlbr | In vitro: H2O2 and NO measurements | Immunostimulatory | Induced greatest release of NO in peritoneal macrophages. | |
| Odabasoglu et al. [15], 2006, Turkey | Usnea longissima | In vivo: SOD, CAT, GR, GPx, MPx, NOS, GSH, and LPO | Gastroprotective | Increased SOD, GPx, GSH and cNOS activities and reduced CAT, GR, LPO, iNOS and MPx activities | |
| Dévéhat et al. [16], 2007, France | Usnea articulata | In vitro: DPPH and SAS | No significant activity | – | |
| Jin, Li and He [22], 2008, China | Usnea longissma | In vitro: MTT, TNF-α and NO | Anti-inflammatory | Dose-dependent inhibitory effect on LPS-induced TNF-α and NO production in macrophages RAW 264.7, associated with decreased synthesis of TNF-α mRNA and iNOS protein | |
| De Paz et al. [21], 2010, Spain | Xanthoparmelia conspersa | In vitro: MTT, ORAC and ROS determination | Antioxidant and neuroprotective | Reduced radical oxygen species (ROS) production on hydrogen peroxide-induced damage in U373 MG cells | |
| Bačkorová et al. [25], 2011, Slovakia | Purchased from Sigma Chemical | In vitro: MTT, HTCA, viability, cell proliferation and detachment, cell cycle transition and apoptotic nuclear morphology | Anticarcinogenic | Anti-proliferative action on A2780, MCF-7, SK-BR-3, HT-29, HCT-116 p53−/−, HL-60 and Jurkat. Higher pro-apoptotic activity, supported by the suppression of viability and cell proliferation, correlated more strongly with an increased number of floating cells | |
| Thadhani et al. [7], 2011, Sri-Lanka | Parmotrema grayana | In vitro: SOR, NOR, and DPPH | No significant activity | – | |
| Behera, Mahadik and Morey [17], 2012, India | Usnea complanata | In vivo: FRSA, NOR, LPO, ACE and HMGR | Cardioprotective | Moderate to strong antioxidant activity, concentration-dependent manner, on the FRSA, NOR) and in LPO. Poor fobrinolytic potencial | |
| Bessadottir et al. [20], 2012, Iceland | Cladonia arbuscula | In vitro: ATP estimation, immunocytochemistry, western blot, visualization of lysosomes and transfection with tfLC3 construct | Autophagy and pH-determined drug distribution | Induced the formation of autophagosomes in human cancer cells, but had minimal effects on normal human fibroblasts. UA-treated cells showed reduced ATP levels and activation of AMP kinase as well as signs of cellular stress. UA is thus likely to trigger autophagosome formation both by energy depletion and stress conditions. | |
| Brisdelli et al. [23], 2013, Italy | Cladonia lepidophora Ahti & Kashiw | In vitro: MTT, CAS 3, 8 and 9, ROS determination and DPPH | Anticarcinogenic | Anti-proliferative effect against MCF-7, HeLa, HCT-116 | |
| Rabelo et al. [27], 2012, Brazil | Purchased from Sigma Chemical | In vitro: TRAP/TAR, OHRS, NOS, TBARS, SOD, CAT, MTT, DFCH-DA | Antioxidant and neurotoxicological | Induced cell detachment and loss of viability at higher concentrations (20 µg/mL) of SH-SY5Y cells alone or in the presence of H2O2 or 1% of FBS, related to the increase of intracellular ROS, inducing an oxidative stress scenario | |
| Polat et al. [26], 2013, Turkey | Purchased from Sigma Chemical | In vitro: TAC, TOS, CA and MN | Antioxidant | Increased TAC in low doses and TOS in a high dose | |
| Ranković et al. [19], 2012, Serbia | Usnea barbata | In vitro: DPPH, SAS, reducing power, MIC, MTT and flow cytometry | Antioxidant, antimicrobial and anticarcinogenic | Very strong antioxidant and antimicrobial activities. Antiproliferative activity correlated with an increase in the number of cells in the sub-G1 phase whiled the percentage of cells in the S-phase and G2/M phase remained unchanged compared to the controls. Interestingly, LS174 cells treated with the tested samples showed a significant increase of the sub-G1 phase and concomitant decrease in G2/M was observed, supporting a G1 phase arrest. These results suggested that the compound have a prominent ability to induce apoptosis in FemX and LS174 cells. | |
| Atranorim(depside) | Marante et al. [24], 2003, Spain | Lethariella canariensis (Parmeliaceae) | In vitro: MIC, MTT and LPO | Antibacterial, anticarcinogenic and antioxidant | Moderate antibacterial activity against Staphylococcus aureus. Anti-proliferative effect against U937 and HL-60 and a dose-dependent antioxidant activity (100–250 µM) by decreasing H2O2/FeCl2 and inhibiting LPO, protecting from oxidative damage by the inhibition of both ROS and free radicals |
| Dévéhat et al. [16], 2007, France | Usnea articulata; | In vitro: DPPH and SAS | No significant activity | – | |
| Papadopoulou et al. [29], 2007, Greece | Hypotrachyna revoluta | In vitro: CO(II)/EDTA induced luminol hemiluminescence | Antioxidant | Antioxidant effect due to an additional hydroxyl group on the aromatic ring were the most active ones | |
| Bačkorová et al. [25], 2011, Slovakia | Purchased from Sigma Chemical Co. | In vitro: MTT, HTCA, viability, cell proliferation and detachment, cell cycle transition and apoptotic nuclear morphology | Anticarcinogenic | Evoked cytotoxicity in HL-60, A2780, MCF-7, SK-BR-3, HT-29, HCT-116 p53−/−, HCT-116 p53+/+ and Jurkat, triggered by higher pro-apoptotic activity (except on A2780 cell line), and supported by the inhibition of clonogenic ability and cell proliferation | |
| Thadhani et al. [7], 2011, Sri-Lanka | Parmotrema grayana | In vitro: SOR, NOR, and DPPH | No significant activity | – | |
| Melo et al. [8], 2011, Brazil | Cladina kalbii | In vitro: TRAP/TAR, TBARS, HRS, NOS, CAT, SOD, MTT, and LPO | Antioxidant, cytoprotective and pro-oxidative | Superoxide dismutase-like and scavenging activity of peroxyl radicals. Induce cytoprotection in the presence of toxic concentrations of H2O2 on the SH-SY5Y cells. Conversely, it presented a pro-oxidant capacity in a lipid-rich system, enhancing TBARS and also enhanced production of NO and H2O2 in higher concentrations | |
| Barreto et al. [30], 2013, Brazil | Cadina Kalbi | In vivo: Myofibroblast field and macroscopic and histological analyses | Wound healing | Topical application of atranorin reduced wound areas, induced earlier granulation tissue formation, increased cell proliferation, improved collagenization and modulated the myofi broblasts differentiation when compared to control animals. | |
| Kosanić et al. [28], 2014, Serbia | Cladonia furcata | In vitro: DPPH, SAS, reducing power, MIC, MTT and flow cytometry | Antioxidant, antimicrobial and anticarcinogenic | Very strong antioxidant and antimicrobial activities. Antiproliferative activity accompanied by a stronger increase in the percentage of the sub-G1 population and concomitant decrease in G2/M of FemX and LS174 cell lines, leading to a G0/G1 cell cycle block and inducing apoptosis in a cell cycle-dependent manner | |
| Diffractaic acid (depside) | Santos et al. [18], 2004, Brazil | Usnea subvacata Motyka | In vitro: H2O2 and NO measurements | Immunostimulatory | Induced greatest release of NO in peritoneal macrophages |
| Bayir et al. [33], 2006, Turkey | Usnea longissima | In vivo: SOD, GPx, GSH, LPO, MPx, NOS, iNOS cNOSand CAT | Antioxidant and gastroprotective | Decreased MPx, iNOS and LPO and increased cNOS, SOD, GPx and GSH, inhibiting neutrophil infiltration into gastric mucosal tissues | |
| Odabasoglu et al. [36], 2012, Turkey | Usnea longissima | In vivo: TUNEL, GSH, SOD, iNOS, MPx, CAS 2, CAS 3, CAS 8, and CAS 9 | Anticarcinogenic | Reduced the iNOS and MPx activities and increased SOD, GSH level and caspases (2, 3, 8 and 9) activities in tissue surrounding titanium implants | |
| Brisdelli et al. [23], 2013, Italy | Protousnea magellanica (Mont.) Krog | In vitro: MTT, CAS 3, 8 and 9, ROS determination and DPPH | Anticarcinogenic | Antiproliferative activity against HCT-116 cells and reduction of viability in MCF-7 and HeLa cells | |
| Lecanoric acid (depside) | Santos et al. [18], 2004, Brazil | Usnea subvacata Motyka | In vitro: H2O2 and NO measurements | No significant activity | – |
| Jayaprakasha and Rao [31], 2000, India | Parmotrea stuppeum | In vitro: β-carotene/linoleate model | Antioxidant | Moderate antioxidant activity | |
| Thadhani et al. [7], 2011, Sri-Lanka | Parmotrema grayana | In vitro: SOR, NOR, and DPPH | Antioxidant | Presented a high SOR activity, comparable to the standards of Propyl gallate (PG) and Butyrated hydroxyanisole (BHA). And moderate activity on NOR and DPPH. | |
| Lopes et al. [32], 2008, Brazil | Parmotrema tinctorum | In vitro: DPPH | Antioxidant | Moderate activity with an IC50 of 42.87 ± 1.20 | |
| Stictic acid (depsidone) | De Paz et al. [21], 2010, Spain | Xanthoparmelia camtschadalis | In vitro: MTT, ORAC and ROS determination | Antioxidant and neuroprotective | Protective effect against U373MG cell line by decreasing ROS production induced by H2O2 |
| Papadopoulou et al. [29], 2007, Greece | Hypotrachyna revoluta | In vitro: CO(II)/EDTA induced luminol chemiluminescence | Antioxidant | Noteworthy antioxidant activity | |
| Dévéhat et al. [16], 2007, France | Usnea articulata | In vitro: DPPH and SAS | No significant activity | – | |
| Lobaric acid (depsidones) | Bhattarai et al. [37], 2013. Republic of Korea | Stereocaulon alpinum | In vitro: Paper disk diffusion, MTT and DPPH | Antibacterial and antioxidant | Activity against gram-positive bacteria Staphylococcus aureus and Bacillus subtilis and moderate scavenge activity in a dose-dependent manner without any toxic effects |
| Brisdelli et al. [23], 2013, Italy | Stereocaulon alpinum Laurer ex Funck | In vitro: MTT, CAS 3, 8 and 9, ROS determination and DPPH | Anticarcinogenic | Anti-proliferative activity against HeLa and HCT cells lines | |
| Thadhani et al. [7], 2011, Sri-Lanka | Cladonia sp. | In vitro: SOR, NOR, and DPPH | Antioxidant | Promising antioxidant activity in SOR assay | |
| Methyl orsenillate (Benzenoid) | Jayaprakasha and Rao [31], 2000, India | Parmotrea stuppeum | In vitro: β-carotene/linoleate model | Antioxidant | Moderate antioxidant activity |
| Orsenillic acid (Benzoic acid derivative) | |||||
| Orcinol (1) (Benzenoid) | Thadhani et al. [7], 2011, Sri-Lanka | Parmotrema grayana | In vitro: SOR, NOR, and DPPH | Antioxidant | Activity in NOR assay |
| Orsellinic acid (2) (Benzenoid) | Parmotrema grayana, | Activity in NOR assay | |||
| Methyl orsellinate (3) (Benzoic acid derivative) | Heterodermia obscurata | Activity in NOR assay | |||
| Methyl haematommate (4) Benzoic acid derivative) | Heterodermia obscurata | Activity in NOR assay | |||
| Methyl β-orcinolcarboxylate (5) (Benzoic acid derivative) | Heterodermia obscurata | Activity in NOR assay | |||
| Methyl β-orsellinate (Benzoic acid derivative) | Marante et al. [24], 2003, Spain | Lethariella canariensis | In vitro: MIC, MTT and LPO | Antibacterial | Activity against Staphilococcus aureus |
| Protocetraric acid (depsidone) | Manojlović et al. [38], 2012, Serbia | Parmelia caperata | In vitro: DPPH, reducing power, SAS, MIC and MTT | Antibacterial and anticarcinogenic | Highly antibacterial active and presented strong anticancer activity toward FemX and LS174 cell lines. These activities could be due to its higher phenol content. |
| Santos et al. [18], 2004, Brazil | Usnea subvacata Motyka | In vitro: H2O2 and NO measurements | Immunostimulatory | Higher activity in increasing NO release in macrophage cells. | |
| Fumarprotocetraric acid (depsidone) | Cladonia verticillaris Roddi | Higher activity in increasing NO and H2O2 release in macrophage cells | |||
| Dévéhat et al. [16], 2007, France | Usnea articulata | In vitro: DPPH and SAS | Antioxidant | High activity in the SAS and moderate in the DPPH | |
| Kosanić et al. [28], 2014, Serbia | Cladonia rangiferina | In vitro: DPPH, SAS, reducing power, MIC, MTT and flow cytometry | Antioxidant, antimicrobial and anticarcinogenic | Strong antioxidant and antimicrobial activities. Antiproliferative activity accompanied by a stronger increase in the percentage of the sub-G1 population and concomitant decrease in G2/M of FemX and LS174 cell lines, inducing apoptosis in a cell cycle-dependent manner | |
| Cryptostictinolide (Compound 2-C19H16O8 - depsidone) | Dévéhat et al. [16], 2007, Australia | Antioxidant | Moderate activity in DPPH | ||
| Compound 1 (C19H14O8, identical to stictic acid–depsidone) | No significant activity | Moderate activity in DPPH | |||
| Cryptostictic acid (Depsidone) | No significant activity | – | |||
| Menegazziaic acid (Depsidone) | No significant activity | – | |||
| Constictic acid (Depsidone) | No significant activity | – | |||
| 3-O-methylconsalazinic acid (Depsidone) | No significant activity | – | |||
| Barbatic acid (Depside) | No significant activity | – | |||
| Ergosterol peroxide (Terpenoid) | No significant activity | – | |||
| Peristictic acid (Depsidone) | Antioxidant | – | |||
| Norstictic acid (depsidone) | Antioxidant | High SAS activity | |||
| Ranković et al. [19], 2012, Serbia | Toninia candida | In vitro: DPPH, SAS, reducing power, MIC, MTT and flow cytometry | Antioxidant, antimicrobial and anticarcinogenic | Stronger antioxidant and antimicrobial activities. Antiproliferative activity correlated with an increase in the number of cells in the sub-G1 phase whiled the percentage of cells in the S-phase and G2/M phase remained unchanged compared to the controls. Interestingly, LS174 cells treated with the tested samples showed a significant increase of the sub-G1 phase and concomitant decrease in G2/M was observed, supporting a G1 phase arrest. These results suggested that the compound have a prominent ability to induce apoptosis in FemX and LS174 cells. | |
| Cryptostictinolide (depsidone) | Papadopoulou et al. [29], 2007, Greece | Hypotrachyna revoluta (Flörke) Hale | In vitro: CO(II)/EDTA induced luminol chemiluminescence | No significant activity | – |
| Hypotrachynic acid (depside) | Antioxidant | Noteworthy antioxidant activity | |||
| Deoxystictic acid (depsidone) | Antioxidant | Noteworthy antioxidant activity | |||
| 8'-methylconstictic acid (depsidone) | Antioxidant | Noteworthy antioxidant activity | |||
| 8'-methylstictic acid (depsidone) | Antioxidant | Noteworthy antioxidant activity | |||
| 8'-methylmenegazziaic acid (depsidone) | Antioxidant | Noteworthy antioxidant activity | |||
| 8'-ethylstictic acid (depsidone) | Antioxidant | Noteworthy antioxidant activity | |||
| Atranol (benzoic acid derivative) | Marante et al. [24], 2003, Spain | Lethariella canariensis | In vitro: MIC, MTT and LPO | Anticarcinogenic | Inhibited the proliferation of U937 and HL-60 and presented dose-dependent antioxidant activity |
| Chloroatranol (benzoic acid derivative) | Anticarcinogenic and antioxidant | Inhibited the proliferation of U937 and HL-60 and presented dose-dependent antioxidant activity | |||
| Hematommic acid (benzoic acid derivative) | Antioxidant | Dose-dependent antioxidant activity | |||
| Chlorohematommic acid (benzoic acid derivative) | Antioxidant | Dose-dependent antioxidant activity | |||
| Methyl chlorohematommate (benzoic acid derivative) | Antioxidant | Dose-dependent antioxidant activity | |||
| Ethyl hematommate (benzoic acid derivative) | Anticarcinogenic and antioxidant | Inhibited the proliferation of U937 and HL-60 and presented dose-dependent antioxidant activity | |||
| Ethyl chlorohematommate (benzoic acid derivative) | Antioxidant | Dose-dependent antioxidant activity | |||
| Chloroatranorin (benzoic acid derivative) | Antioxidant | Dose-dependent antioxidant activity | |||
| Methyl hematommate (benzoic acid derivative) | Anticarcinogenic and antioxidant | Inhibited the proliferation of U937 and HL-60 and presented dose-dependent antioxidant activity | |||
| Thadhani et al. [7], 2011, Sri-Lanka | Cladonia sp. | In vitro: SOR, NOR, and DPPH | Antioxidant | Promising antioxidant activity in NOR | |
| Montagnetal (benzoic acid derivative) | Antioxidant | Promising antioxidant activity in NOR | |||
| Divericatic acid (depside) | Antioxidant | Significant level of activity in SOR | |||
| Erythrin (depside) | Antioxidant | Promising antioxidant activity in NOR | |||
| Sekikiac acid (depside) | Antioxidant | Significant level of activity in SOR | |||
| Zeorin (Terpenoid) | Antioxidant | Significant level of activity in SOR | |||
| Lobastin (depsidone) | Bhattarai et al. [37], 2013. Republic of Korea | Stereocaulon alpinum | In vitro: Paper disk diffusion, MTT and DPPH | Antibacterial and Antioxidant | Active against Gram-positive bacteria, B. subtilis and S. aureus. Moderate antioxidant activity compared with the synthetic commercial standard BHT |
| Sphaerophorin (depside) | Russo et al. [44], 2008, Chile | Sphaerophorus globosus, Psoroma reticulatum, P. pulchrum, P. balladium | In vitro: DNA cleavage induced by H2O2 UV-photolysis, DNA single-strand breaks induced by Angeli’s salt, SAS, TUNEL, Comet, CAS 3, ROS determination; SAS, MTT | Antioxidant and Anticarcinogenic | The compounds suppressed the formation of lin DNA and induced a partial recovery of scDNA; showed a dose-dependent superoxide scavenging effect; exhibited a significant inhibitory effect on M14 cell; produced DNA damage, inducing a programmed cell death; increased cas-3 and ROS in a concentrantion-dependent manner. |
| Pannarin (depsidone) | |||||
| Psoromic acid (depsidone) | Behera, Mahadik and Morey [17], 2012, India | Usnea complanata | In vivo: FRSA, NOR, LPO, ACE and HMGR | Cardioprotective | Moderate to strong antioxidant activity, concentration-dependent manner, on the FRSA, NOR and in LPO. Poor fibrinolytic potential |
| Isophysodic acid (depsidone) | Pavlović et al. [39], 2013, Serbia | Hypogymnia physodes | In vitro: CCK-8, ROS determination, MMP | No significant activity | – |
| Physodalic acid (depsidone) | Immunoprotective | Induced thymocytes toxicity mainly through increased ROS levels and decreased MMP | |||
| Stojanović et al. [41], 2014, Serbia | In vitro: MTT | No significant activity | – | ||
| 3-hydroxyphysodic acid (depsidone) | Pavlović et al. [39], 2013, Serbia | In vitro: CCK-8, ROS determination, MMP | Immunoprotective | Induced thymocytes toxicity that may lead to intracellular low energy levels with resulted cytotoxicity | |
| Stojanović et al. [41], 2014, Serbia | In vitro: MTT | Anticarcinogenic | Anti-proliferative action on HeLa cells | ||
| Physodic acid (depsidone) | Pavlović et al. [39], 2013, Serbia | In vitro: CCK-8, ROS determination, MMP | Immunoprotective | Induced thymocytes toxicity mainly through increased ROS levels and decreased MMP | |
| Stojanović et al. [41], 2014, Serbia | In vitro: MTT | Anticarcinogenic | Anti-proliferative action on HeLa cells | ||
| Kosanić et al. [40], 2013, Serbia | Pseudoevernia furfuraceae (L.) Zopf | In vitro: DPPH, Reducing power, SAS, MIC, MTT and flow citometry | Antioxidant, antimicrobial and anticarcinogenic | Both compounds showed high antioxidant activity on reducing power and SAS assays, correlated with a high content of total phenol of the acetone extracts of the species from which they were isolated. Very strong antimicrobial (MIC) assay activity against B. mycoides, B. subtilis, E. coli, K. neumonia, S. aureus, A. flavus, A. fumigatus, C. albicans, P. purpurescens and P. verrucosum, and cytotoxic activities against FemX and LS 174 cell lines. | |
| Evernic acid (depside) | |||||
| Parietin (quinone) | Bačkorová et al. [25], 2011,Slovakia | Xanthoria parietina | In vitro: MTT, HTCA, viability, cell proliferation and detachment, cell cycle transition and apoptotic nuclear morphology | Anticarcinogenic | Evoked cytotoxicity in A2780, Jurkat and HT-29 human cancer cell lines. Only inhibited some clonogenic ability of HeLa and MCF-7 cell lines. |
| Gyrophoric acid (depside) | Umbilicaria hirsuta | Anticarcinogenic | Anti-proliferative effect on HL-60, A2780 and Jurkat cells and and slightly pro-apoptotic. | ||
| Vicanicin (depsidones) | Brisdelli et al. [23], 2013, Italy | Psoroma pallidum Nyl., P. pulchrum Malme | In vitro: MTT, CAS 3, 8 and 9, ROS determination and DPPH | Anticarcinogenic | Induced a significant loss of viability in a dose-dependent manner in HeLa and HCT-116 cells |
| Variolaric acid | Ochrolechia deceptionis (Hue) Darb. | No significant activity | – | ||
| Protolichesterinic acid (depsidones) | Cornicularia aculeata (Schreb.) Ach. | Anticarcinogenic | Stronger cytotoxic activity related to its ability to induce apoptosis in HeLa cells by activating an extrinsic cas-8/-3-mediated as well as intrinsic cas-9/-3-mediated pathway. | ||
| Salazinic acid (depsidone) | De Paz et al. [21], 2010, Spain | Xanthoparmelia camtschadalis | In vitro: MTT, ORAC and ROS determination | Antioxidant and neuroprotective | Protective effect against U373MG cell line by decreasing ROS production induced by H2O2 |
| Manojlović et al. [38], 2012, Serbia | Parmelia caperata | In vitro: DPPH, reducing power, SAS, MIC and MTT | Antibacterial and anticarcinogenic | Active against B. mycoides, B. subtilis, E. coli, K. neumonia, S. aureus, A. flavus, A. fumigatus, C. albicans, P. purpurescens and P. verrucosum and presented anti-proliferative activity toward FemX and LS174 cell lines. | |
| Santos et al. [18], 2004, Brazil | Rimelia cetrata | In vitro: H2O2 and NO measurements | Immunostimulatory | Activated the release of H2O2 and NO in the culture of mice peritoneal macrophages. | |
| Hypostictic acid (depsidone) | Pseudoparmelia sphaerosphora (Nyl) Hale | No significant activity | – | ||
| Biruloquinone (quinone) | Luo et al. [46], 2013, China | Cladonia macilenta | In vitro: MTT and AChE inhibittory | Neuroprotective | Improved viability the H2O2 and β-amyloid injured PC12 cells. Classified as a a mixed-II inhibitor |
| Canarione (quinone) | Kinoshita et al. [42], 2010, Japan | Lethariella sernanderi, L. cashmeriana, and L. sinensis | In vitro: Cu(II) reducing activity | Antioxidant | Among them, 7-chlororubrocashmeriquinone showed the strongest potential, although canarione, 7-chlorocanarione also demonstrated high antioxidant activities |
| Rubrocashmeriquinone (quinone) | |||||
| 7-Chlororubrocashmeriquinone (quinone) | |||||
| 7-chlorocanarione (quinone) | |||||
| Ramalin (nitrogen compound) | Paudel et al. [43], 2011, Republic of Korea | Ramalina terebrata | In vitro: DPPH, ABTS•+, Fe3+ reducing power, SAS, and tyrosinase inhibitory In vivo: MTT, H2O2 and iNOS | Antioxidant and anticarcinogenic | Scavenged DPPH, ABTS•+, NO and H2O2 radicals. Presented capacity in reducing Fe3+ to Fe2+ ions and inhibited tyrosinase activity in murine macrophage |
| 1,8-dihydrixy-3-hydroxymethyl-5-methylxanthone (xantone) | Takenaka et al. [44], 2000, Japan | Pyrenula japonica | Not tested | – | – |
| 1,2,8-trihydroxy-5-methoxy-3-methylxanthone (xantone) | In vitro: DPPH | Antioxidant | Higher scavenging than those well-known antioxidants, α-tocopherol and BHT | ||
| 1,7-dihydroxy-3-methylxanthone (xantone) | In vitro: DPPH | – | Low scavenging activity | ||
| 1,5,8-trihydroxy-3-methylxanthone (xantone) | In vitro: DPPH | Antioxidant | Higher scavenging than those well-known antioxidants, α-tocopherol and BHT | ||
| 1,8-dihydroxy-5-methoxy-3-methylxanthone (xantone) | In vitro: DPPH | – | Low scavenging activity | ||
| Emodin (xantone) | Not tested | – | – | ||
| Sclerotiorin (xantone) | Not tested | – | – |
3. Final Considerations
4. Methods
4.1. Search Strategy
4.2. Study Selection
4.3. Data Extraction
5. Conclusions
Supplementary Mterials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Hale, L.J. The pattern of growth of Clytia johnstoni. J. Embryol. Exp. Morphol. 1973, 29, 283–309. [Google Scholar]
- Thadhani, V.M.; Choudhary, M.I.; Ali, S.; Omar, I.; Siddique, H.; Karunaratne, V. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. [Google Scholar] [CrossRef]
- Melo, M.G.D.; Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; Pasquali, M.A.B.; Rabelo, T.K.; Rocha, R.F.; Quintans-Junior, L.J.; Araújo, A.A.S.; Silva, F.A.; et al. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. In Vitro 2011, 25, 462–468. [Google Scholar]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C 2010, 65, 157–173. [Google Scholar]
- Schmitt, I.; Lumbsch, H.T. Molecular phylogeny of the Pertusariaceae supports secondary chemistry as an important systematic character set in lichen-forming ascomycetes. Mol. Phylogenet. Evol. 2004, 33, 43–55. [Google Scholar] [CrossRef]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Nunes, P.S.; Albuquerque-Junior, R.L.; Cavalcante, D.R.; Dantas, M.D.; Cardoso, J.C.; Bezerra, M.S.; Souza, J.C.; Serafini, M.R.; Quitans-Junior, L.J.; Bonjardim, L.R.; et al. Collagen-based films containing liposome-loaded usnic acid as dressing for dermal burn healing. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Nunes, P.S.; Bezerra, M.S.; Costa, L.P.; Cardoso, J.C.; Albuquerque-Junior, R.L.; Rodrigues, M.O.; Barin, G.B.; Silva, F.A.; Araujo, A.A.S. Thermal characterization of usnic acid/collagen-based films. J. Therm. Anal. Calorim. 2010, 99, 1011–1014. [Google Scholar] [CrossRef]
- Barreto, R.S.S.; Albuquerque-Júnior, R.L.C.; Araújo, A.A.S.; Almeida, J.R.G.S.; Santos, M.R.V.; Barreto, A.S.; DeSantana, J.M.; Siqueira-Lima, P.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. A Systematic Review of the Wound-Healing Effects of Monoterpenes and Iridoid Derivatives. Molecules 2014, 19, 846–862. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C.J. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef]
- Dévéhat, F.; Tomasi, S.; Elix, J.A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. Stictic Acid Derivatives from the Lichen Usnea articulata and Their Antioxidant Activities. J. Nat. Prod. 2007, 70, 1218–1220. [Google Scholar] [CrossRef]
- Behera, B.C.; Mahadik, N.; Morey, M. Antioxidative and cardiovascular-protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation. Pharm. Biol. 2012, 50, 968–979. [Google Scholar] [CrossRef]
- Santos, L.C.; Honda, N.K.; Carlos, I.Z.; Vilegas, W. Intermediate reactive oxygen and nitrogen from macrophages induced by Brazilian lichens. Fitoterapia 2004, 75, 473–479. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological Activities of Toninia candida and Usnea barbata Together with Their Norstictic Acid and Usnic Acid Constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef]
- Bessadottir, M.; Egilsson, M.; Einarsdottir, E.; Magnusdottir, I.H.; Ogmundsdottir, M.H.; Omarsdottir, S.; Ogmundsdottir, H.M. Proton-Shuttling Lichen Compound Usnic Acid Affects Mitochondrial and Lysosomal Function in Cancer Cells. PLoS One 2012, 7, e51296. [Google Scholar] [CrossRef]
- De Paz, G.A.; Raggio, J.; Gómez-Serranillos, M.P.; Palomino, O.M.; González-Burgos, E.; Carretero, M.E.; Crespo, A. HPLC isolation of antioxidant constituents from Xanthoparmelia spp. J. Pharm. Biomed. Anal. 2010, 53, 165–171. [Google Scholar] [CrossRef]
- Jin, J.Q.; Li, C.Q.; He, L.C. Down-regulatory effect of usnic acid on nuclear factor-kappaB-dependent tumor necrosis factor-alpha and inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Phytother. Res. 2008, 22, 1605–1609. [Google Scholar] [CrossRef]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Bozzi, A.; Amicosante, G.; Celenza, G. Cytotoxic activity and antioxidant capacity of purified lichen metabolites: An in vitro study. Phytother. Res. 2013, 27, 431–437. [Google Scholar] [CrossRef]
- Marante, F.J.T.; Castellano, A.G.; Rosas, F.E.; Aguiar, J.Q.; Barrera, J.J.B. Identification and quantitation of allelochemicals from the lichen Lethariella canariensis: Phytotoxicity and antioxidative activity. Chem. Ecol. 2003, 29, 2049–2071. [Google Scholar] [CrossRef]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef]
- Polat, Z.; Aydin, E.; Türkez, H.; Aslan, A. In vitro risk assessment of usnic acid compound. Toxicol. Ind. Health 2013. [Google Scholar] [CrossRef]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Vasques, L.M.; Dos Santos, J.P.; Da Rocha, R.F.; Pasquali, M.A.; Rybarczyk-Filho, J.L.; Araújo, A.A.; Moreira, J.C.; Gelain, D.P. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. In Vitro 2012, 26, 304–314. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Rančić, A.; Manojlović, N. Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT Food Sci. Technol. 2014, 59, 518–525. [Google Scholar] [CrossRef]
- Papadopoulou, P.; Tzakou, O.; Vagias, C.; Kefalas, P.; Roussis, V. Beta-orcinol metabolites from the lichen Hypotrachyna revoluta. Molecules 2007, 12, 997–1005. [Google Scholar]
- Barreto, R.S.S.; Albuquerque-Júnior, R.L.C.; Pereira-Filho, R.N.; Quintans, J.S.S.; Barreto, A.S.; DeSantana, J.M.; Santana-Filho, V.J.; Santos, M.R.V.; Bonjardim, L.R.; Araújo, A.A.S.; et al. Evaluation of wound healing activity of atranorin, a lichen secondary metabolite, on rodents. Braz. J. Pharmacogn. 2013, 23, 310–319. [Google Scholar]
- Jayaprakasha, G.K.; Rao, L.J. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z. Naturforsch. C 2000, 55, 1018–1022. [Google Scholar]
- Lopes, T.I.; Coelho, R.G.; Yoshida, N.C.; Honda, N.K. Radical-scavenging activity of orsellinates. Chem. Pharm. Bull. 2008, 56, 1551–1554. [Google Scholar]
- Bayir, Y.; Odabasoglu, F.; Cakir, A.; Aslan, A.; Suleyman, H.; Halici, M.; Kazaz, C. The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine 2006, 13, 584–590. [Google Scholar]
- Liu, W.; Xu, Z.; Yang, T.; Deng, Y.; Xu, B.; Feng, S.; Li, Y. The protective role of tea polyphenols against methylmercury-induced neurotoxic effects in rat cerebral cortex via inhibition of oxidative stress. Free Radic. Res. 2014, 48, 849–863. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar]
- Odabasoglu, F.; Yildirim, O.S.; Aygun, H.; Halici, Z.; Halici, M.; Erdogan, F.; Cadirci, E.; Cakir, A.; Okumus, Z.; Aksakal, B.; et al. Diffractaic acid, a novel proapoptotic agent, induces with olive oil both apoptosis and antioxidative systems in Ti-implanted rabbits. Eur. J. Pharmacol. 2012, 674, 171–178. [Google Scholar] [CrossRef]
- Bhattarai, H.D.; Kim, T.; Oh, H.; Yim, J.H.J. A new pseudodepsidone from the Antarctic lichen Stereocaulon alpinum and its antioxidant, antibacterial activity. J. Antibiot 2013, 66, 559–561. [Google Scholar]
- Manojlović, N.; Ranković, B.; Kosanić, M.; Vasiljević, P.; Stanojković, T. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 2012, 19, 1166–1172. [Google Scholar] [CrossRef]
- Pavlovic, V.; Stojanovic, I.; Jadranin, M.; Vajs, V.; Djordjević, I.; Smelcerovic, A.; Stojanovic, G. Effect of four lichen acids isolated from Hypogymnia physodes on viability of rat thymocytes. Food Chem. Toxicol. 2013, 51, 160–164. [Google Scholar] [CrossRef]
- Kosanić, M.; Manojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Stojanović, I.; Najman, S.; Jovanović, O.; Petrović, G.; Najdanović, J.; Vasiljević, P.; Šmelcerović, A. Effects of Depsidones from Hypogymnia physodes on HeLa Cell Viability and Growth. Folia Biol. 2014, 60, 89–94. [Google Scholar]
- Kinoshita, K.; Togawa, T.; Hiraishi, A.; Nakajima, Y.; Koyama, K.; Narui, T.; Wang, L.S.; Takahashi, K. Antioxidant activity of red pigments from the lichens Lethariella sernanderi, L. cashmeriana, and L. sinensis. J. Nat. Med. 2010, 64, 85–88. [Google Scholar] [CrossRef]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef]
- Takenaka, Y.; Tanahashi, T.; Nagakura, N.; Hamada, N. Production of xanthones with free radical scavenging properties, emodin and sclerotiorin by the cultured lichen mycobionts of Pyrenula japonica. Z. Naturforsch. C 2000, 55, 910–914. [Google Scholar]
- Russo, A.; Piovano, M.; Lombardo, L.; Garbarino, J.; Cardile, V. Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci. 2008, 83, 468–474. [Google Scholar] [CrossRef]
- Luo, H.; Li, C.; Kim, J.C.; Liu, Y.; Jung, J.S.; Koh, Y.J.; Hur, J.S. Biruloquinone, an Acetylcholinesterase Inhibitor Produced by Lichen-Forming Fungus Cladonia macilenta. J. Microbiol. Biotechnol. 2013, 23, 161–166. [Google Scholar] [CrossRef]
- Boustie, J.; Grube, M. Lichens—A promising source of bioactive secondary metabolites. Plant Genet. Resour. 2005, 3, 273–287. [Google Scholar] [CrossRef]
- Cowan, D.A.; Green, T.G.A.; Wilson, A.T. Lichen metabolism—Aspects of light and dark physiology. New Phytol. 1979, 83, 761–769. [Google Scholar]
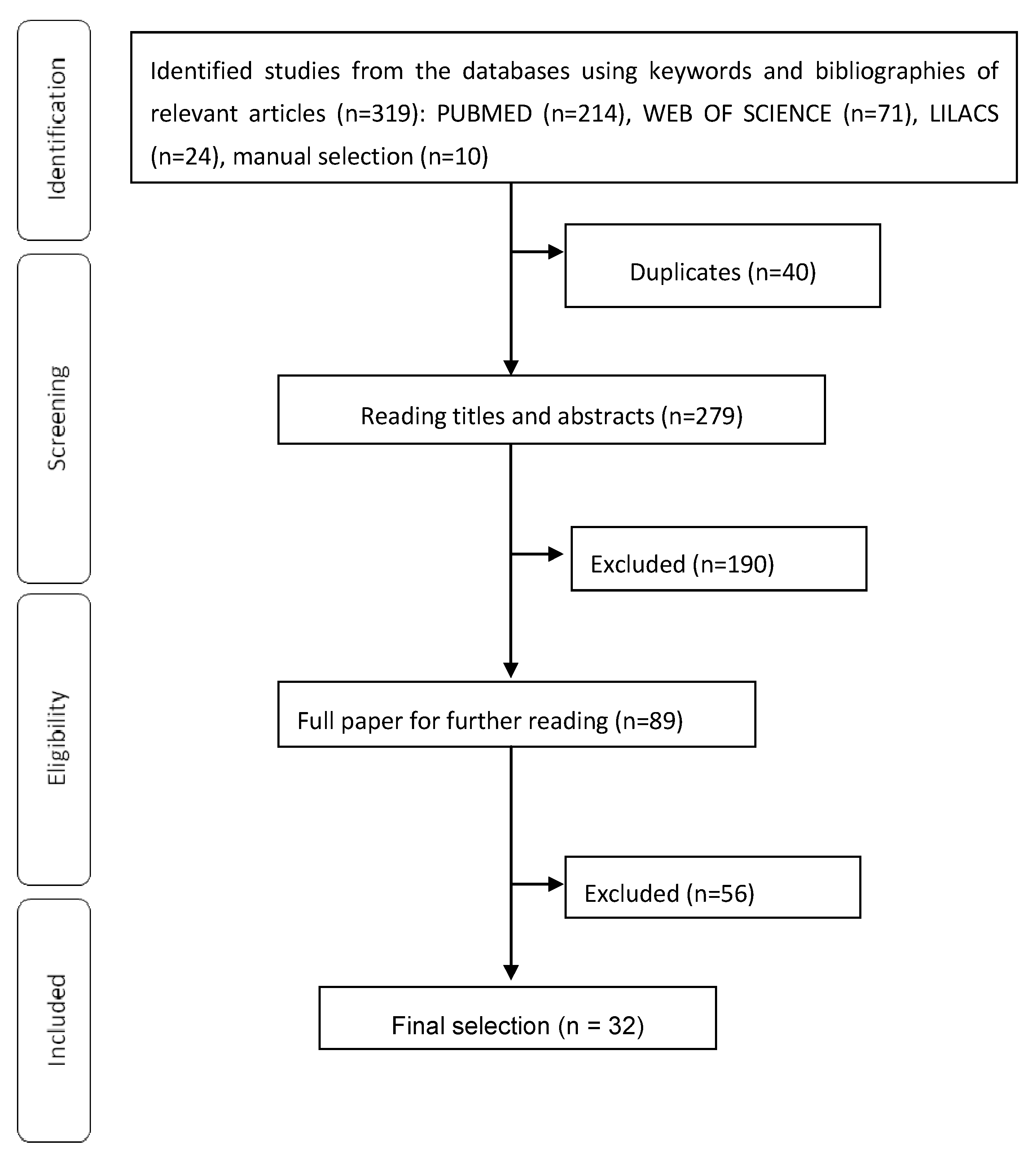
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules 2014, 19, 14496-14527. https://doi.org/10.3390/molecules190914496
White PAS, Oliveira RCM, Oliveira AP, Serafini MR, Araújo AAS, Gelain DP, Moreira JCF, Almeida JRGS, Quintans JSS, Quintans-Junior LJ, et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules. 2014; 19(9):14496-14527. https://doi.org/10.3390/molecules190914496
Chicago/Turabian StyleWhite, Pollyanna A. S., Rita C. M. Oliveira, Aldeidia P. Oliveira, Mairim R. Serafini, Adriano A. S. Araújo, Daniel P. Gelain, Jose C. F. Moreira, Jackson R. G. S. Almeida, Jullyana S. S. Quintans, Lucindo J. Quintans-Junior, and et al. 2014. "Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review" Molecules 19, no. 9: 14496-14527. https://doi.org/10.3390/molecules190914496
APA StyleWhite, P. A. S., Oliveira, R. C. M., Oliveira, A. P., Serafini, M. R., Araújo, A. A. S., Gelain, D. P., Moreira, J. C. F., Almeida, J. R. G. S., Quintans, J. S. S., Quintans-Junior, L. J., & Santos, M. R. V. (2014). Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules, 19(9), 14496-14527. https://doi.org/10.3390/molecules190914496




