Effect of Redox Modulating NRF2 Activators on Chronic Kidney Disease
Abstract
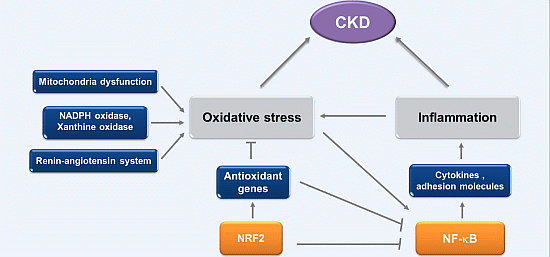
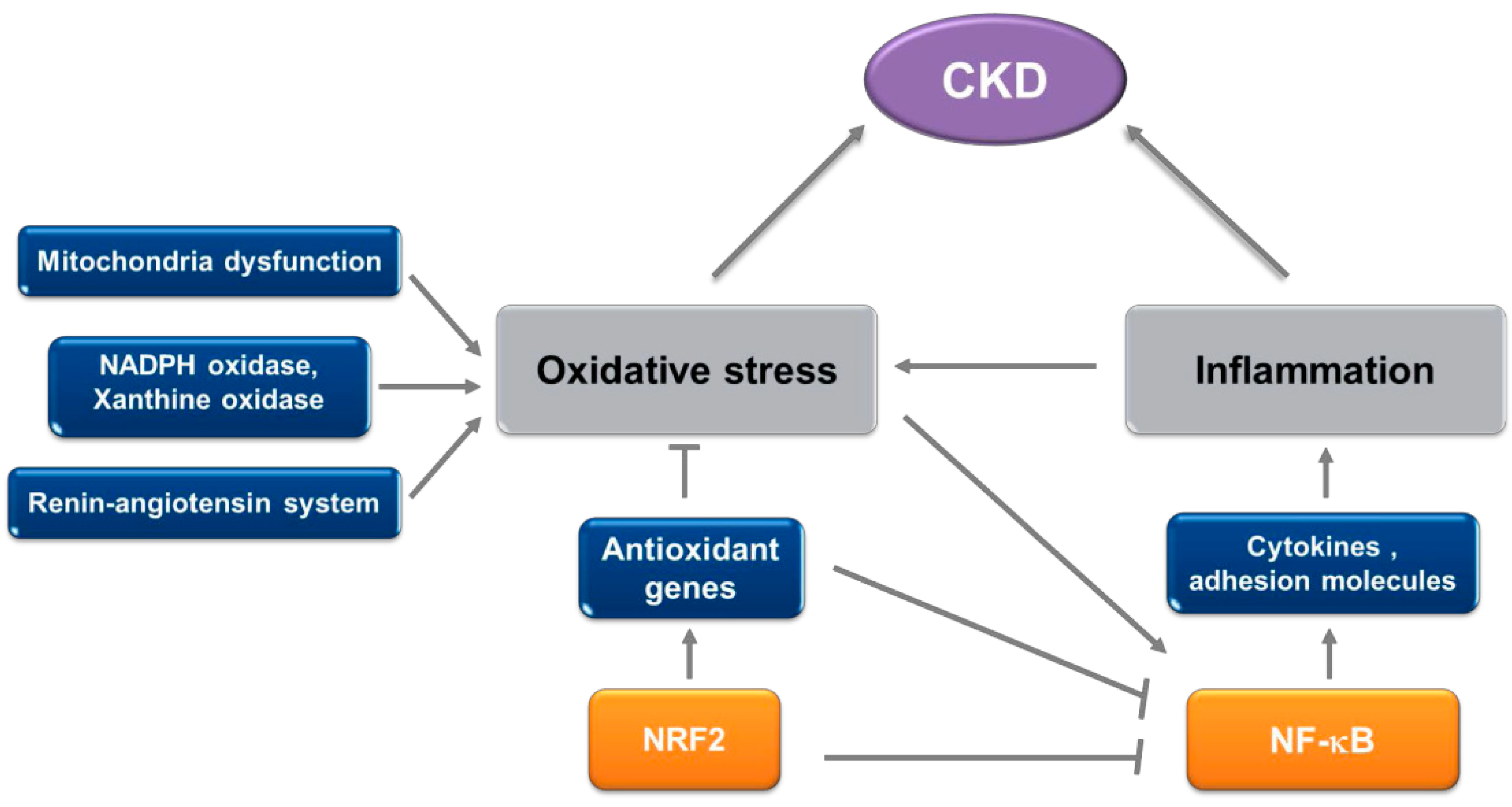
:1. Chronic Kidney Disease (CKD)
2. Oxidative Stress and Inflammation in CKD
2.1. Oxidative Stress in CKD
2.2. Inflammation in CKD
2.3. Biological Markers of Oxidative Stress and Inflammation in CKD
| Type of Markers | Group | Specific Marker | Refs. |
|---|---|---|---|
| Oxidative markers | Lipid | F2-isoprostanes | [56,57,58] |
| Malondialdehyde (MDA) | [59,60] | ||
| Thiobarbituric acid- reactive substance | [61] | ||
| Protein | Carbonyls | [62,63] | |
| Advanced glycation end-products (AGEs) | [64] | ||
| Advanced oxidation protein products (AOPP) | [65,66] | ||
| Oxidized low density lipoproteins (OxLDL) | [67] | ||
| DNA | 8-Oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG) | [46,68] | |
| DNA strand breaks | [69,70] | ||
| Inflammatory markers | C-reactive protein (CRP) | [71,72] | |
| IL-1 | [73] | ||
| IL-6 | [72] | ||
| TNFα | [73,74] |
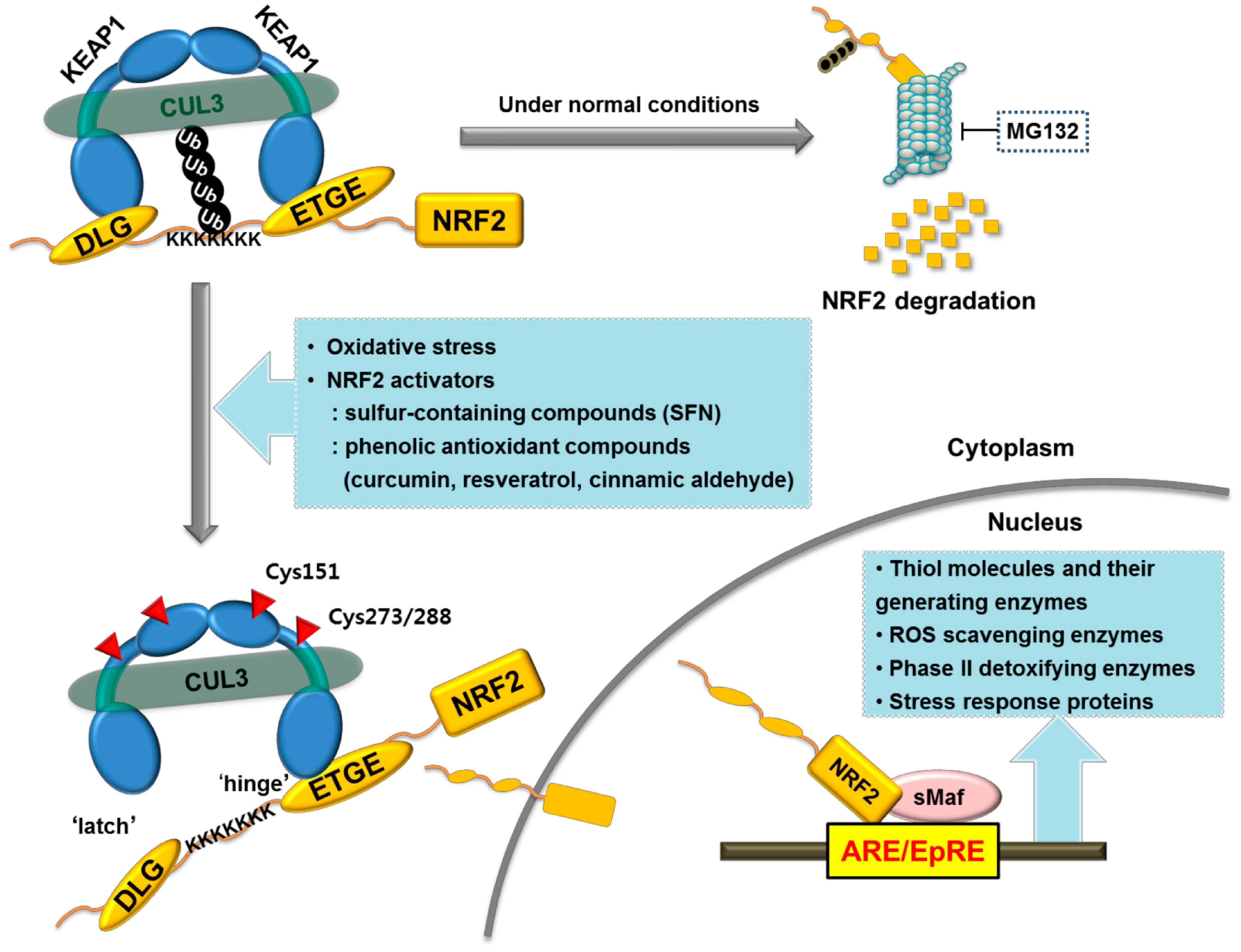
3. Involvement of NRF2 Signaling in CKD Pathology
3.1. NRF2 as a Crucial Regulator of the Antioxidant Defense System
| Functional Classification | Gene Name | Description | Species | |
|---|---|---|---|---|
| Antioxidant proteins | GCLC | γ-Glutamate-cysteine ligase, catalytic subunit | m | h |
| GCLM | γ-Glutamate-cysteine ligase, modifier subunit | m | h | |
| GSR | Glutathione reductase | m | h | |
| GPx1 | Glutathione peroxidase 1 (or 4) | m | ||
| GPx2 | Glutathione peroxidase 2 | m | h | |
| TXNRD | Thioredoxin reductase | m | h | |
| TXN | Thioredoxin | m | h | |
| PRDX1 &6 | Peroxiredoxin 1 (or 6) | m | h | |
| CAT | Catalase | m | h | |
| SOD | Superoxide dismutase | m | h | |
| SRXN1 | Sulfiredoxin 1 | m | h | |
| GGT1 | γ-Glutamyltransferase 1 | h | ||
| GLRX | Glutaredoxin | h | ||
| Phase I oxidation, reduction and hydrolysis enzymes | ALDH3A1 | Aldehyde dehydrogenase 3A1 | m | h |
| ADH7 | Alcohol dehydrogenase 7 | m | ||
| AKR1B1 | Aldo-keto reductase 1B1 | m | h | |
| AKR1C1 | Aldo-keto reductase 1C1 | h | ||
| CBR1 | Carbonyl reductase 1 | m | ||
| EPHX1 | Microsomal epoxide hydrolase 1 | m | h | |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 | m | h | |
| CYP2B9 | Cytochrome p450, 2B9 | |||
| Phase II detoxifying enzymes | GSTM1 | Glutathione S-transferase class mu 1 (or 2,4,5,6) | m | |
| GSTM3 | Glutathione S-transferase class mu 3 | m | h | |
| GSTA1 | Glutathione S-transferase class alpha 1 (or 2,3,4) | m | ||
| MGST1 | Microsomal glutathione S-transferase 2 | m | h | |
| MGST2 | Microsomal glutathione S-transferase 3 | m | ||
| UGT1A6 | UDP glucuronosyltransferase 1A6 | h | ||
| UGT2B1 | UDP glucuronosyltransferase 2B1 | m | ||
| UGT2B5 | UDP glucuronosyltransferase 2B5 | m | ||
| NADPH-generating enzymes | ME1 | Malic enzyme 1 | m | h |
| G6PD | Glucose-6-phosphate 1-dehydrogenase | m | h | |
| PGD | 6-Phosphogluconate dehydrogenase | m | h | |
| Drug transporters | ABCB6 | ATP-binding cassette, subfamily B, member 6 | m | h |
| ABCC1 | ATP-binding cassette, subfamily C, member 1 | m | ||
| ABCC2 | ATP-binding cassette, subfamily C, member 2 | m | h | |
| ABCC3 | ATP-binding cassette, subfamily C, member 3 | m | h | |
| ABCC4 | ATP-binding cassette, subfamily C, member 4 | m | ||
| ABCC5 | ATP-binding cassette, subfamily C, member 5 | m | ||
| Heme and metal metabolism (stress response protein) | HO-1 | Heme oxygenase-1 | m | h |
| FTH1 | Ferritin, heavy polypeptide 1 | m | h | |
| FTL1 | Ferritin, light polypeptide 1 | m | h | |
| MT1 | Metallothionein 1 | m | h | |
| MT2 | Metallothionein 2 | m | h | |
| Protein degradation | UbC | Ubiquitin C | m | |
| PSMB5 | Proteasome 26S PSMB5 subunit | m | ||
| Lipid metabolism | ACOT7 | Acetyl-CoA thioesterase 7 | m | |
| ACOX1 | Acetyl-CoA oxidase 1 | m | ||
| LIPH | Lipase, member H | m | ||
| CES1G | Carboxylesterase 1G | m | ||
3.2. NRF2 as a Multi-organ Protector against Oxidative Damages
3.3. NRF2 as Anti-inflammatory Modulator
4. Role of the NRF2 System in CKD
5. Naturally Occurring NRF2 Activators and CKD
5.1. SFN
| NRF2 Activators | Chemical Structure |
|---|---|
| Sulforaphane (SFN) |  |
| Resveratrol |  |
| Curcumin |  |
| Cinnamic aldehyde (CA) |  |
| Bardoxolone methyl |  |
5.2. Resveratrol
5.3. Curcumin
5.4. CA
6. Experience and Promise from Bardoxolone Methyl for CKD Management
7. Conclusions
Abbreviation
| CKD | chronic kidney disease |
| T2DM | type 2 diabetes mellitus |
| DN | diabetic nephropathy |
| GFR | glomerular filtration rate |
| ECM | extracellular matrix |
| UUO | unilateral ureteral obstruction |
| RAS | renin-angiotensin system |
| ACE | angiotensin-converting enzyme |
| STZ | streptozotocin |
| AGE | advanced glycation end products |
| ROS | reactive oxygen species |
| 8-oxo-dG | 8-oxo-7,8-dihydro-2'-deoxyguanosine |
| GSH | glutathione |
| GCL | γ-glutamate cysteine ligase |
| SOD | superoxide dismutase |
| GPx | glutathione peroxidase |
| NQO1 | NAD(P)H quinone oxidoreductase-1 |
| GST | glutathione S-transferase |
| HO-1 | heme oxygenase-1 |
| UGT | UDP-glucuronosyl transferase |
| TNFα | tumor necrosis factor-α |
| IL | interleukin |
| MDA | malondialdehyde |
| SFN | sulforaphane |
| CA | cinnamic aldehyde |
Acknowledgments
Conflicts of Interest
References
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; de Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, F.; Bookhart, B.K.; Vekeman, F.; Corral, M.; Duh, M.S.; Bailey, R.A.; Piech, C.T.; Lefebvre, P. Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: A managed care perspective. J. Manag. Care Pharm. 2009, 15, 312–322. [Google Scholar] [PubMed]
- Roshan, B.; Stanton, R.C. A story of microalbuminuria and diabetic nephropathy. J. Nephropathol. 2013, 2, 234–240. [Google Scholar] [PubMed]
- Saito, H. Toxico-pharmacological perspective of the NRF2-KEAP1 defense system against oxidative stress in kidney diseases. Biochem. Pharmacol. 2013, 85, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.D.; Bakris, G.L. Hypertensive nephropathy: Prevention and treatment recommendations. Expert Opin. Pharmacother. 2010, 11, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am. J. Kidney Dis. 2004, 43, S1–S290. [Google Scholar]
- Vaziri, N.D. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin. Nephrol. 2004, 24, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Hakim, R.M. Oxidative stress in uremia. Curr. Opin. Nephrol. Hypertens. 2003, 12, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, Y.; Zhang, P.; Huang, S.; Zhu, C.; Ding, G.; Liu, B.; Yang, T.; Zhang, A. Mitochondrial dysfunction accounts for aldosterone-induced epithelial-to-mesenchymal transition of renal proximal tubular epithelial cells. Free Radic. Biol. Med. 2012, 53, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Kurahashi, T.; Saito, Y.; Otsuki, N.; Kwon, M.; Ohtake, H.; Yamakawa, M.; Yamada, K.I.; Miyata, S.; Tomita, Y.; et al. Kidney fibrosis is independent of the amount of ascorbic acid in mice with unilateral ureteral obstruction. Free Radic. Res. 2014, 48, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics 2009, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Dicus, M.; Ho, N.D.; Boroujerdi-Rad, L.; Sindhu, R.K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003, 63, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Fortuno, A.; Beloqui, O.; San Jose, G.; Moreno, M.U.; Zalba, G.; Diez, J. Increased phagocytic nicotinamide adenine dinucleotide phosphate oxidase-dependent superoxide production in patients with early chronic kidney disease. Kidney Int. Suppl. 2005, 99, S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Perianayagam, M.C.; Liangos, O.; Kolyada, A.Y.; Wald, R.; MacKinnon, R.W.; Li, L.; Rao, M.; Balakrishnan, V.S.; Bonventre, J.V.; Pereira, B.J.; et al. NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J. Am. Soc. Nephrol. 2007, 18, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Tapia, E.; Soto, A.; Avila-Casado, C.; Franco, M.; Wessale, J.L.; Zhao, L.; Johnson, R.J. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron. Physiol. 2008, 108, 69–78. [Google Scholar]
- Kosugi, T.; Nakayama, T.; Heinig, M.; Zhang, L.; Yuzawa, Y.; Sanchez-Lozada, L.G.; Roncal, C.; Johnson, R.J.; Nakagawa, T. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am. J. Physiol. Renal. Physiol. 2009, 297, F481–F488. [Google Scholar] [CrossRef] [PubMed]
- Omori, H.; Kawada, N.; Inoue, K.; Ueda, Y.; Yamamoto, R.; Matsui, I.; Kaimori, J.; Takabatake, Y.; Moriyama, T.; Isaka, Y.; et al. Use of xanthine oxidase inhibitor febuxostat inhibits renal interstitial inflammation and fibrosis in unilateral ureteral obstructive nephropathy. Clin. Exp. Nephrol. 2012, 16, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Shevalye, H.; Lupachyk, S.; Watcho, P.; Stavniichuk, R.; Khazim, K.; Abboud, H.E.; Obrosova, I.G. Prediabetic nephropathy as an early consequence of the high-calorie/high-fat diet: Relation to oxidative stress. Endocrinology 2012, 153, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sato, T.; Rodriguez-Iturbe, B.; Vaziri, N.D. Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. J. Pharmacol. Exp. Ther. 2011, 337, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Vio, C.P.; Jeanneret, V.A. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int. Suppl. 2003, 86, S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Araki, E. Investigation of a novel mechanism of diabetic complications: Impacts of mitochondrial reactive oxygen species. Rinsho. Byori. 2008, 56, 712–719. [Google Scholar] [PubMed]
- Hinerfeld, D.; Traini, M.D.; Weinberger, R.P.; Cochran, B.; Doctrow, S.R.; Harry, J.; Melov, S. Endogenous mitochondrial oxidative stress: Neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J. Neurochem. 2004, 88, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Brezniceanu, M.L.; Liu, F.; Wei, C.C.; Tran, S.; Sachetelli, S.; Zhang, S.L.; Guo, D.F.; Filep, J.G.; Ingelfinger, J.R.; Chan, J.S. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007, 71, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, G.; Nakano, K.; Sawada, M.; Uno, K.; Shibayama, Y.; Ienaga, K.; Kondo, M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991, 40, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Milena, F.J.; Mora, C.; Leon, C.; Garcia, J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am. J. Nephrol. 2006, 26, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Fukui, M.; Ebihara, I.; Osada, S.; Nagaoka, I.; Tomino, Y.; Koide, H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes 1993, 42, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Shikata, K.; Wada, J.; Horiuchi, S.; Makino, H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: Aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia 1999, 42, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Radeke, H.H.; Meier, B.; Topley, N.; Floge, J.; Habermehl, G.G.; Resch, K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990, 37, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Takamura, T.; Kaneko, S. Induction of reactive oxygen species from isolated rat glomeruli by protein kinase c activation and TNF-alpha stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 2007, 80, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, T.M.; Janssen, U.; Grone, H.J.; Ostendorf, T.; Kunter, U.; Schmidt, H.; Brabant, G.; Floege, J. Early events leading to renal injury in obese zucker (fatty) rats with type ii diabetes. Kidney Int. 2000, 57, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Guerra, A.F.; Vargas-Robles, H.; Lozano Nuevo, J.J.; Escalante-Acosta, B.A. Correlation between circulating adhesion molecule levels and albuminuria in type-2 diabetic hypertensive patients. Kidney Blood Press Res. 2009, 32, 106–109. [Google Scholar]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Greiber, S.; Muller, B.; Daemisch, P.; Pavenstadt, H. Reactive oxygen species alter gene expression in podocytes: Induction of granulocyte macrophage-colony-stimulating factor. J. Am. Soc. Nephrol. 2002, 13, 86–95. [Google Scholar] [PubMed]
- Guijarro, C.; Egido, J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001, 59, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, C.K.; Antunes, G.R.; Mattar, A.L.; Malheiros, D.M.; Vieira, J.M., Jr.; Zatz, R. Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am. J. Physiol. Renal. Physiol. 2007, 292, F92–F99. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Mizuiri, S.; Arita, M.; Hemmi, H. Nuclear factor-kappab activation in diabetic rat kidney: Evidence for involvement of p-selectin in diabetic nephropathy. Tohoku J. Exp. Med. 2005, 206, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.T.; Kuo, M.C.; Chiu, Y.W.; Chang, J.M.; Guh, J.Y.; Chen, H.C. Increased glomerular and extracellular malondialdehyde levels in patients and rats with focal segmental glomerulosclerosis. Eur. J. Clin. Investig. 2005, 35, 245–250. [Google Scholar] [CrossRef]
- Grone, H.J.; Grone, E.F.; Malle, E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab. Investig. 2002, 82, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakamura, T.; Sato, E.; Kawagoe, Y.; Hikichi, Y.; Ueda, Y.; Node, K. Renovascular protective effects of erythropoietin in patients with chronic kidney disease. Intern. Med. 2011, 50, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004, 66, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thevenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jungers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar]
- Ongajooth, L.; Ongajyooth, S.; Likidlilid, A.; Chantachum, Y.; Shayakul, C.; Nilwarangkur, S. Role of lipid peroxidation, trace elements and anti-oxidant enzymes in chronic renal disease patients. J. Med. Assoc. Thai. 1996, 79, 791–800. [Google Scholar] [PubMed]
- Shurtz-Swirski, R.; Mashiach, E.; Kristal, B.; Shkolnik, T.; Shasha, S.M. Antioxidant enzymes activity in polymorphonuclear leukocytes in chronic renal failure. Nephron 1995, 71, 176–179. [Google Scholar]
- Lee, S.J.; Choi, M.G.; Kim, D.S.; Kim, T.W. Manganese superoxide dismutase gene polymorphism (V16A) is associated with stages of albuminuria in Korean type 2 diabetic patients. Metabolism 2006, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, T.; Wang, N.; Wang, F.; Li, M.; Jiang, J.; Zhao, R.; Li, L.; Zhao, W.; Zhu, Q.; et al. The manganese superoxide dismutase Val16Ala polymorphism is associated with decreased risk of diabetic nephropathy in Chinese patients with type 2 diabetes. Mol. Cell Biochem. 2009, 322, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, T.; Tanaka, Y.; Piao, L.; Nagasaka, K.; Sakai, K.; Ogihara, T.; Nakajima, K.; Watada, H.; Kawamori, R. The polymorphism of manganese superoxide dismutase is associated with diabetic nephropathy in Japanese type 2 diabetic patients. J. Hum. Genet. 2003, 48, 138–141. [Google Scholar] [PubMed]
- Yilmaz, M.I.; Saglam, M.; Caglar, K.; Cakir, E.; Sonmez, A.; Ozgurtas, T.; Aydin, A.; Eyileten, T.; Ozcan, O.; Acikel, C.; et al. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. 2006, 47, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.F.; Shintani, A.; Ikizler, T.A.; Himmelfarb, J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J. Am. Soc. Nephrol. 2008, 19, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Morrow, J.D.; Roberts, L.J.; Evanson, J.A.; Becker, B.; Hakim, R.M.; Shyr, Y.; Himmelfarb, J. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin. Nephrol. 2002, 58, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.J.; Walter, M.F.; Adhikarla, R.; Gross, J.; Dallal, G.E.; Levin, N.W.; Blumberg, J.B. Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 2001, 59, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Atamer, A.; Kocyigit, Y.; Ecder, S.A.; Selek, S.; Ilhan, N.; Ecder, T.; Atamer, Y. Effect of oxidative stress on antioxidant enzyme activities, homocysteine and lipoproteins in chronic kidney disease. J. Nephrol. 2008, 21, 924–930. [Google Scholar] [PubMed]
- Apeland, T.; Mansoor, M.A.; Seljeflot, I.; Bronstad, I.; Goransson, L.; Strandjord, R.E. Homocysteine, malondialdehyde and endothelial markers in dialysis patients during low-dose folinic acid therapy. J. Intern. Med. 2002, 252, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Caimi, G.; Carollo, C.; Montana, M.; Iatrino, R.; Bondi, B.; Lo Presti, R. Nitric oxide metabolites, leukocyte activation markers and oxidative status in dialyzed subjects. Blood Purif. 2009, 27, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Brzeszczynska, J.; Kruszynska, I.; Gwozdzinski, K. Investigation of albumin properties in patients with chronic renal failure. Free Radic. Res. 2009, 43, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Saito, A.; Kurokawa, K.; van Ypersele de Strihou, C. Advanced glycation and lipoxidation end products: Reactive carbonyl compounds-related uraemic toxicity. Nephrol. Dial. Transplant 2001, 16 (Suppl. 4), 8–11. [Google Scholar]
- Sakata, N.; Imanaga, Y.; Meng, J.; Tachikawa, Y.; Takebayashi, S.; Nagai, R.; Horiuchi, S. Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis 1999, 142, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar]
- Valli, A.; Suliman, M.E.; Meert, N.; Vanholder, R.; Lindholm, B.; Stenvinkel, P.; Watanabe, M.; Barany, P.; Alvestrand, A.; Anderstam, B. Overestimation of advanced oxidation protein products in uremic plasma due to presence of triglycerides and other endogenous factors. Clin. Chim. Acta 2007, 379, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Donck, J.; Landeloos, M.; Brouwers, E.; Luijtens, K.; Arnout, J.; Lesaffre, E.; Vanrenterghem, Y.; Collen, D. Correlation between oxidized low density lipoproteins and von willebrand factor in chronic renal failure. Thromb. Haemost. 1996, 76, 663–669. [Google Scholar] [PubMed]
- Tarng, D.C.; Wen Chen, T.; Huang, T.P.; Chen, C.L.; Liu, T.Y.; Wei, Y.H. Increased oxidative damage to peripheral blood leukocyte DNA in chronic peritoneal dialysis patients. J. Am. Soc. Nephrol. 2002, 13, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Ersson, C.; Thorman, R.; Rodhe, Y.; Moller, L.; Hylander, B. DNA damage in salivary gland tissue in patients with chronic kidney disease, measured by the comet assay. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Domenici, F.A.; Vannucchi, M.T.; Jordao, A.A., Jr.; Meirelles, M.S.; Vannucchi, H. DNA oxidative damage in patients with dialysis treatment. Ren. Fail. 2005, 27, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Jhangri, G.S.; Curhan, G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005, 68, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Fried, L.F.; Crump, C.; Bleyer, A.J.; Manolio, T.A.; Tracy, R.P.; Furberg, C.D.; Psaty, B.M. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003, 107, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.J.; Shapiro, L.; King, A.J.; Falagas, M.E.; Strom, J.A.; Dinarello, C.A. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994, 45, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Rimm, E.B.; Pai, J.K.; Rexrode, K.M.; Cannuscio, C.C.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Kidney dysfunction, inflammation, and coronary events: A prospective study. J. Am. Soc. Nephrol. 2004, 15, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, C.L.; Stenvinkel, P.; Dekker, F.W.; Carrero, J.J. Monitoring of inflammation in patients on dialysis: Forewarned is forearmed. Nat. Rev. Nephrol. 2011, 7, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Herbelin, A.; Nguyen, A.T.; Zingraff, J.; Urena, P.; Descamps-Latscha, B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 1990, 37, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor NRF2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. KEAP1 represses nuclear activation of antioxidant responsive elements by NRF2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. KEAP1 recruits neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Padmanabhan, B.; Kobayashi, A.; Shang, C.; Hirotsu, Y.; Yokoyama, S.; Yamamoto, M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 2007, 27, 7511–7521. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An NRF2/small Maf heterodimer mediates the induction of phase ii detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kleeberger, S.R. NRF2 protects against airway disorders. Toxicol. Appl. Pharmacol. 2010, 244, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The NRF2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Kwak, M.K. The NRF2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Cho, C.Y.; Thimmulappa, R.K.; Zhen, L.; Srisuma, S.S.; Kensler, T.W.; Yamamoto, M.; Petrache, I.; Tuder, R.M.; Biswal, S. Genetic ablation of NRF2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004, 114, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Sato, H.; Nishimura, N.; Takahashi, S.; Itoh, K.; Yamamoto, M. Accelerated DNA adduct formation in the lung of the nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001, 173, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The NRF2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Itoh, K.; Enomoto, A.; Hirayama, A.; Yamaguchi, N.; Kobayashi, M.; Morito, N.; Koyama, A.; Yamamoto, M.; Takahashi, S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001, 60, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription factor NRF2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Park, H.M.; Jung, K.A.; Choi, H.G.; Kim, J.A.; Kim, D.D.; Kim, S.G.; Kang, K.W.; Ku, S.K.; Kensler, T.W.; et al. The NRF2-heme oxygenase-1 system modulates cyclosporin a-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic. Biol. Med. 2010, 48, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The protective role of NRF2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, A.N. Dietary cancer-chemopreventive compounds: From signaling and gene expression to pharmacological effects. Trends Pharmacol. Sci. 2005, 26, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.R.; Gao, Z.H.; Qu, X.J. NRF2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010, 34, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, A.N. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic. Biol. Med. 2004, 36, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in KEAP1 are required for KEAP1-dependent ubiquitination of NRF2 and for stabilization of NRF2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Paton, V. Potent induction of carcinogen defence enzymes with sulforaphane, a putative prostate cancer chemopreventive agent. Prostate Cancer Prostatic Dis. 1999, 2, S8. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Hebbar, V.; Kim, B.R.; Chen, C.; Winnik, B.; Buckley, B.; Soteropoulos, P.; Tolias, P.; Hart, R.P.; Kong, A.N. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J. Pharmacol. Exp. Ther. 2004, 310, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Williamson, G.; Garner, R.C.; Lappin, G.; Langouet, S.; Bao, Y. Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis 2003, 24, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Basten, G.P.; Bao, Y.; Williamson, G. Sulforaphane and its glutathione conjugate but not sulforaphane nitrile induce UDP-glucuronosyl transferase (UGT1A1) and glutathione transferase (GSTA1) in cultured cells. Carcinogenesis 2002, 23, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of NRF2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar] [PubMed]
- Rose, P.; Faulkner, K.; Williamson, G.; Mithen, R. 7-methylsulfinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis 2000, 21, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of KEAP1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar]
- Dinkova-Kostova, A.T.; Talalay, P. Relation of structure of curcumin analogs to their potencies as inducers of phase 2 detoxification enzymes. Carcinogenesis 1999, 20, 911–914. [Google Scholar]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of NRF2 and KEAP1 in KEAP1-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Pedruzzi, L.M.; Stockler-Pinto, M.B.; Leite, M., Jr.; Mafra, D. NRF2-KEAP1 system versus NF-kappaB: The good and the evil in chronic kidney disease? Biochimie 2012, 94, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. NRF2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006, 116, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Magilnick, N.; Lee, C.; Kalmaz, D.; Ou, X.; Chan, J.Y.; Lu, S.C. NRF1 and NRF2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell. Biol. 2005, 25, 5933–5946. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.; Qiao, L.; Wang, X. Disruption of nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-alpha, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010, 2010, 238321. [Google Scholar]
- Jun, C.D.; Kim, Y.; Choi, E.Y.; Kim, M.; Park, B.; Youn, B.; Yu, K.; Choi, K.S.; Yoon, K.H.; Choi, S.C.; et al. Gliotoxin reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice: Evidence of the connection between heme oxygenase-1 and the nuclear factor-kappaB pathway in vitro and in vivo. Inflamm. Bowel. Dis. 2006, 12, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; MacEwan, D.J.; O'Connell, M.A. Lipopolysaccharide-induced expression of nad(p)h: Quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 2008, 181, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhauser, C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Raina, D.; Meyer, C.; Kharbanda, S.; Kufe, D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J. Biol. Chem. 2006, 281, 35764–35769. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Jacintho, J.D. Systemic lupus erythematosus and other autoimmune diseases from endogenous and exogenous agents: Unifying theme of oxidative stress. Mini Rev. Med. Chem. 2003, 3, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. NRF2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-kappaB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the NRF2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Lee, G.; Chung, H.S.; Bae, H. Curcumin attenuates lupus nephritis upon interaction with regulatory t cells in new zealand black/white mice. Br. J. Nutr. 2013, 110, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ye, Y.; Min, S.Y.; Zhu, J.; Khobahy, E.; Zhou, J.; Yan, M.; Hemachandran, S.; Pathak, S.; Zhou, X.J.; et al. Targeting multiple signaling axes and oxidative stress using a synthetic triterpenoid prevents murine lupus nephritis. Arthritis Rheumatol. 2014. [Google Scholar] [CrossRef]
- Yoh, K.; Hirayama, A.; Ishizaki, K.; Yamada, A.; Takeuchi, M.; Yamagishi, S.; Morito, N.; Nakano, T.; Ojima, M.; Shimohata, H.; et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in nrf2-deficient mice. Genes Cells 2008, 13, 1159–1170. [Google Scholar] [PubMed]
- Ryoo, I.G.; Ha, H.; Kwak, M.K. Inhibitory role of the KEAP1-NRF2 pathway in TGFbeta1-stimulated renal epithelial transition to fibroblastic cells: A modulatory effect on smad signaling. PLoS One 2014, 9, e93265. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Shin, D.H.; Kang, K.S.; Kwak, M.K. Involvement of NRF2-GSH signaling in TGFbeta1-stimulated epithelial-to-mesenchymal transition changes in rat renal tubular cells. Arch. Pharm. Res. 2014. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired NRF2-KEAP1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Renal. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Nicholas, S.B.; Norris, K.C.; Vaziri, N.D. Role of impaired NRF2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol. Dial. Transplant. 2013, 28, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.; Sharma, T.K.; Singh, I.; Singh, N.; Ghalaut, V.S.; Vardey, S.K.; Shankar, V. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N. Am. J. Med. Sci. 2013, 5, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Fassett, R.G.; Coombes, J.S.; Kunde, D.A.; Ahuja, K.D.; Robertson, I.K.; Ball, M.J.; Geraghty, D.P. Relationship between antioxidant enzyme genotype and activity and kidney function: A case-control study. Clin. Nephrol. 2012, 78, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Puchades, M.J.; Saez, G.; Munoz, M.C.; Gonzalez, M.; Torregrosa, I.; Juan, I.; Miguel, A. Study of oxidative stress in patients with advanced renal disease and undergoing either hemodialysis or peritoneal dialysis. Clin. Nephrol. 2013, 80, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, F.; Zhang, L.; Cao, Y.; Liu, W.; Hao, J.; Liu, Q.; Duan, H. Modulation of NRF2 expression alters high glucose-induced oxidative stress and antioxidant gene expression in mouse mesangial cells. Cell Signal. 2011, 23, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Kraft, D.C.; Deocaris, C.C.; Wadhwa, R.; Rattan, S.I. Preincubation with the proteasome inhibitor mg-132 enhances proteasome activity via the NRF2 transcription factor in aging human skin fibroblasts. Ann. New York Acad. Sci. 2006, 1067, 420–424. [Google Scholar]
- Luo, Z.F.; Qi, W.; Feng, B.; Mu, J.; Zeng, W.; Guo, Y.H.; Pang, Q.; Ye, Z.L.; Liu, L.; Yuan, F.H. Prevention of diabetic nephropathy in rats through enhanced renal antioxidative capacity by inhibition of the proteasome. Life Sci. 2011, 88, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, B.; Bai, Y.; Miao, X.; Chen, Q.; Sun, W.; Tan, Y.; Luo, P.; Zhang, C.; Zheng, S.; et al. Potential role for NRF2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E87–E99. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kim, Y.J.; Kang, D.H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of NRF2 through activation of NF-kappaB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Jeong, W.S.; Kong, A.N. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat. Res. 2004, 555, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of phase ii enzymes by sulforaphane: Implications for its cardioprotective potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Gangopadhyay, H.; Das, D.K. Broccoli: A unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J. Agric. Food Chem. 2008, 56, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Innamorato, N.G.; Rojo, A.I.; Garcia-Yague, A.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor NRF2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the NRF2-KEAP1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltran, C.E.; Calderon-Oliver, M.; Martinez-Abundis, E.; Tapia, E.; Zarco-Marquez, G.; Zazueta, C.; Pedraza-Chaverri, J. Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD(P)H: Quinone oxidoreductase 1 and gamma glutamyl cysteine ligase: Studies in mitochondria isolated from rat kidney and in LLC-PK1 cells. Toxicol. Lett. 2010, 199, 80–92. [Google Scholar]
- Guerrero-Beltran, C.E.; Calderon-Oliver, M.; Tapia, E.; Medina-Campos, O.N.; Sanchez-Gonzalez, D.J.; Martinez-Martinez, C.M.; Ortiz-Vega, K.M.; Franco, M.; Pedraza-Chaverri, J. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2010, 192, 278–285. [Google Scholar]
- Yoon, H.Y.; Kang, N.I.; Lee, H.K.; Jang, K.Y.; Park, J.W.; Park, B.H. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the NRF2-dependent phase 2 enzyme. Biochem. Pharmacol. 2008, 75, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic potential of NRF2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Bai, Y.; Miao, X.; Luo, P.; Chen, Q.; Tan, Y.; Rane, M.J.; Miao, L.; Cai, L. Prevention of diabetic nephropathy by sulforaphane: Possible role of NRF2 upregulation and activation. Oxid. Med. Cell Longev. 2012, 2012, 821936. [Google Scholar]
- Chung, S.D.; Lai, T.Y.; Chien, C.T.; Yu, H.J. Activating NRF-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One 2012, 7, e47299. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Karbalay-Doust, S.; Poorshahid, M. Stereological survey of the ameliorative effects of sulforaphane and quercetin on renal tissue in unilateral ureteral obstruction in rats. Acta Clin. Croat. 2012, 51, 555–562. [Google Scholar] [PubMed]
- Bertelli, A.A.; Das, D.K. Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Zhang, X.; Zhang, W.; Zhen, H.N. New enlightenment of french paradox: Resveratrol’s potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol. Ther. 2007, 6, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lee, J.Y.; Hwang, D.H. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr. Rev. 2011, 69, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Ardito, R.; Faraglia, B.; Boninsegna, A.; Wolf, F.I.; Cittadini, A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat. Res. 2001, 496, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Mokni, M.; Elkahoui, S.; Limam, F.; Amri, M.; Aouani, E. Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem. Res. 2007, 32, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lim, J.H.; Youn, H.H.; Hong, Y.A.; Yang, K.S.; Park, H.S.; Chung, S.; Ko, S.H.; Shin, S.J.; Choi, B.S.; et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia 2013, 56, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pang, S.; Deng, B.; Qian, L.; Chen, J.; Zou, J.; Zheng, J.; Yang, L.; Zhang, C.; Chen, X.; et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-kappaB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int. J. Biochem. Cell Biol. 2012, 44, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anjaneyulu, M.; Kulkarni, S.K.; Chopra, K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 2006, 76, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Ricardo, S.D.; Bertram, J.F.; Nikolic-Paterson, D.J. Resveratrol inhibits renal fibrosis in the obstructed kidney: Potential role in deacetylation of Smad3. Am. J. Pathol. 2010, 177, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tian, S.; Han, J.; Xiong, P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren. Fail. 2014, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NRF2-KEAP1 signaling. Biochim Biophys. Acta 2011, 1812, 719–731. [Google Scholar]
- Huang, K.; Huang, J.; Xie, X.; Wang, S.; Chen, C.; Shen, X.; Liu, P.; Huang, H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the NRF2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 2013, 65, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Brasnyo, P.; Molnar, G.A.; Mohas, M.; Marko, L.; Laczy, B.; Cseh, J.; Mikolas, E.; Szijarto, I.A.; Merei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Castilla, P.; Davalos, A.; Teruel, J.L.; Cerrato, F.; Fernandez-Lucas, M.; Merino, J.L.; Sanchez-Martin, C.C.; Ortuno, J.; Lasuncion, M.A. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008, 87, 1053–1061. [Google Scholar] [PubMed]
- Castilla, P.; Echarri, R.; Davalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gomez-Coronado, D.; Ortuno, J.; Lasuncion, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Bates, T.E.; Mancuso, C.; Cornelius, C.; Ventimiglia, B.; Cambria, M.T.; di Renzo, L.; de Lorenzo, A.; Dinkova-Kostova, A.T. Curcumin and the cellular stress response in free radical-related diseases. Mol. Nutr. Food Res. 2008, 52, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Killian, P.; Pfeffer, U.; Nerlich, A.G. Novel aspects for the application of curcumin in chemoprevention of various cancers. Front. Biosci. (Schol Ed.) 2010, 2, 697–717. [Google Scholar]
- Osawa, T. Nephroprotective and hepatoprotective effects of curcuminoids. Adv. Exp. Med. Biol. 2007, 595, 407–423. [Google Scholar] [PubMed]
- Gonzalez-Salazar, A.; Molina-Jijon, E.; Correa, F.; Zarco-Marquez, G.; Calderon-Oliver, M.; Tapia, E.; Zazueta, C.; Pedraza-Chaverri, J. Curcumin protects from cardiac reperfusion damage by attenuation of oxidant stress and mitochondrial dysfunction. Cardiovasc. Toxicol. 2011, 11, 357–364. [Google Scholar]
- Reyes-Fermin, L.M.; Gonzalez-Reyes, S.; Tarco-Alvarez, N.G.; Hernandez-Nava, M.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Neuroprotective effect of alpha-mangostin and curcumin against iodoacetate-induced cell death. Nutr. Neurosci. 2012, 15, 34–41. [Google Scholar]
- Soetikno, V.; Suzuki, K.; Veeraveedu, P.T.; Arumugam, S.; Lakshmanan, A.P.; Sone, H.; Watanabe, K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov. Today 2013, 18, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF-kappaB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. (Lond) 2011, 8, 35. [Google Scholar] [CrossRef]
- Soetikno, V.; Watanabe, K.; Sari, F.R.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Arozal, W.; Sukumaran, V.; Lakshmanan, A.P.; Arumugam, S.; et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-alpha and PKC-beta1 activity in streptozotocin-induced type I diabetic rats. Mol. Nutr. Food Res. 2011, 55, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Khan, Z.A.; Farhangkhoee, H.; Chakrabarti, S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 2009, 25, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, K.; Lan, T.; Xie, X.; Shen, X.; Liu, P.; Huang, H. Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol. Cell Endocrinol. 2013, 365, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am. J. Physiol. Renal. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, G.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Li, Y.; Yan, Y.; Wang, Z.; Li, X.; et al. Attenuation of high-glucose-induced inflammatory response by a novel curcumin derivative B06 contributes to its protection from diabetic pathogenic changes in rat kidney and heart. J. Nutr. Biochem. 2013, 24, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Huang, Y.; Wang, Z.; Fang, Q.; Sun, Y.; Tong, C.; Peng, K.; Wang, Y.; Miao, L.; Cai, L.; et al. Inhibition of MAPK-mediated ACE expression by compound C66 prevents STZ-induced diabetic nephropathy. J. Cell Mol. Med. 2014, 18, 231–241. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates NRF2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the NRF2-KEAP1 pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Marquez, G.; Molina-Jijon, E.; Cristobal-Garcia, M.; Santamaria, J.; Garcia-Nino, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin induces NRF2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid. Med. Cell Longev. 2012, 2012, 269039. [Google Scholar]
- Tapia, E.; Zatarain-Barron, Z.L.; Hernandez-Pando, R.; Zarco-Marquez, G.; Molina-Jijon, E.; Cristobal-Garcia, M.; Santamaria, J.; Pedraza-Chaverri, J. Curcumin reverses glomerular hemodynamic alterations and oxidant stress in 5/6 nephrectomized rats. Phytomedicine 2013, 20, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.; Raju, Y.S.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D 2008, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial activity of leaf essential oils and their constituents from cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Chung, Y.L.; Wu, M.L.; Chuang, S.M. Cinnamaldehyde enhances NRF2 nuclear translocation to upregulate phase II detoxifying enzyme expression in HepG2 cells. J. Agric. Food Chem. 2011, 59, 5164–5171. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-inflammatory activities of cinnamomum cassia constituents in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2012, 2012, 429320. [Google Scholar]
- Wondrak, G.T.; Cabello, C.M.; Villeneuve, N.F.; Zhang, S.; Ley, S.; Li, Y.; Sun, Z.; Zhang, D.D. Cinnamoyl-based NRF2-activators targeting human skin cell photo-oxidative stress. Free Radic. Biol. Med. 2008, 45, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Chang, W.T.; Shih, Y.W.; Huang, J.S. Cinnamaldehyde impairs high glucose-induced hypertrophy in renal interstitial fibroblasts. Toxicol. Appl. Pharmacol. 2010, 244, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bhatti, R.; Singh, A.; Singh Ishar, M.P. Ameliorative effect of the cinnamon oil from cinnamomum zeylanicum upon early stage diabetic nephropathy. Planta Med. 2010, 76, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor NRF2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, A.; Yon, J.; Day, P.J.; Richardson, C.; Tickle, I.J.; Williams, P.A.; Callahan, J.F.; Carr, R.; Concha, N.; Kerns, J.K.; et al. Structure of the BTB domain of KEAP1 and its interaction with the triterpenoid antagonist CDDO. PLoS One 2014, 9, e98896. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Wang, Y.; Senitko, M.; Meyer, C.; Wigley, W.C.; Ferguson, D.A.; Grossman, E.; Chen, J.; Zhou, X.J.; Hartono, J.; et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes NRF2, PPARgamma, and HO-1. Am. J. Physiol. Renal. Physiol. 2011, 300, F1180–F1192. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aleksunes, L.M.; Goedken, M.J.; Chen, C.; Reisman, S.A.; Manautou, J.E.; Klaassen, C.D. Coordinated induction of NRF2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity. Toxicol. Appl. Pharmacol. 2008, 231, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Sharma, A.; Stefanovic, N.; Yuen, D.Y.; Karagiannis, T.C.; Meyer, C.; Ward, K.W.; Cooper, M.E.; de Haan, J.B. A derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner, lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes 2014. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Reisman, S.A.; Vaziri, N.D.; Khazaeli, M.; Yuan, J.; Meyer, C.J. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores NRF2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica 2014, 44, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Krauth, M.; Huff, J.W.; Ferguson, D.A.; Ruiz, S.; Meyer, C.J.; Warnock, D.G. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b-4 CKD. Am. J. Nephrol. 2011, 33, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef] [PubMed]
- De Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choi, B.-h.; Kang, K.-S.; Kwak, M.-K. Effect of Redox Modulating NRF2 Activators on Chronic Kidney Disease. Molecules 2014, 19, 12727-12759. https://doi.org/10.3390/molecules190812727
Choi B-h, Kang K-S, Kwak M-K. Effect of Redox Modulating NRF2 Activators on Chronic Kidney Disease. Molecules. 2014; 19(8):12727-12759. https://doi.org/10.3390/molecules190812727
Chicago/Turabian StyleChoi, Bo-hyun, Kyung-Shin Kang, and Mi-Kyoung Kwak. 2014. "Effect of Redox Modulating NRF2 Activators on Chronic Kidney Disease" Molecules 19, no. 8: 12727-12759. https://doi.org/10.3390/molecules190812727
APA StyleChoi, B.-h., Kang, K.-S., & Kwak, M.-K. (2014). Effect of Redox Modulating NRF2 Activators on Chronic Kidney Disease. Molecules, 19(8), 12727-12759. https://doi.org/10.3390/molecules190812727