Effects of Heat Acclimation on Photosynthesis, Antioxidant Enzyme Activities, and Gene Expression in Orchardgrass under Heat Stress
Abstract
:1. Introduction
2. Results
2.1. Physiological Responses to Heat Stress Following Heat Preconditioning and Non-Preconditioning
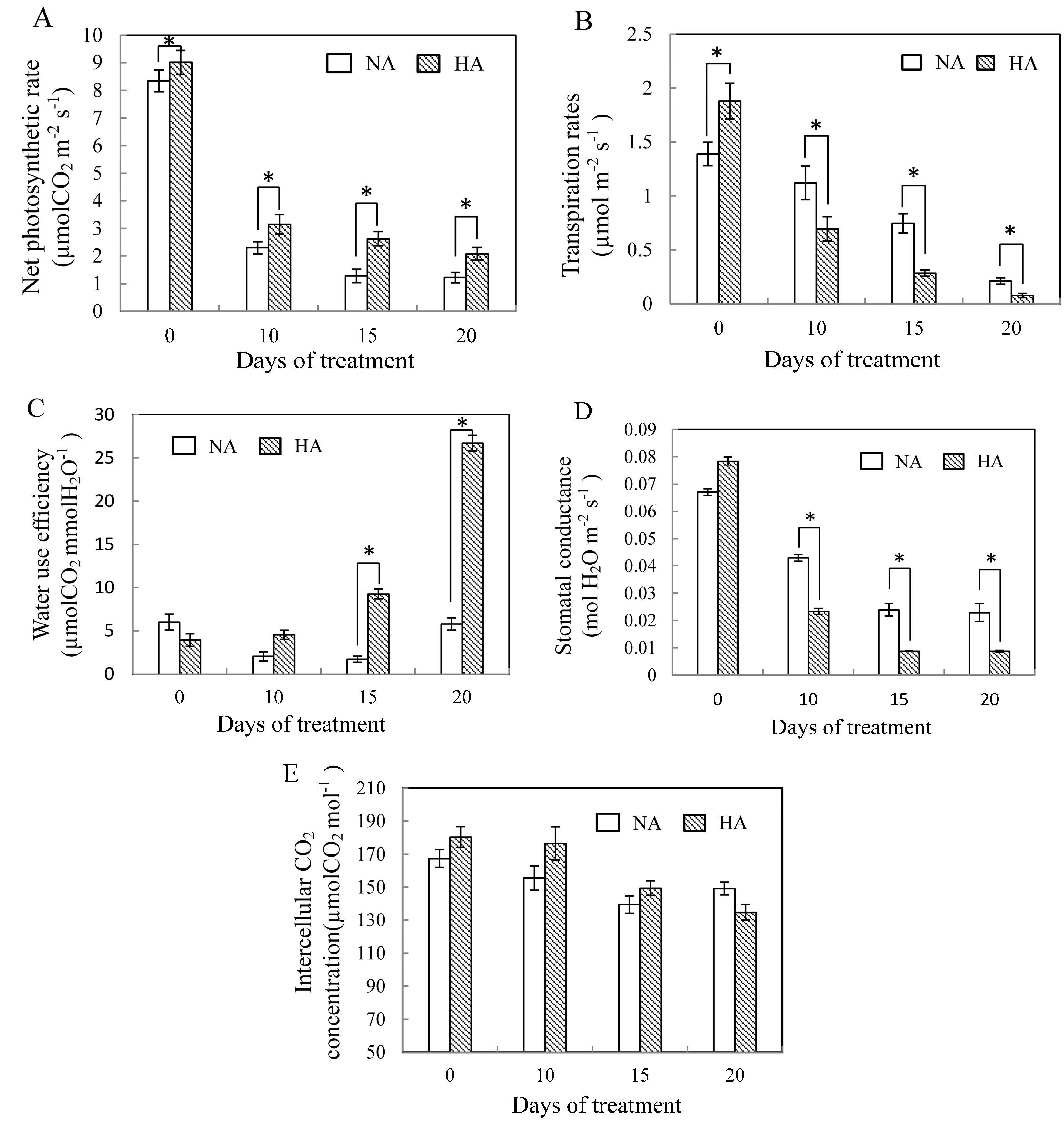
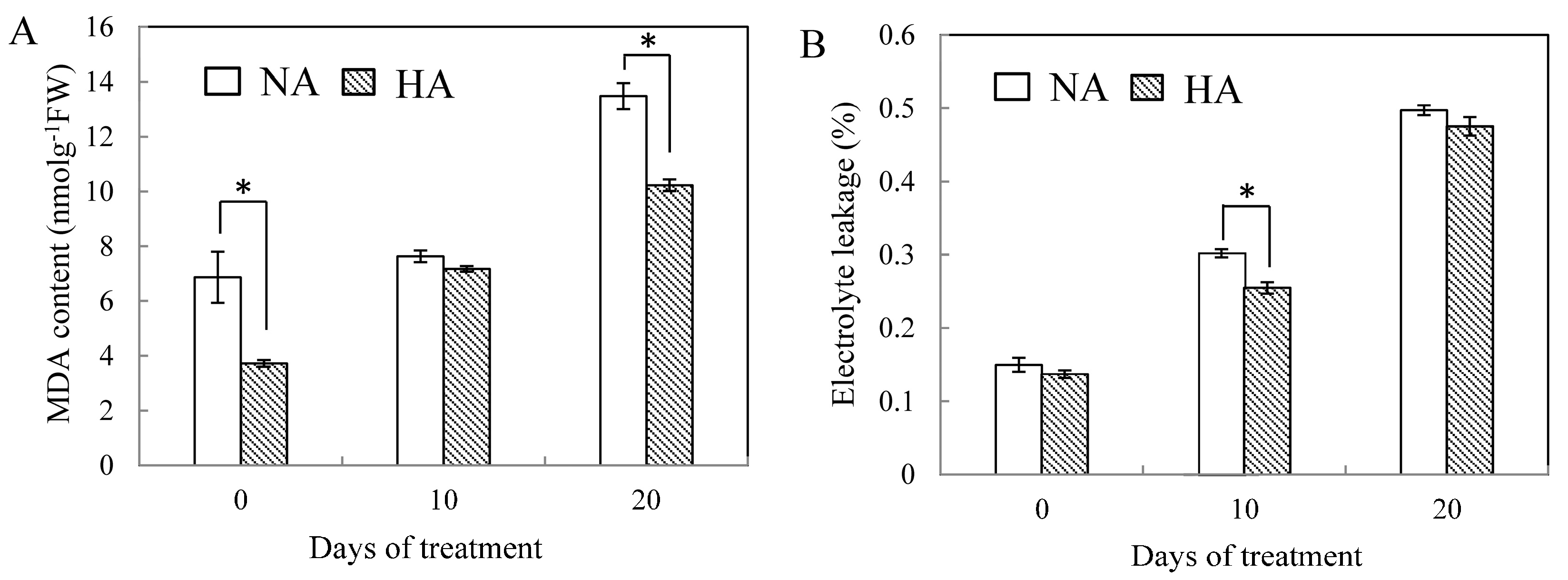
2.2. Effects of Preconditioning and Non-preconditioning on Antioxidant Enzyme Activity Under Heat Stress
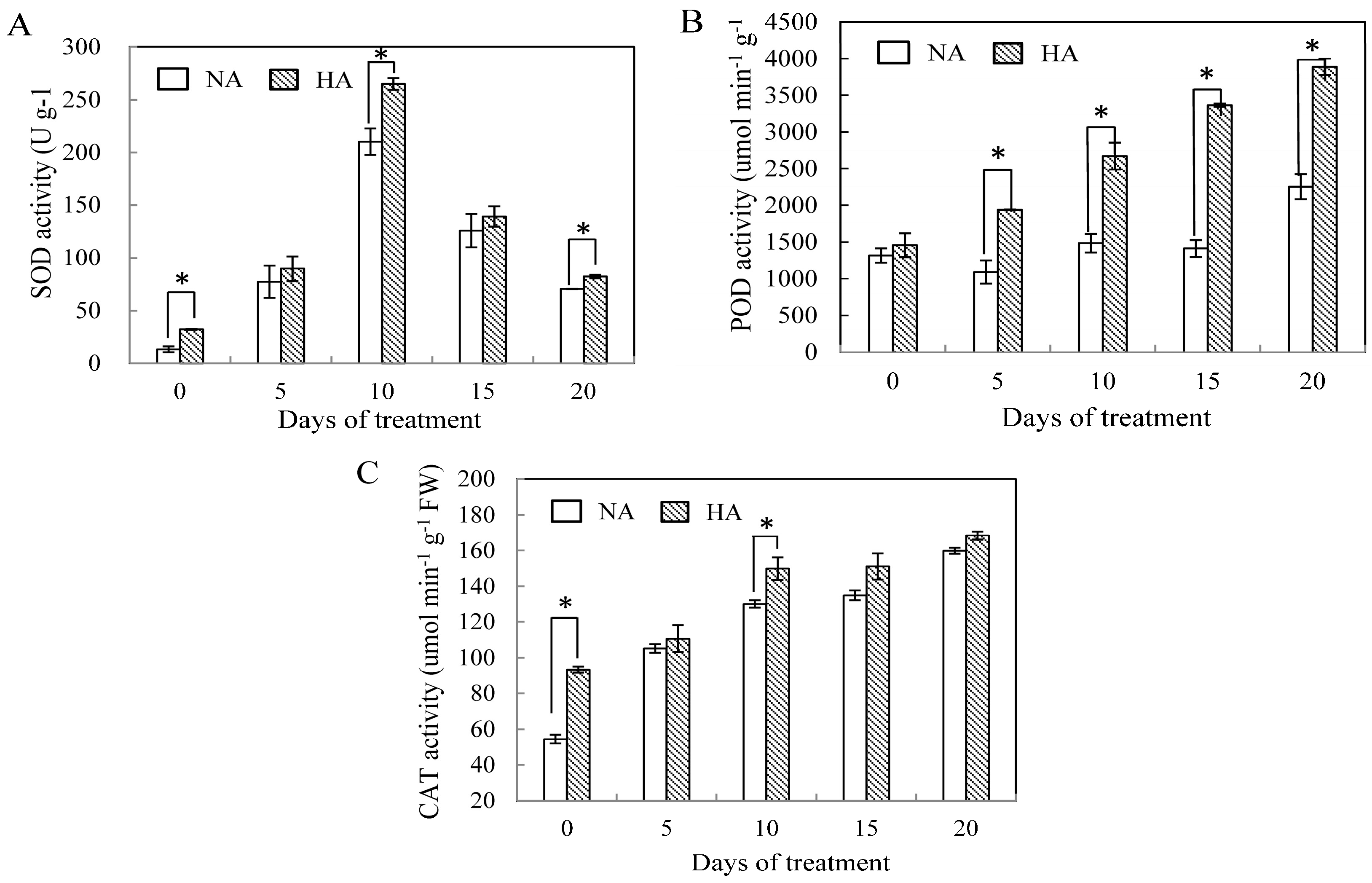
2.3. Assessment of Antioxidant Enzyme Gene Expression under Heat Stress
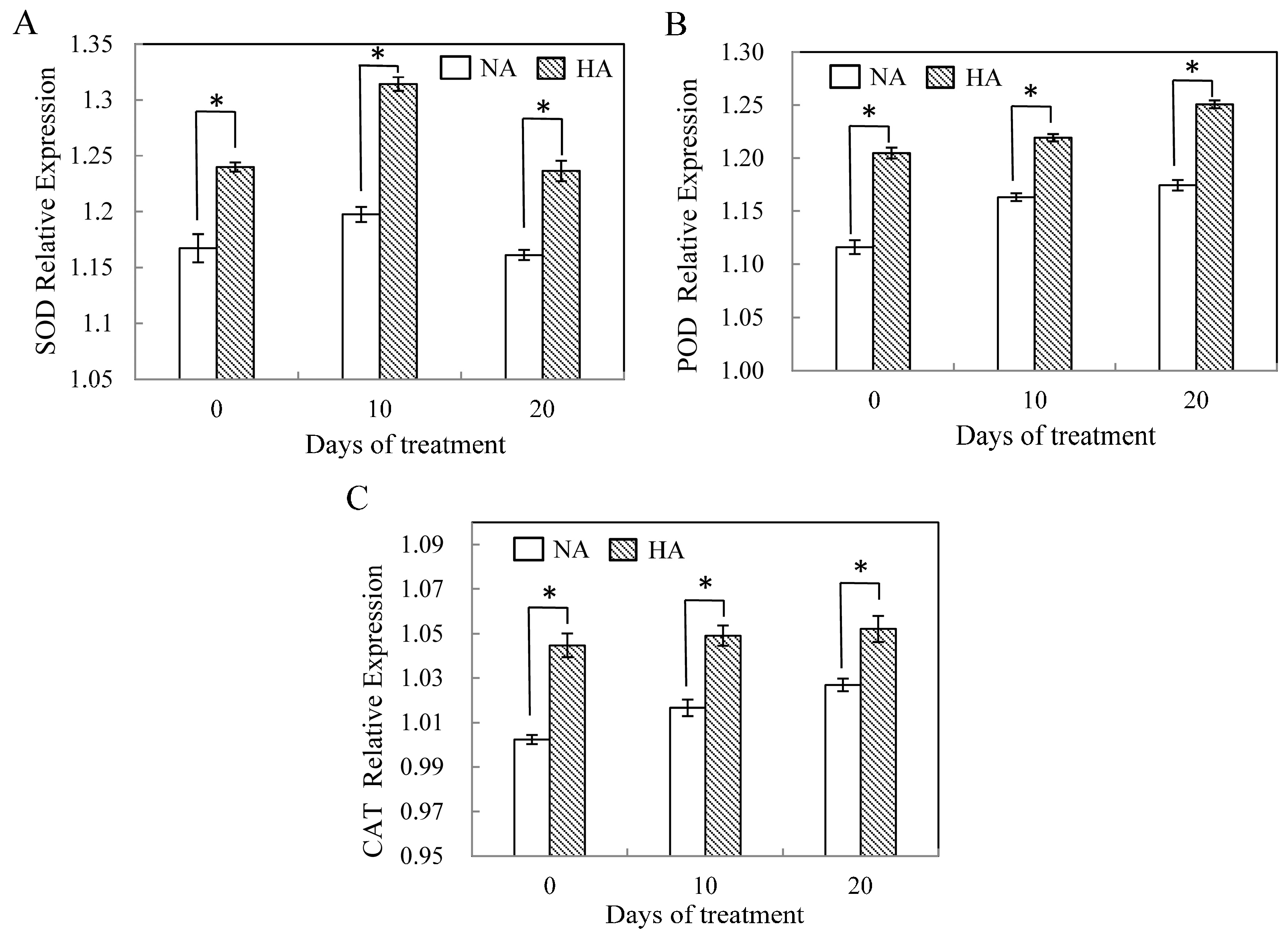
3. Discussion
4. Experimental Section
4.1. Plant Materials and Treatments
4.2. Photosynthetic Characteristics and Cell Membrane Stability
4.3. Antioxidant Enzymes and Lipid Peroxidation
4.4. Gene Expression
| Gene | Species | Accession | Sequence of Primers |
|---|---|---|---|
| Cu/ZnSOD | Orchardgrass | HO131072.1 | F: 5′-GGCATACTGTCACTCTAAG-3′ |
| R: 5′- CAACTCCAGGTCATATCG-3′ | |||
| CAT | Orchardgrass | HO179331.1 | F: 5′-GCGACGGCATGAAGTTCCC-3′ |
| R: 5′-CGGTTGACGAGCGTGTAGGTG-3′ | |||
| POD | Orchardgrass | HO125605.1 | F: 5′-GCCTCCGTGCTGCTCGAC-3′ |
| R: 5′-CCAGGATGTCGGAGCAGGAGA-3′ | |||
| β-actin | Orchardgrass | HO135499.1 | F: 5′-GTGTTGGATTCTGGTGATGGTGT-3′ |
| R: 5′-GGCAGTGGTGGTGAAGGAGTAA-3′ |
4.5. Statistical Analysis
5. Conclusions
Abbreviations
| CAT | Catalase |
| Ci | Intercellular CO2 concentration |
| EL | Electrolyte leakage |
| Gs | Stomatal conductance |
| HA | Heat acclimated |
| MDA | Malondialdehyde |
| NA | Non-acclimated |
| Pn | Net photosynthetic rate |
| POD | Guaiacol peroxidase |
| SOD | Superoxide dismutase |
| Tr | Transpiration rates |
| WUE | Water use efficiency |
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solomon, S. Climate Change 2007-the Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; p. 851. [Google Scholar]
- Lobell, D.B.; Field, C.B. Global scale climate-crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2007, 2, 014002. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–123. [Google Scholar]
- Sinsawat, V.; Leiner, J.; Stamp, P.; Fracheboud, Y. Effect of heat stress on the photosynthetic apparatus in maize (Zea mays L.) grown at control or high temperature. Environ. Exp. Bot. 2004, 52, 123–129. [Google Scholar] [CrossRef]
- Horowitz, M. Heat acclimation: Phenotypic plasticity and cues to the underlying molecular mechanisms. J. Therm. Biol. 2001, 26, 357–363. [Google Scholar]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Gratani, L.; Pesoli, P.; Crescente, M.; Aichner, K.; Larcher, W. Photosynthesis as a temperature indicator in Quercus ilex L. Glob. Planet. Chang. 2000, 24, 153–163. [Google Scholar] [CrossRef]
- Dias, A.; Semedo, J.; Ramalho, J.; Lidon, F. Bread and durum wheat under heat stress: A comparative study on the photosynthetic performance. J. Environ. Qual. 2011, 197, 50–56. [Google Scholar]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar]
- Sairam, R.K.; Vasanthan, B.; Arora, A. Calcium regulates Gladiolus flower senescence by influencing antioxidative enzymes activity. Acta Physiol. Plant 2011, 33, 1897–1904. [Google Scholar]
- Signorelli, S.; Casaretto, E.; Sainz, M.; Díaz, P.; Monza, J.; Borsani, O. Antioxidant and photosystem II responses contribute to explain the drought–heat contrasting tolerance of two forage legumes. Plant Physiol. Bioch. 2013, 70, 195–203. [Google Scholar]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar]
- Stewart, A.V.; Ellison, N.W. Dactylis. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 73–87. [Google Scholar]
- Li, C.-Z.; Wang, G.-X. Interactions between reactive oxygen species, ethylene and polyamines in leaves of Glycyrrhiza inflata seedlings under root osmotic stress. Plant Growth Regul. 2004, 42, 55–60. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar]
- Gulen, H.; Eris, A. Some physiological changes in strawberry (Fragaria × Ananassa ‘Camarosa’) plants under heat stress. J. Hortic. Sci. Biotech. 2003, 78, 894–898. [Google Scholar]
- Xu, L.; Han, L.; Huang, B. Antioxidant enzyme activities and gene expression patte rns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. J. Am. Soc. Hortic. Sci. 2011, 136, 247–255. [Google Scholar]
- Monk, L.S.; Fagerstedt, K.V.; Crawford, R.M. Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol. Plant 1989, 76, 456–459. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar]
- Georgieva, K. Some mechanisms of damage and acclimation of the photosynthetic apparatus due to high temperature. Bulg. J. Plant Physiol. 1999, 25, 89–99. [Google Scholar]
- Raison, J.K.; Roberts, J.K.; Berry, J.A. Correlations between the thermal stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of the higher plant, Nerium oleander, to growth temperature. BBA-Biomembranes 1982, 688, 218–228. [Google Scholar] [CrossRef]
- Zhao, X.; Nishimura, Y.; Fukumoto, Y.; Li, J. Effect of high temperature on active oxygen species, senescence and photosynthetic properties in cucumber leaves. Environ. Exp. Bot. 2011, 70, 212–216. [Google Scholar]
- Schulze, E.-D.; Hall, A. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In Physiological Plant Ecology II; Springer: Berlin/Heidelberg, Germany, 1982; pp. 181–230. [Google Scholar]
- Guo, Y.-P.; Zhou, H.-F.; Zhang, L.-C. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci. Hortic-Amst. 2006, 108, 260–267. [Google Scholar]
- Pastenes, C.; Horton, P. Effect of high temperature on photosynthesis in beans (I. Oxygen evolution and chlorophyll fluorescence). Plant Physiol. 1996, 112, 1245–1251. [Google Scholar]
- Pastenes, C.; Horton, P. Effect of high temperature on photosynthesis in beans (II. CO2 assimilation and metabolite contents). Plant Physiol. 1996, 112, 1253–1260. [Google Scholar]
- Türkan, İ.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005, 56, 417–423. [Google Scholar]
- Medeiros, C.D.; Neto, J.R.F.; Oliveira, M.T.; Rivas, R.; Pandolfi, V.; Kido, E.A.; Baldani, J.I.; Santos, M.G. Photosynthesis, antioxidant activities and transcriptional responses in two sugarcane (Saccharum officinarum L.) cultivars under salt stress. Acta Physiol. Plant 2014, 36, 447–459. [Google Scholar] [CrossRef]
- Payton, P.; Allen, R.D.; Trolinder, N.; Holaday, A.S. Over-expression of chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum L., cv. Coker 312) does not alter the reduction of photosynthesis after short exposures to low temperature and high light intensity. Photosynth. Res. 1997, 52, 233–244. [Google Scholar]
- Chinevere, T.D.; Kenefick, R.W.; Cheuvront, S.N.; Lukaski, H.C.; Sawka, M.N. Effect of heat acclimation on sweat minerals. Med. Sci. Sports Exerc. 2008, 40, 886–891. [Google Scholar]
- Wang, D.; Li, X.F.; Zhou, Z.J.; Feng, X.P.; Yang, W.J.; Jiang, E.A. Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol. Plantarum 2010, 139, 55–67. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Method. Enzymol. 1955, 2, 764–775. [Google Scholar]
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 2001, 41, 436–442. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, X.X.; Huang, L.K.; Zhang, X.Q.; Li, Z.; Peng, Y. Effects of Heat Acclimation on Photosynthesis, Antioxidant Enzyme Activities, and Gene Expression in Orchardgrass under Heat Stress. Molecules 2014, 19, 13564-13576. https://doi.org/10.3390/molecules190913564
Zhao XX, Huang LK, Zhang XQ, Li Z, Peng Y. Effects of Heat Acclimation on Photosynthesis, Antioxidant Enzyme Activities, and Gene Expression in Orchardgrass under Heat Stress. Molecules. 2014; 19(9):13564-13576. https://doi.org/10.3390/molecules190913564
Chicago/Turabian StyleZhao, Xin Xin, Lin Kai Huang, Xin Quan Zhang, Zhou Li, and Yan Peng. 2014. "Effects of Heat Acclimation on Photosynthesis, Antioxidant Enzyme Activities, and Gene Expression in Orchardgrass under Heat Stress" Molecules 19, no. 9: 13564-13576. https://doi.org/10.3390/molecules190913564
APA StyleZhao, X. X., Huang, L. K., Zhang, X. Q., Li, Z., & Peng, Y. (2014). Effects of Heat Acclimation on Photosynthesis, Antioxidant Enzyme Activities, and Gene Expression in Orchardgrass under Heat Stress. Molecules, 19(9), 13564-13576. https://doi.org/10.3390/molecules190913564






