Synthesis and Pharmacological Evaluation of Modified Adenosines Joined to Mono-Functional Platinum Moieties
Abstract
:1. Introduction
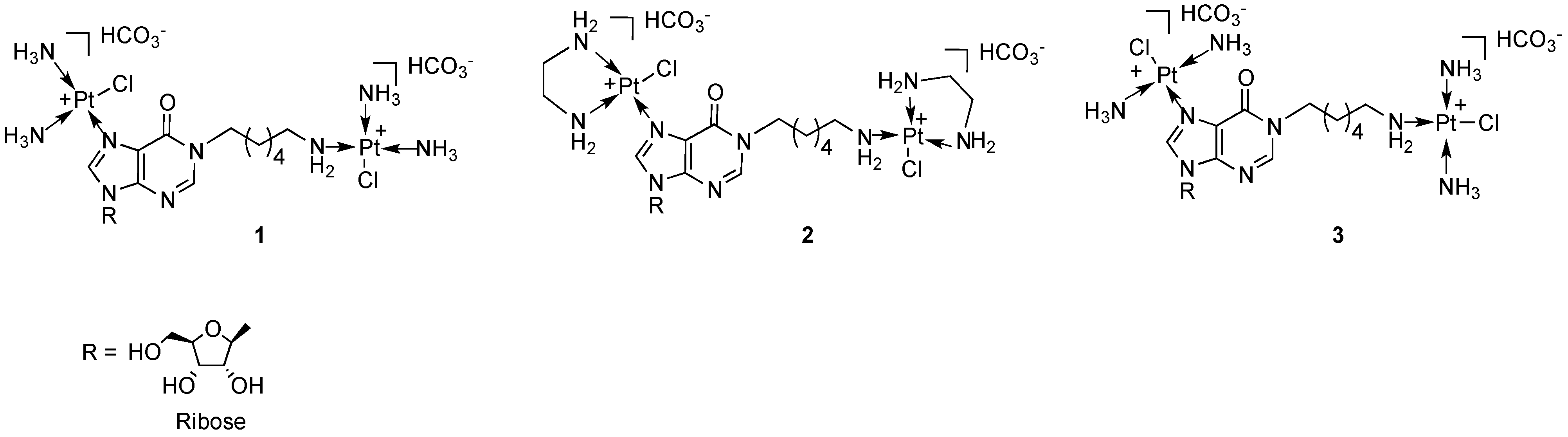
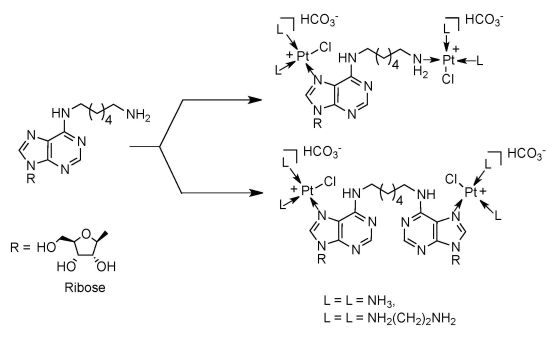
2. Results and Discussion
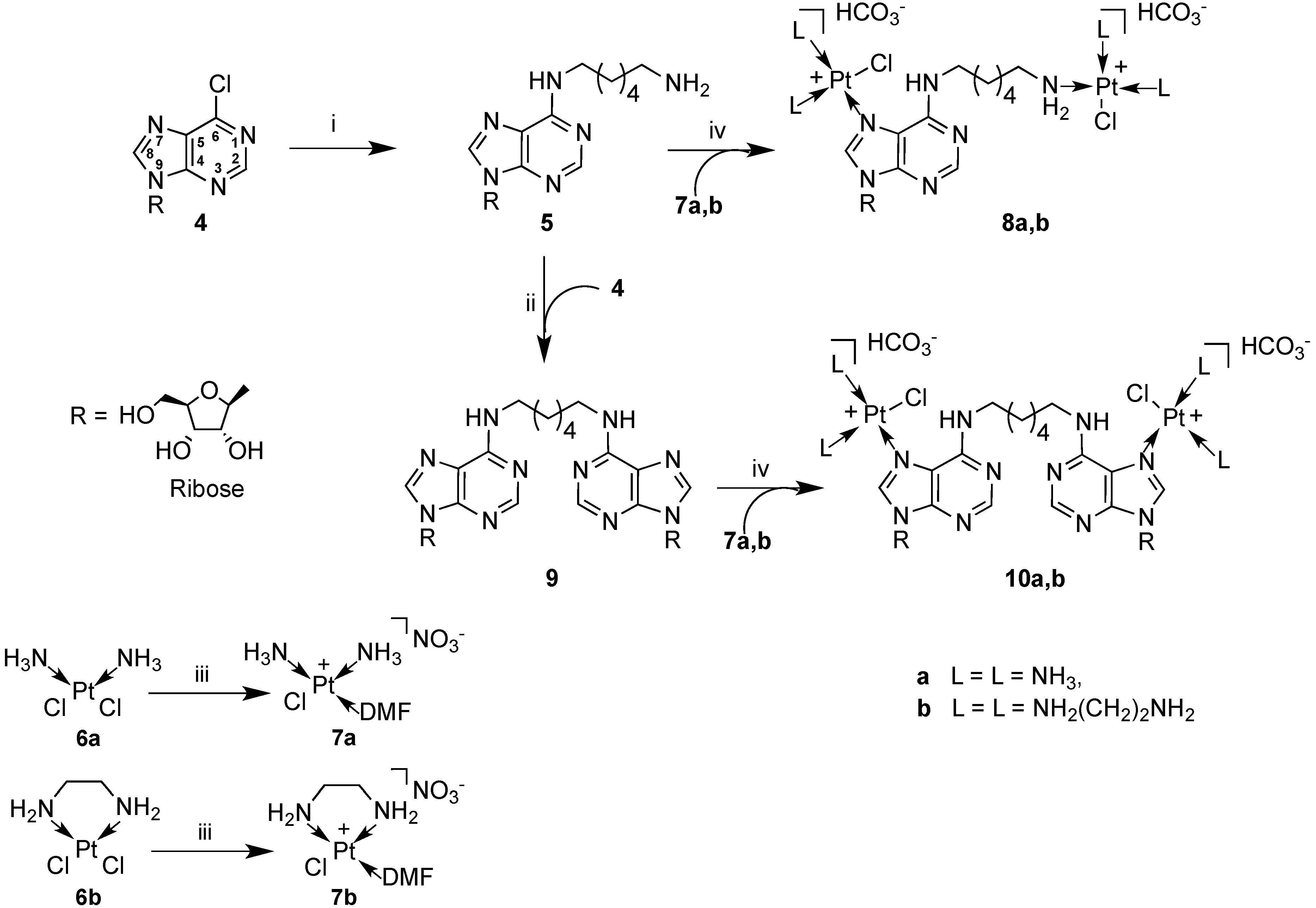
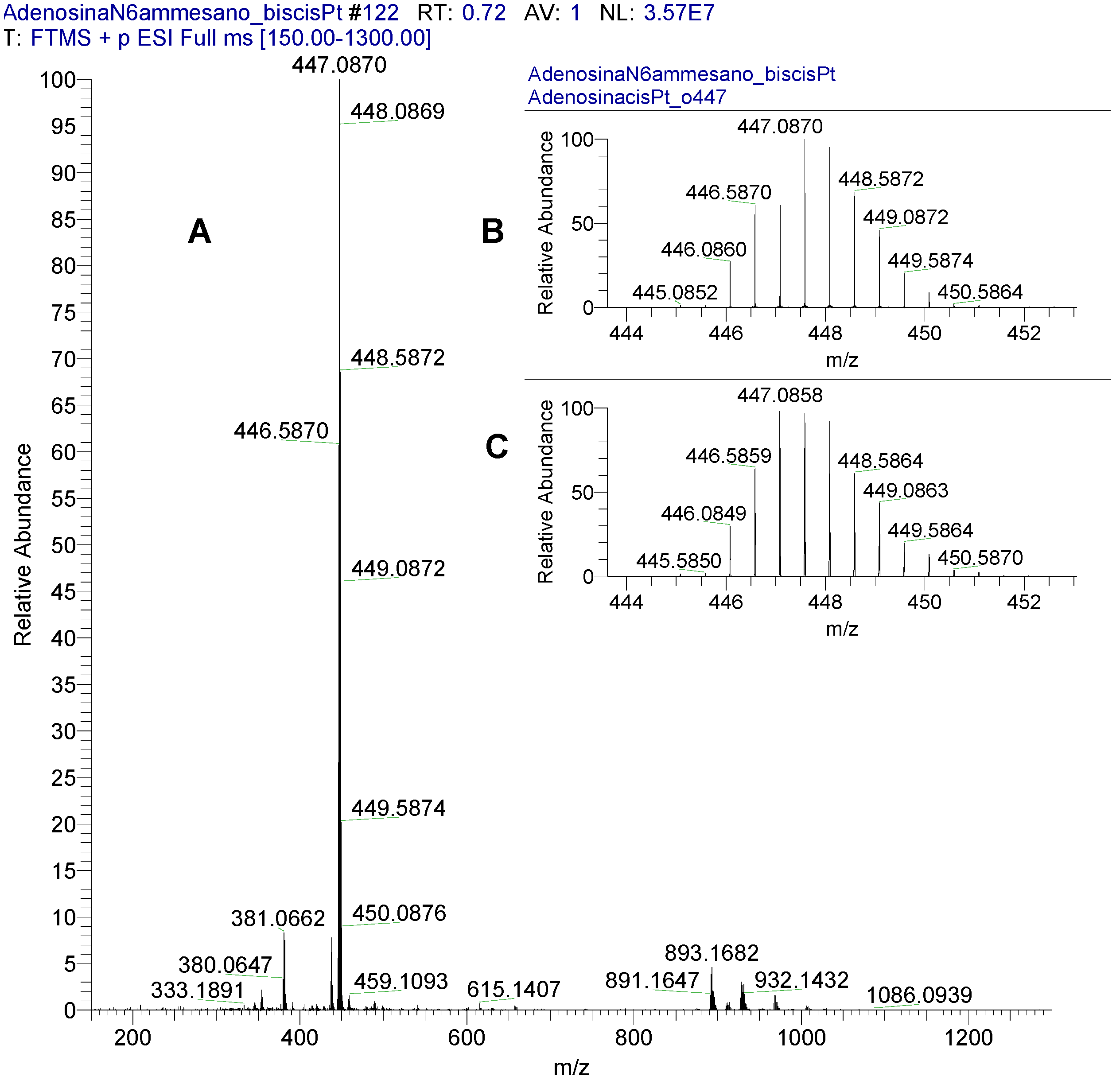
2.1. Synthesis and Characterization
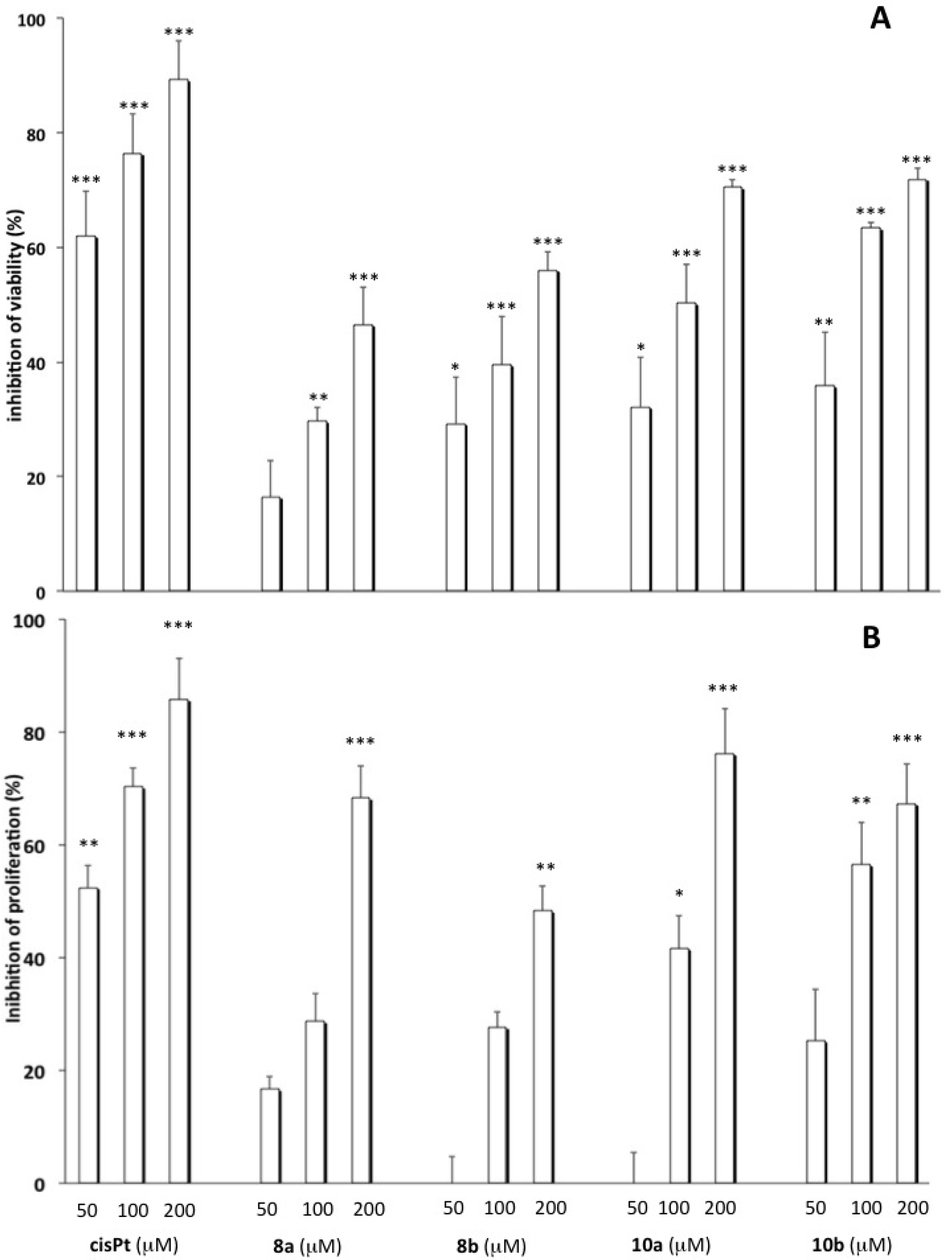
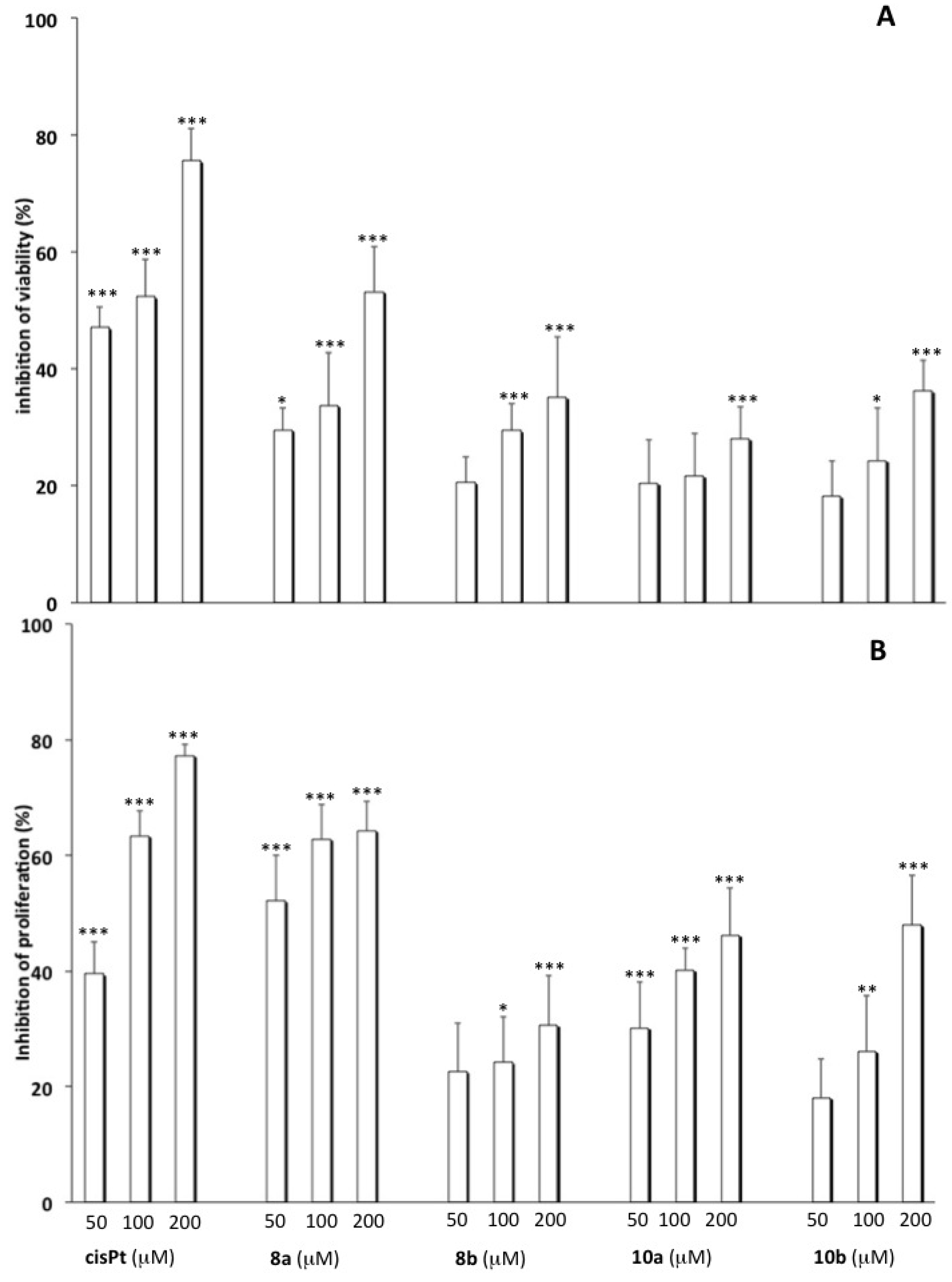
2.2. Cytotoxicity Studies
3. Experimental Section
3.1. General Methods
3.2. General Procedure for the Preparation of Dinuclear Platinum Complexes 8a,b and 10a,b
3.3. MTT Viability Assay
3.4. BrdU Cell Proliferation Assay
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosenberg, B.; Vancamp, L.; Krigas, T. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Kelland, L.R.; Farrell, N. Platinum Based Drugs in Cancer Therapy; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Allardyce, C.S.; Dorcier, A.; Scolaro, C.; Dyson, P.J. Development of organometallic (organo-transition metal) pharmaceuticals. Appl. Organometal. Chem. 2005, 19, 1–10. [Google Scholar]
- Wexselblatt, E.; Yavin, E.; Gibson, D. Cellular interactions of platinum drugs. Inorg. Chim. Acta 2012, 393, 75–83. [Google Scholar] [CrossRef]
- Harper, B.W.; Krause-Heuer, A.M.; Grant, M.P.; Manohar, M.; Garbutcheon-Singh, K.B.; Aldrich-Wright, J.R. Advances in Platinum Chemotherapeutics. Chem. Eur. J. 2010, 16, 7064–7077. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H. Metal Ions in Biological Systems: Metal Complexes in Tumor Diagnosis and as Anticancer Agents; CRC Press: London, UK, 2004. [Google Scholar]
- Florea, A.M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Fan, D.; Yang, X.; Wang, X.; Zhang, S.; Mao, J.; Ding, J.; Lin, L.; Guo, Z. A dinuclear monofunctional platinum(II) complex with an aromatic linker shows low reactivity towards glutathione but high DNA binding ability and antitumor activity. J. Biol. Inorg. Chem. 2007, 12, 655–665. [Google Scholar] [CrossRef]
- Boulikas, T. Clinical overview on Lipoplatin™: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Kettunen, M. Platinum group antitumor chemistry: Design and development of new anticancer drugs complementary to cisplatin. Curr. Med. Chem. 2006, 13, 1337–1357. [Google Scholar] [CrossRef]
- Arnesano, F.; Natile, G. Mechanistic insight into the cellular uptake and processing of cisplatin 30 years after its approval by FDA. Coord. Chem. Rev. 2009, 253, 2070–2081. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xing, Z.; Liu, D.; Sun, J.; Li, X.; Zhang, Y. Status of Bi- and Multi-Nuclear Platinum Anticancer Drug Development. Anti Cancer Agents Med. Chem. 2010, 10, 272–282. [Google Scholar] [CrossRef]
- Wheate, N.; Collins, J.G. Multi-nuclear platinum complexes as anti-cancer drugs. Coord. Chem. Rev. 2003, 241, 133–145. [Google Scholar] [CrossRef]
- Roberts, J.D.; Peroutka, J.; Farrell, N. Cellular pharmacology of polynuclear platinum anti-cancer agents. J. Inorg. Biochem. 1999, 77, 51–57. [Google Scholar] [CrossRef]
- Pratesi, G.; Perego, P.; Polizzi, D.; Righetti, S.C.; Supino, R.; Caserini, C.; Manzotti, C.; Giuliani, F.C.; Pezzoni, G.; Tognella, S.; et al. A novel charged trinuclear platinum complex effective against cisplatin-resistant tumours: Hypersensitivity of p53-mutant human tumour xenografts. Br. J. Cancer 1999, 80, 1912–1919. [Google Scholar] [CrossRef]
- Kasparkova, J.; Zehnulova, J.; Farrell, N.; Brabec, V. DNA interstrand cross-links of the novel antitumor trinuclear platinum complex BBR3464. Conformation, recognition by high mobility group domain proteins, and nucleotide excision repair. J. Biol. Chem. 2002, 277, 48076–48086. [Google Scholar]
- Jansen, B.A.J.; Wielaard, P.; Kalayda, G.V.; Ferrari, M.; Molenaar, C.; Tanke, H.J.; Brouwer, J.; Reedijk, J. Dinuclear platinum complexes with N,N'-bis(aminoalkyl)-1,4-diaminoanthraquinones as linking ligands. Part I. Synthesis, cytotoxicity, and cellular studies in A2780 human ovarian carcinoma cells. J. Biol. Inorg. Chem. 2004, 9, 403–413. [Google Scholar] [CrossRef]
- Mitova, V.; Bogomilova, A.; Shestakova, P.; Momekov, G.; Momekova, D.; Abbas, R.K.; Koseva, N. Synthesis of a new polynuclear platinum (ii) complex and its prodrug forms. Evaluation of their cytotoxic properties. J. Chem. Technol. Metall. 2013, 48, 17–27. [Google Scholar]
- Cincinelli, R.; Musso, L.; Dallavalle, S.; Artali, R.; Tinelli, S.; Colangelo, D.; Zunino, F.; de Cesare, M.; Beretta, G.L.; Zaffaroni, N. Design, modeling, synthesis and biological activity evaluation of camptothecin-linked platinum anticancer agents. Eur. J. Med. Chem. 2013, 63, 387–400. [Google Scholar]
- Robillard, M.S.; Valentijn, A.; Meeuwenoord, N.J.; van der Marel, G.A.; van Boom, J.H.; Reedijk, J. The First Solid-Phase Synthesis of a Peptide-Tethered Platinum (II) Complex. Angew. Chem. Int. Ed. 2000, 112, 3226–3229. [Google Scholar]
- Robillard, M.S.; van Alphen, S.; Meeuwenoord, N.J.; Jansen, B.A.J.; van der Marel, G.A.; van Boom, J.H.; Reedijk, J. Solid-phase synthesis of peptide-platinum complexes using platinum-chelating building blocks derived from amino acids. New J. Chem. 2005, 29, 220–225. [Google Scholar] [CrossRef]
- Holmes, R.J.; McKeage, M.J.; Murray, V.; Denny, W.A.; McFadyen, W.D. cis-Dichloroplatinum (II) complexes tethered to 9-aminoacridine-4-carboxamides: Synthesis and action in resistant cell lines in vitro. J. Inorg. Biochem. 2001, 85, 209–217. [Google Scholar]
- Cai, L.; Lim, K.; Ren, S.; Cadena, R.S.; Beck, W.T. Synthesis and in Vitro Antitumor Activity of Oligonucleotide-Tethered and Related Platinum Complexes. J. Med. Chem. 2001, 44, 2959–2965. [Google Scholar] [CrossRef]
- Schmidt, K.S.; Boudvillain, M.; Schwartz, A.; van der Marel, G.A.; van Boom, J.H.; Reedijk, J.; Lippert, B. Monofunctionally trans-Diammine Platinum (II)-Modified Peptide Nucleic Acid Oligomers: A New Generation of Potential Antisense Drugs. Chem. Eur. J. 2002, 8, 5566–5570. [Google Scholar] [CrossRef]
- Butler, J.S.; Sadler, P.J. Targeted delivery of platinum-based anticancer complexes. Curr. Opin. Chem. Biol. 2013, 17, 175–188. [Google Scholar] [CrossRef]
- Sengupta, P.; Basu, S.; Soni, S.; Pandey, A.; Roy, B.; Oh, M.S.; Chin, K.T.; Paraskar, A.S.; Sarangi, S.; Connor, Y. Cholesterol-tethered platinum II-based supramolecular nanoparticle increases antitumor efficacy and reduces nephrotoxicity. Proc. Natl. Acad. Sci. USA 2012, 109, 11294–11299. [Google Scholar]
- Coluccia, M.; Boccarelli, A.; Cermelli, C.; Portolani, M.; Natile, G. Platinum(II)-Acyclovir Complexes: Synthesis, Antiviral and Antitumour Activity. Metal-Based Drugs. 1995, 2, 249–256. [Google Scholar]
- Nervi, C.; Vigna, M.A.; Cavigiolio, G.; Ravera, M.; Osella, D. Synthesis and characterization of functionalized thymidine as a potential carrier for cisplatin-like drugs. Inorg. Chim. Acta 2005, 358, 2799–2803. [Google Scholar] [CrossRef]
- Štarha, P.; Popa, I.; Trávníček, Z.; Vančo, J. N6-Benzyladenosine Derivatives as Novel N-Donor Ligands of Platinum(II) Dichlorido Complexes. Molecules 2013, 18, 6990–7003. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Piccialli, V.; Amato, J.; Borbone, N.; D’Atri, V.; D’Alessio, F.; Di Noto, R.; Ruffo, F.; Salvatore, F.; et al. Solid-phase synthesis and pharmacological evaluation of novel nucleoside-tethered dinuclear platinum (II) complexes. Bioorg. Med. Chem. Lett. 2011, 21, 5835–5838. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Piccialli, G.; Mayol, L. Facile Solid-Phase Synthesis of AICAR 5'-Monophosphate (ZMP) and Its 4-N-Alkyl Derivatives. Eur. J. Org. Chem. 2010, 2010, 1517–1524. [Google Scholar]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Bucci, E.; Piccialli, V.; Mayol, L. Synthesis of 4-N-alkyl and ribose-modified AICAR analogues on solid support. Tetrahedron 2008, 64, 6475–6481. [Google Scholar] [CrossRef]
- Oliviero, G.; D Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Piccialli, G.; Mayol, L. A solid-phase approach to the synthesis of N-1-alkyl analogues of cyclic inosine-diphosphate-ribose (cIDPR). Tetrahedron 2010, 66, 1931–1936. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-Phase Synthesis of a New Diphosphate 5-Aminoimidazole-4-carboxamide Riboside (AICAR) Derivative and Studies toward Cyclic AICAR Diphosphate Ribose. Molecules 2011, 16, 8110–8118. [Google Scholar]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A Facile Synthesis of 5'-Fluoro-5'-deoxyacadesine (5'-F-AICAR): A Novel Non-phosphorylable AICAR Analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Synthesis of New Acadesine (AICA-riboside) Analogues Having Acyclic D-Ribityl or 4-Hydroxybutyl Chains in Place of the Ribose. Molecules 2013, 18, 9420–9431. [Google Scholar]
- Park, G.Y.; Wilson, J.J.; Song, Y.; Lippard, S.J. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. Proc. Natl. Acad. Sci. USA 2012, 109, 11987–11992. [Google Scholar]
- Johnstone, T.C.; Alexander, S.M.; Lin, W.; Lippard, S.J. Effects of Monofunctional Platinum Agents on Bacterial Growth: A Retrospective Study. J. Am. Chem. Soc. 2014, 136, 116–118. [Google Scholar] [CrossRef]
- Gregg, A.; Bottle, S.E.; Devine, S.M.; Figler, H.; Linden, J.; White, P.; Pouton, C.W.; Urmaliya, V.; Scammells, P.J. Dual acting antioxidant A1 adenosine receptor agonists. Bioorg. Med. Chem. Lett. 2007, 17, 5437–5441. [Google Scholar]
- Agathocleous, D.C.; Page, P.B.C.; Cosstick, R.; Galpin, J.J.; McLennan, A.G.; Prescott, M. Synthesis of bridged nucleosides. Tetrahedron 1990, 46, 2047–2058. [Google Scholar] [CrossRef]
- Amo-Ochoa, P.; González, V.M.; Pérez, J.M.; Masaguer, J.R.; Alonso, C.; Navarro-Ranninger, C. Cytotoxicity, DNA binding, and reactivity against nucleosides of platinum (II) and (IV) spermine compounds. J. Inorg. Biochem. 1996, 64, 287–299. [Google Scholar] [CrossRef]
- Van Rijt, S.; van Zutphen, S.; den Dulk, H.; Brouwer, J.; Reedijk, J. Structure–activity relationship studies for three new asymmetric cis-platinum(II) aminoethanol-based complexes. Inorg. Chim. Acta 2006, 359, 4125–4129. [Google Scholar]
- Girault, J.O.; Chottard, G.; Lallemand, J.Y.; Chottard, J.C. Interaction of cis-[Pt(NH3)2(H2O)2](NO3)2 with ribose and deoxyribose diguanosine phosphates. Biochemistry 1982, 21, 1352–1356. [Google Scholar]
- Arpalahti, J.; Klika, K.D.; Sillanpää, R.; Kivekäs, R. Dynamic processes in platinum(II)-adenosine complexes. Preparation, NMR spectroscopic characterisation and crystal structure of isomeric Pt II (dien)-adenosine complexes. J. Chem. Soc. Dalton Trans. 1998, 1397–1402. [Google Scholar]
- Wang, S.; Chu, W.; Wang, Y.; Liu, S.; Zhang, J.; Li, S.; Wei, H.; Zhou, G.; Qin, X. Synthesis, characterization and cytotoxicity of Pt(II), Pd(II), Cu(II) and Zn(II) complexes with 4’-substituted terpyridine. Appl. Organometal. Chem. 2013, 27, 373–379. [Google Scholar] [CrossRef]
- Li, L.J.; Wang, C.; Qiao, Y.; Yang, X.Y.; Hua, X.X.; Du, J.L. Platinum(II) complexes of reduced amino acid ester Schiff bases: Synthesis, characterization, and antitumor activity. Res. Chem. Intermed. 2014, 40, 413–424. [Google Scholar]
- Fan, S.; Smith, M.L.; Rivet, D.J.; Duba, D.; Zhan, Q.; Kohn, K.W.; Fornace, A.J.; O’Connor, P.M. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995, 55, 1649–1654. [Google Scholar]
- De Stefano, D.; Tommonaro, G.; Malik, S.A.; Iodice, C.; de Rosa, S.; Maiuri, M.C.; Carnuccio, R. Cacospongionolide and Scalaradial, Two Marine Sesterterpenoids as Potent Apoptosis-Inducing Factors in Human Carcinoma Cell Lines. PLoS One 2012, 7, e33031. [Google Scholar]
- Sample Availability: Samples of the compounds 8a,b and 10a,b are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D'Errico, S.; Oliviero, G.; Borbone, N.; Piccialli, V.; Pinto, B.; De Falco, F.; Maiuri, M.C.; Carnuccio, R.; Costantino, V.; Nici, F.; et al. Synthesis and Pharmacological Evaluation of Modified Adenosines Joined to Mono-Functional Platinum Moieties. Molecules 2014, 19, 9339-9353. https://doi.org/10.3390/molecules19079339
D'Errico S, Oliviero G, Borbone N, Piccialli V, Pinto B, De Falco F, Maiuri MC, Carnuccio R, Costantino V, Nici F, et al. Synthesis and Pharmacological Evaluation of Modified Adenosines Joined to Mono-Functional Platinum Moieties. Molecules. 2014; 19(7):9339-9353. https://doi.org/10.3390/molecules19079339
Chicago/Turabian StyleD'Errico, Stefano, Giorgia Oliviero, Nicola Borbone, Vincenzo Piccialli, Brunella Pinto, Francesca De Falco, Maria Chiara Maiuri, Rosa Carnuccio, Valeria Costantino, Fabrizia Nici, and et al. 2014. "Synthesis and Pharmacological Evaluation of Modified Adenosines Joined to Mono-Functional Platinum Moieties" Molecules 19, no. 7: 9339-9353. https://doi.org/10.3390/molecules19079339
APA StyleD'Errico, S., Oliviero, G., Borbone, N., Piccialli, V., Pinto, B., De Falco, F., Maiuri, M. C., Carnuccio, R., Costantino, V., Nici, F., & Piccialli, G. (2014). Synthesis and Pharmacological Evaluation of Modified Adenosines Joined to Mono-Functional Platinum Moieties. Molecules, 19(7), 9339-9353. https://doi.org/10.3390/molecules19079339