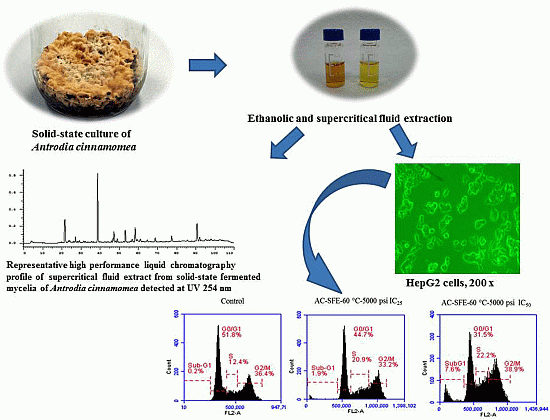
Comparison of the Apoptotic Effects of Supercritical Fluid Extracts of Antrodia cinnamomea Mycelia on Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Triterpenoid Content of AC Extracts
| Extract 1 | Yeild (%) | Total Triterpenoids 2 | Content (mg/g Extract, DW) 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| S-3000-40 1 | 11.83 ± 0.75 de | 22.83 ± 3.99 e | 2.95 ± 0.02 d | 5.88 ± 0.34 e | 0.10 ± 0.01 bcd | 0.02 ± 0.006 d | 0.21 ± 0.03 bc | 0.01 ± 0.004 b | 0.11 ± 0.01 de |
| S-3000-60 1 | 13.15 ± 0.64 d | 40.17 ± 1.94 d | 3.86 ± 0.31 c | 6.58 ± 0.25 cd | 0.12 ± 0.03 bc | 0.05 ± 0.004 c | 0.23 ± 0.05 bc | ND 4 | 0.98 ± 0.02 a |
| S-3000-80 1 | 10.43 ± 0.90 e | 21.31 ± 2.50 cd | 3.86 ± 0.09 c | 6.23 ± 0.07 d | 0.07 ± 0.01 cd | 0.04 ± 0.001 cd | 0.20 ± 0.07 bc | ND | 0.07 ± 0.04 e |
| S-4000-40 1 | 15.76 ± 0.46 c | 33.48 ± 3.61 cd | 3.96 ± 0.05 c | 7.98 ± 0.12 b | 0.18 ± 0.02 b | 0.02 ± 0.007 d | 0.29 ± 0.01 ab | 0.002 ± 0.0002 c | 0.19 ± 0.01 d |
| S-4000-60 1 | 16.09 ± 1.22 c | 49.88 ± 2.88 b | 4.75 ± 0.53 b | 8.69 ± 0.34 a | 0.31 ± 0.31 a | 0.04 ± 0.003 cd | 0.34 ± 0.08 a | 0.03 ± 0.001 a | 0.34 ± 0.08 c |
| S-4000-80 1 | 16.97 ± 1.47 bc | 42.12 ± 5.06 a | 4.20 ± 0.34 bc | 6.89 ± 0.34 c | 0.34 ± 0.12 a | 0.12 ± 0.004 b | 0.26 ± 0.05 ab | ND | 0.37 ± 0.11 bc |
| S-5000-40 1 | 16.40 ± 1.06 bc | 35.39 ± 4.02 e | 3.86 ± 0.13 c | 7.70 ± 0.21 b | 0.16 ± 0.01 bc | 0.02 ± 0.001 d | 0.27 ± 0.06 ab | 0.03 ± 0.002 a | 0.17 ± 0.03 de |
| S-5000-60 1 | 18.38 ± 0.52 b | 59.70 ± 0.40 a | 3.03 ± 0.05 d | 5.78 ± 0.02 e | 0.37 ± 0.04 a | 0.12 ± 0.03 b | 0.23 ± 0.02 bc | 0.004 ± 0.0003 c | 0.40 ± 0.02 bc |
| S-5000-80 1 | 15.41 ± 2.24 c | 40.18 ± 6.24 cd | 3.34 ± 0.07 d | 6.11 ± 0.15 e | 0.28 ± 0.05 a | 0.10 ± 0.02 b | 0.16 ± 0.01 c | ND | 0.31 ± 0.12 c |
| Ethanol | 32.26 ± 1.34 a | 60.33 ± 0.04 a | 6.98 ± 0.15 a | 8.13 ± 0.07 b | 0.01 ± 0.003 d | 0.32 ± 0.03 a | 0.29 ± 0.06 ab | 0.01 ± 0.002 b | 0.46 ± 0.06 b |
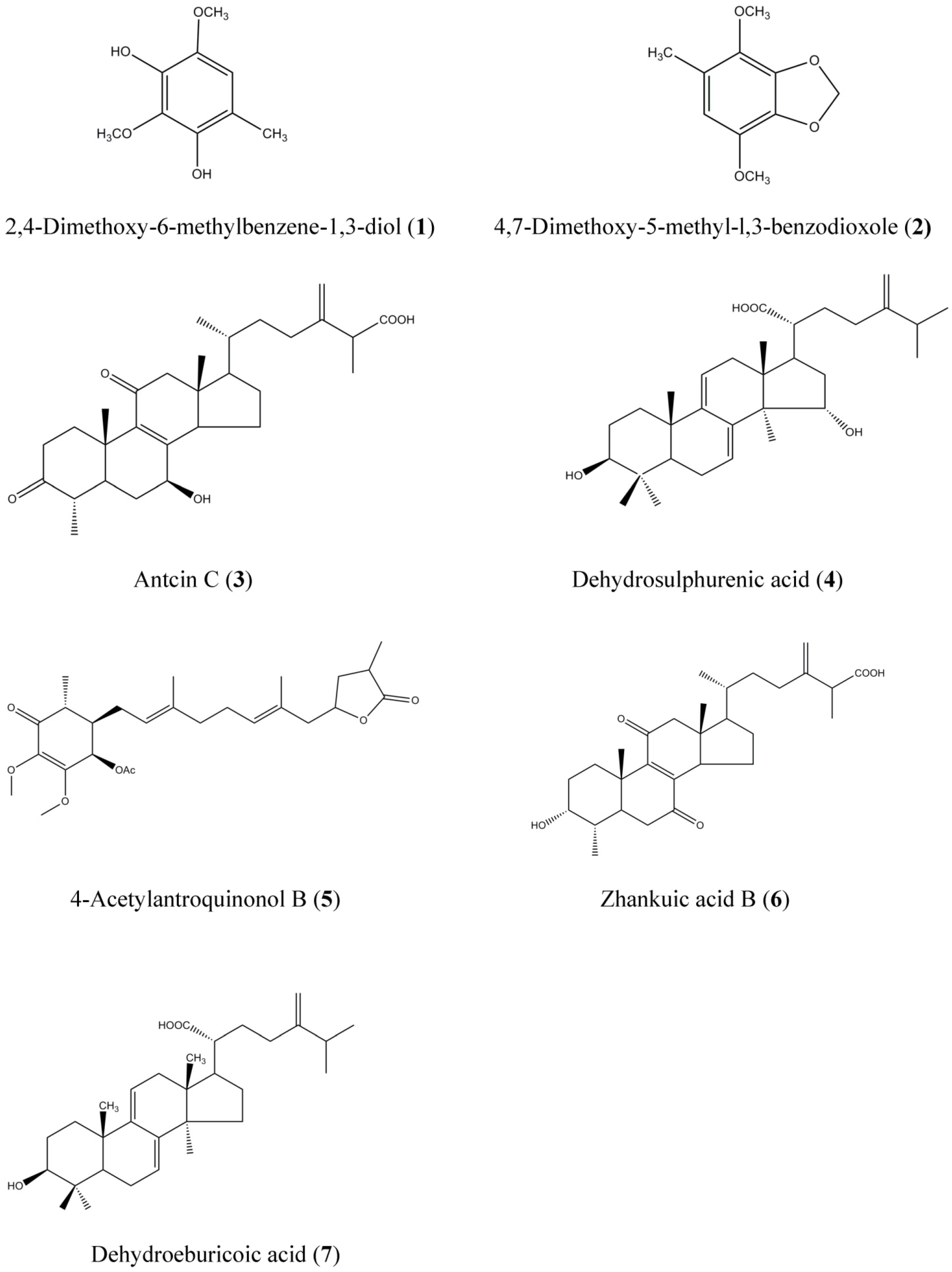
2.2. HPLC and LC-MS/MS Analysis of AC Extracts
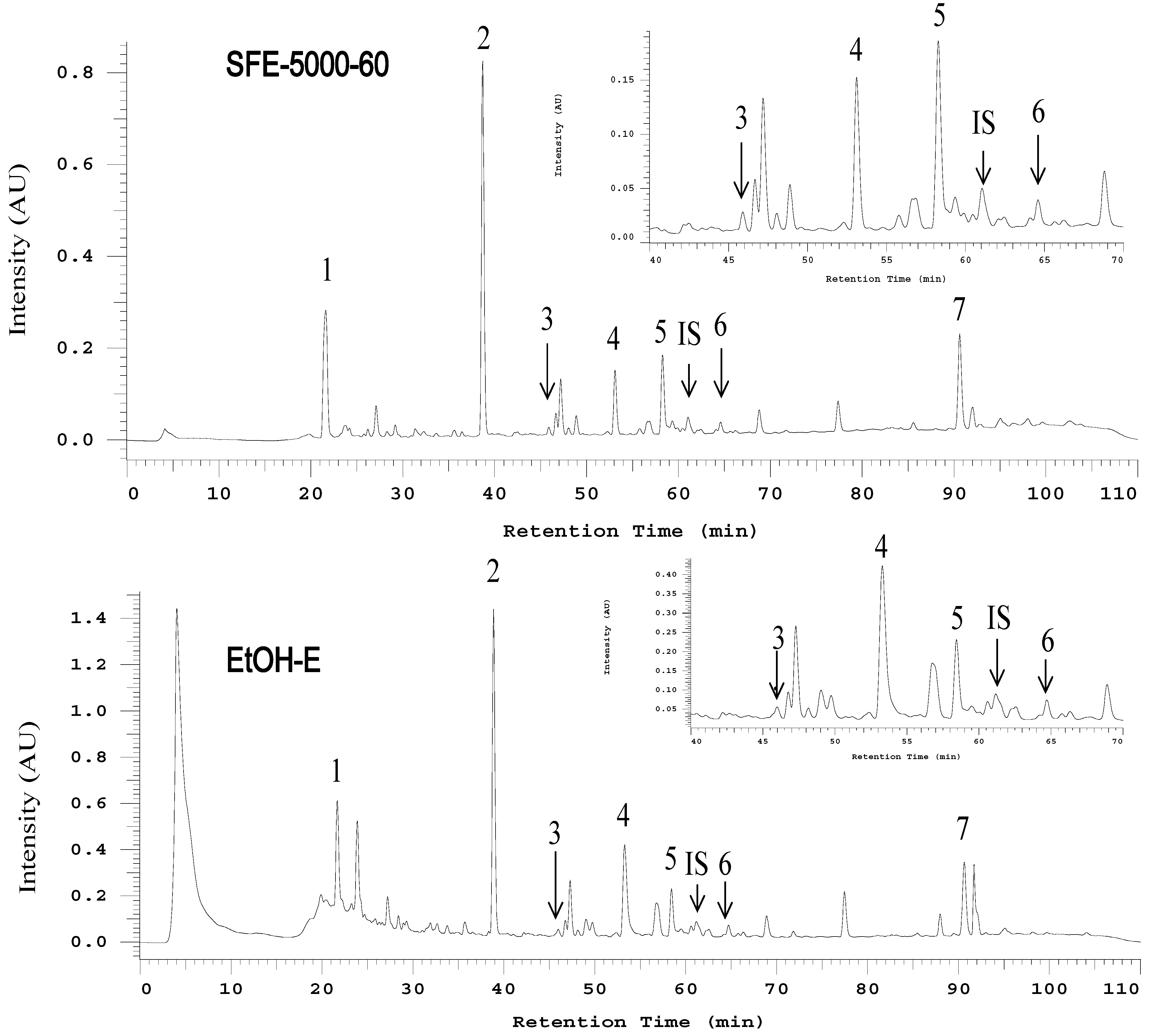

| Peak No. | Compounds a | tR (min) | UV-Vis Absorbance (nm) | [M + H]+ | [M − H]− | MS-MS Major Fragments |
|---|---|---|---|---|---|---|
| 1 | 2,4-Dimethoxy-6-methylbenzene-1,3-diol | 13.6 | 286, 236 | 185 | - b | 153, 137, 170 |
| 2 | 4,7-Dimethoxy-5-methyl-l,3-benzodioxole | 25.8 | 236, 284, 212(sh) | 197 | - | 139, 167, 182, 137 |
| 3 | Antcin C | 37.0 | 255, 288(sh) | - | 469 | 425, 341, 407, 301, 247 |
| 4 | Dehydrosulphurenic acid | 40.1 | 266, 233 | 485 | - | 471, 389 |
| 5 | 4-Acetylantroquinonol B | 40.7 | 264, 232(sh) | 463 | - | 403, 420, 389, 235 |
| 6 | Zhankuic acid B | 44.2 | 256, 233(sh) | - | 469 | 425, 407 |
| 7 | Dehydroeburicoic acid | 69.8 | 246, 254(sh), 231(sh) | 469 | - | 311, 451 |
| Peak No. | Compound Name | Linearity Range (µg/mL) | Equation | R2 |
|---|---|---|---|---|
| 1 | 2,4-Dimethoxy-6-methylbenzene-1,3-diol | 10~600 | y = 0.076x + 0.5363 | 0.9986 |
| 2 | 4,7-Dimethoxy-5-methyl-1,3-benzodioxole | 10~800 | y = 0.0821x − 0.0556 | 0.9955 |
| 3 | Antcin C | 0.1~20 | y = 0.4367x + 0.6518 | 0.9952 |
| 4 | Dehydrosulphurenic acid | 0.1~50 | y = 0.6956x + 0.7382 | 0.9953 |
| 5 | 4-Acetlyantroquinonol B | 0.1~50 | y = 0.5478x − 0.0415 | 0.9921 |
| 6 | Zhankuic acid B | 0.1~20 | y = 0.9429x − 0.0199 | 0.9967 |
| 7 | Dehydroeburicoic acid | 1~50 | y = 0.3893x + 0.8817 | 0.993 |
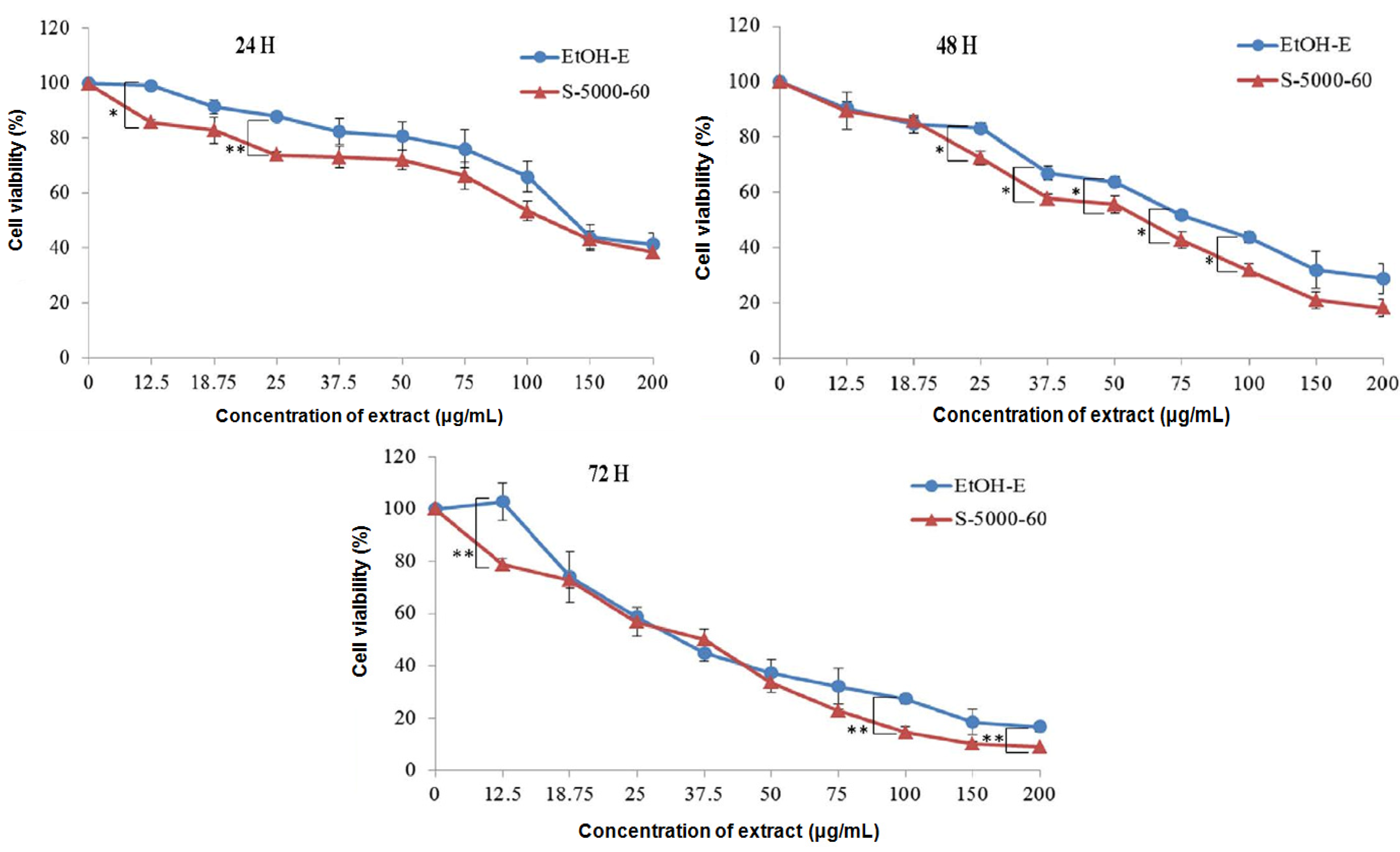
2.3. Inhibitory Effect on Proliferation of HepG2 Cells
| Treatment | Time (hour) | |||||
|---|---|---|---|---|---|---|
| Inhibition | 24 h | 48 h | 72 h | |||
| Concentration 1 | EtOH-E 2 | S-5000-60 3 | EtOH-E | S-5000-60 | EtOH-E | S-5000-60 |
| IC25 | 77.88 a | 41.15 b | 25.67 bc | 26.40 bc | 18.47 c | 14.85 c |
| IC50 | 131.09 a | 116.15 b | 80.04 c | 57.82 c | 48.30 d | 43.96 d |
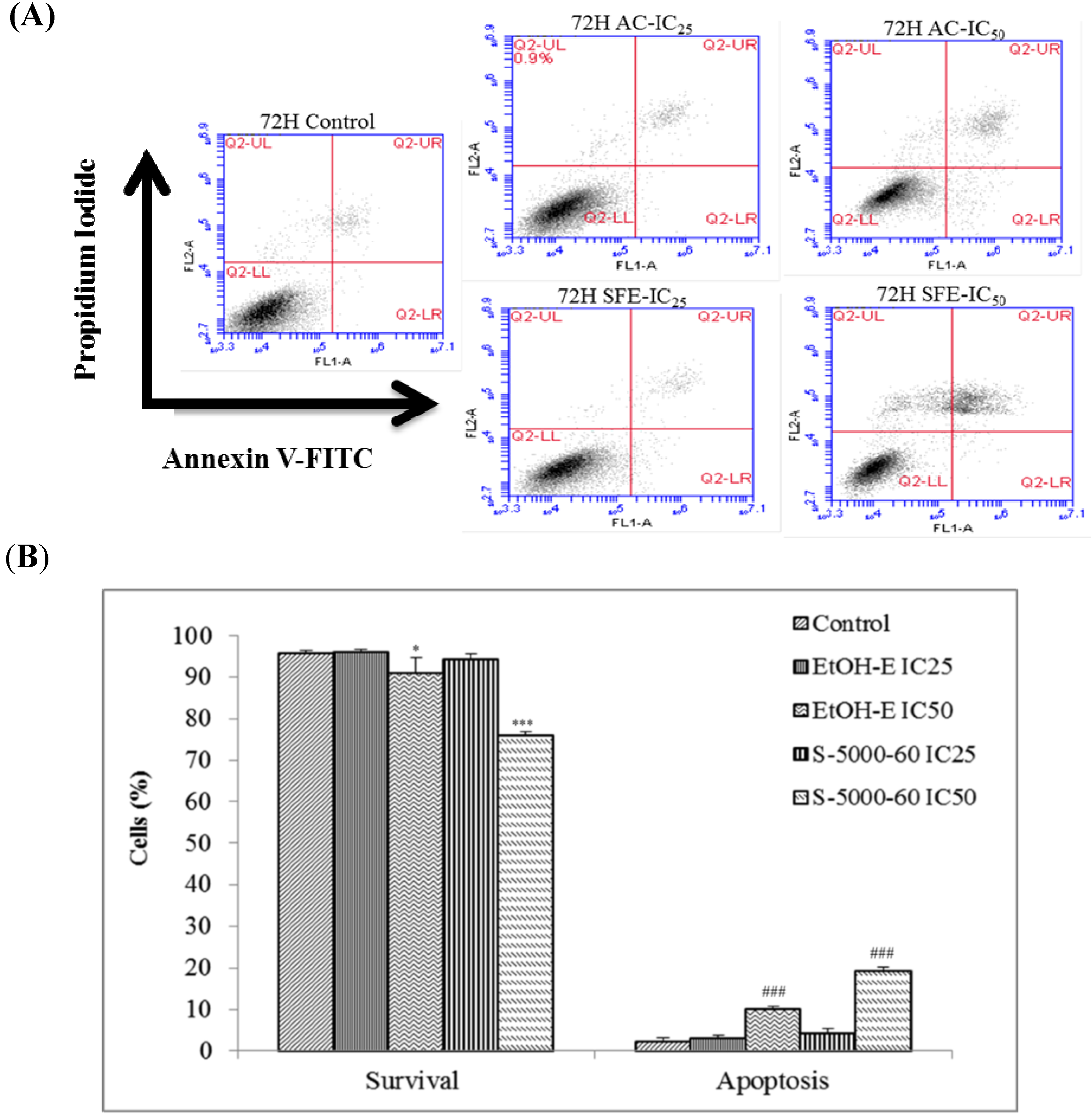
2.4. The Apoptotic Activities of S-5000-60 and EtOH-E Extracts
3. Experimental
3.1. Chemicals, Reagents and Antibodies
3.2. Solid-State Culture of A. cinnamomea
3.3. Supercritical Fluid and Ethanol Solvent Extraction of A. cinnamomea Mycelia
3.4. Determination of Total Triterpenoids
3.5. HPLC and LC-MS/MS Analysis of Extracts
3.5.1. Preparation of Calibration Standards
3.5.2. Quantification of the Major Components in Extracts
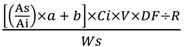
3.5.3. LC-Tandem Mass Spectrometry
3.6. Effects of Extracts on the Proliferation of HumanHepatocellular Carcinoma HepG2 Cells
3.6.1. Cell Culture and Cell Viability Assay
3.6.2. Apoptosis Assay by Annexin V FITC/PI Double-Staining
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, T.T.; Chou, W.N. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol. Res. 1995, 99, 756–758. [Google Scholar]
- Chen, C.C.; Liu, Y.W.; Ker, Y.B.; Wu, Y.Y.; Lai, E.Y.; Chyau, C.C.; Hseu, T.H.; Peng, R.Y. Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J. Agric. Food Chem. 2007, 58, 5007–5012. [Google Scholar]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidant and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid.-Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Cho, C.Y.; Ni, W.C.; Tzeng, T.F.; Ng, L.T.; Kuo, Y.H.; Lin, C.C. Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor kappaB pathway. Food Chem. Toxicol. 2007, 45, 1249–1257. [Google Scholar] [CrossRef]
- Ker, Y.B.; Peng, C.C.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Hepatoprotective Bioactivity of the Glycoprotein, Antrodan, Isolated from Antrodia cinnamomea Mycelia. PLoS One 2014, 9, e93191. [Google Scholar]
- Wu, M.D.; Cheng, M.J.; Wang, W.Y.; Huang, H.C.; Yuan, G.F.; Chen, J.J.; Chen, I.S.; Wang, B.C. Antioxidant activities of extracts and metabolites isolated from the fungus Antrodia cinnamomea. Nat. Prod. Res. 2011, 5, 1488–1496. [Google Scholar]
- Cheng, P.C.; Huang, C.C.; Chiang, P.F.; Lin, C.N.; Li, L.L.; Lee, T.W.; Lin, B.; Chen, I.C.; Chang, K.W.; Fan, C.K.; et al. Radioprotective effects of Antrodia cinnamomea are enhanced on immune cells and inhibited on cancer cells. Int. J. Radiat. Biol. 2014. [Google Scholar] [CrossRef]
- Wu, S.H.; Ryvarden, L.; Chang, T.T. Antrodia camphorata (“niu-chang-chih”), new combination of a medicinal fungus in Taiwan. Bot. Bull. Acad. Sin. 1997, 38, 273–275. [Google Scholar]
- Liu, D.Z.; Liang, H.J.; Chen, C.H.; Su, C.H.; Lee, T.H.; Huang, C.T.; Hou, W.C.; Lin, S.Y.; Zhong, W.B.; Lin, P.J.; et al. Comparative anti-inflammatory characterization of wild fruiting body, liquid-state fermentation, and solid-state culture of Taiwanofungus camphoratus in microglia and the mechanism of its action. J. Ethnopharmacol. 2007, 15, 45–53. [Google Scholar]
- Cherng, I.H.; Wu, D.P.; Chiang, H.C. Triterpenoids from Antrodia cinnamomea. Phytochemistry 1996, 41, 263–267. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Yu, Y.L.; Chen, I.H.; Shen, K.Y.; Huang, R.Y.; Wang, W.R.; Chou, C.J.; Chang, T.T.; Chu, C.L. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation. Eur. J. Immunol. 2009, 39, 2482–2491. [Google Scholar]
- Huang, C.H.; Chang, Y.Y.; Liu, C.W.; Kang, W.Y.; Lin, Y.L.; Chang, H.C.; Chen, Y.C. Fruiting body of Niuchangchih (Antrodia camphorata) protects livers against chronic alcohol consumption damage. J. Agric. Food Chem. 2010, 58, 3859–3866. [Google Scholar]
- Herzi, N.; Bouajila, J.; Camy, S.; Romdhane, M.; Condoret, J.S. Comparison of different methods for extraction from Tetraclinis articulata: Yield, chemical composition and antioxidant activity. Food Chem. 2013, 141, 3537–3545. [Google Scholar]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520. [Google Scholar] [CrossRef]
- Cygnarowicz-Provost, M.; O’Brien, D.J.; Maxwell, R.T.; Hampson, J.W. Supercritical-fluid extraction of fungal lipids using mixed solvents: Experiment and modeling. J. Supercrit. Fluids 1992, 5, 24–30. [Google Scholar]
- Yang, S.S.; Wang, G.J.; Wang, S.Y.; Lin, Y.Y.; Kuo, Y.H.; Lee, T.H. New constituents with iNOS inhibitory activity from mycelium of Antrodia camphorata. Planta Med. 2009, 75, 512–516. [Google Scholar] [CrossRef]
- Lien, H.M.; Lin, H.W.; Wang, Y.J.; Chen, L.C.; Yang, D.Y.; Lai, Y.Y.; Ho, Y.S. Inhibition of anchorage-independent proliferation and G0/G1 cell-cycle regulation in human colorectal carcinoma cells by 4,7-dimethoxy-5-methyl-l,3-benzodioxole isolated from the fruiting body of Antrodia camphorata. Evid.-Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Rao, Y.K.; Wu, C.C.; Huang, C.Y.; Geethangili, M.; Hsu, S.L.; Tzeng, Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem. Res. Toxicol. 2010, 23, 1256–1267. [Google Scholar] [CrossRef]
- Shen, C.C.; Wang, Y.H.; Chang, T.T.; Lin, L.C.; Don, M.J.; Hou, Y.C.; Liou, K.T.; Chang, S.; Wang, W.Y.; Ko, H.C.; et al. Anti-inflammatory ergostanes from the basidiomata of Antrodia salmonea. Planta Med. 2007, 73, 1208–1213. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chiang, B.H. 4-Acetylantroquinonol B isolated from Antrodia cinnamomea arrests proliferation of human hepatocellular carcinoma HepG2 cell by affecting p53, p21 and p27 levels. J. Agric. Food Chem. 2011, 59, 8625–8631. [Google Scholar] [CrossRef]
- Shen, Y.C.; Wang, Y.H.; Chou, Y.C.; Chen, C.F.; Lin, L.C.; Chang, T.T.; Tien, J.H.; Chou, C.J. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Med. 2004, 70, 310–314. [Google Scholar] [CrossRef]
- Du, Y.C.; Chang, F.R.; Wu, T.Y.; Hsu, Y.M.; El-Shazly, M.; Chen, C.F.; Sung, P.J.; Lin, Y.Y.; Lin, Y.H.; Wu, Y.C.; et al. Antileukemia component, dehydroeburicoic acid from Antrodia camphorata induces DNA damage and apoptosis in vitro and in vivo models. Phytomedicine 2012, 19, 788–796. [Google Scholar]
- Tu, S.H.; Wu, C.H.; Chen, L.C.; Huang, C.S.; Chang, H.W.; Chang, C.H.; Lien, H.M.; Ho, Y.S. In vivo antitumor effects of 4,7-dimethoxy-5-methyl-1,3-benzodioxole isolated from the fruiting body of Antrodia camphorata through activation of the p53-mediated p27/Kip1 signaling pathway. J. Agric. Food Chem. 2012, 60, 3612–3618. [Google Scholar]
- Du, Y.C.; Wu, T.Y.; Chang, F.R.; Lin, W.Y.; Hsu, Y.M.; Cheng, F.T.; Lu, C.Y.; Yen, M.H.; Tsui, Y.T.; Chen, H.L.; et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata. J. Pharm. Biomed. Anal. 2012, 58, 182–192. [Google Scholar] [CrossRef]
- Kuo, C.F.; Su, J.D.; Chiu, C.H.; Peng, C.C.; Chang, C.H.; Sung, T.Y.; Huang, S.H.; Lee, W.C.; Chyau, C.C. Anti-inflammatory effects of supercritical carbon dioxide extract and its isolated carnosic acid from Rosmarinus officinalis leaves. J. Agric. Food Chem. 2011, 59, 3674–3685. [Google Scholar] [CrossRef]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar]
- Peng, C.H.; Chang, C.H.; Peng, R.Y.; Chyau, C.C. Improved membrane transport of astaxanthine by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef]
- Wilkins, R.C.; Kutzner, B.C.; Truong, M.; Sanchez-Dardon, J.; McLean, J.R.N. Analysis of radiationiInduced apoptosis in human lymphocytes: Flow cytometry using Annexin V and Propidium Iodide versus the neutral Comet assay. Cytometry 2002, 48, 14–19. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C.P. A novel assay for apoptosis: Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts from A. Cinnamomea mycelia are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, H.-M.; Chiu, C.-H.; Chen, C.-C.; Chang, W.-L.; Chyau, C.-C.; Peng, R.Y. Comparison of the Apoptotic Effects of Supercritical Fluid Extracts of Antrodia cinnamomea Mycelia on Hepatocellular Carcinoma Cells. Molecules 2014, 19, 9033-9050. https://doi.org/10.3390/molecules19079033
Lien H-M, Chiu C-H, Chen C-C, Chang W-L, Chyau C-C, Peng RY. Comparison of the Apoptotic Effects of Supercritical Fluid Extracts of Antrodia cinnamomea Mycelia on Hepatocellular Carcinoma Cells. Molecules. 2014; 19(7):9033-9050. https://doi.org/10.3390/molecules19079033
Chicago/Turabian StyleLien, Hsiu-Man, Chun-Hung Chiu, Chia-Chang Chen, Wan-Lin Chang, Charng-Cherng Chyau, and Robert Y. Peng. 2014. "Comparison of the Apoptotic Effects of Supercritical Fluid Extracts of Antrodia cinnamomea Mycelia on Hepatocellular Carcinoma Cells" Molecules 19, no. 7: 9033-9050. https://doi.org/10.3390/molecules19079033
APA StyleLien, H.-M., Chiu, C.-H., Chen, C.-C., Chang, W.-L., Chyau, C.-C., & Peng, R. Y. (2014). Comparison of the Apoptotic Effects of Supercritical Fluid Extracts of Antrodia cinnamomea Mycelia on Hepatocellular Carcinoma Cells. Molecules, 19(7), 9033-9050. https://doi.org/10.3390/molecules19079033