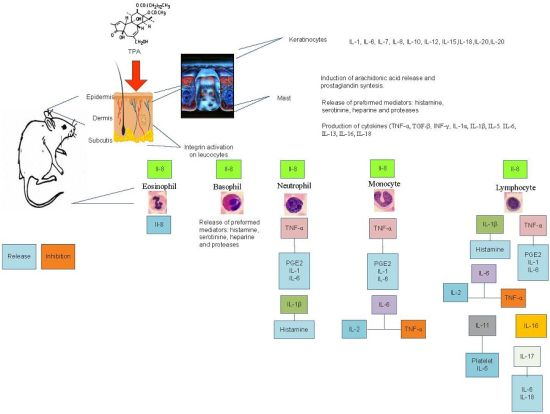
Bioassay-Guided Chemical Study of the Anti-Inflammatory Effect of Senna villosa (Miller) H.S. Irwin & Barneby (Leguminosae) in TPA-Induced Ear Edema
Abstract
:1. Introduction

2. Results and Discussion
2.1. Anti-Inflammatory Effect
| Group | Topical Dose/Ear (mg) | Edema (mg) | % of Inhibition |
|---|---|---|---|
| TPA-Control | Vehicle | 10.23 ± 0.39 | - |
| Indomethacin | 2 | 6.53 ± 0.48 ** | 36.15 ± 4.75 |
| Chloroform Extract | 2 | 4.30 ± 0.55 **,♦ | 57.96 ± 5.21 ♦ |
| Methanol Extract | 2 | 9.11 ± 0.29 | 10.90 ± 2.88 |
| Aqueous extract | 2 | 7.83 ± 0.59 * | 23.42 ± 5.78 |
2.2. Leukocyte Differential Count
| Celular Type | Time | TPA-Control | Indomethacin | Chloroform Extract |
|---|---|---|---|---|
| Neutrophil | 0 | 54.50 ± 9.75 | 37.53 ± 2.91 | 46.72 ± 6.21 |
| 240 | 34.69 ± 4.13 | 40.11 ± 6.84 | 80.72 ± 1.63 * ♦,● | |
| 300 | 38.77 ± 1.72 | 55.88 ± 6.99 | 66.32 ± 2.90 ♦ | |
| 360 | 91.15 ± 2.98 * | 40.37 ± 2.21 | 81.04 ± 1.95 * ♦,● | |
| Monocyt | 0 | 3.17 ± 1.10 | 2.01 ± 0.24 | 1.50 ± 0.62 |
| 240 | 1.23± 0.24 | 2.39 ± 0.60 | 1.75 ± 1.28 | |
| 300 | 1.23 ± 0.24 | 0.67 ± 0.37 | 5.75 ± 1.75 ♦,● | |
| 360 | 1.65 ± 0.30 | 3.32 ± 0.33 | 8.99 ± 1.76 * ♦,● | |
| Lymphocyte | 0 | 54.98 ± 1.24 | 55.37 ± 3.39 | 51.72 ± 6.17 |
| 240 | 50.93 ± 2.53 | 53.75 ± 6.99 | 18.13 ± 1.76 * ♦,● | |
| 300 | 43.00 ± 1.61 | 38.88 ± 6.40 | 30.48 ± 3.30 * | |
| 360 | 35.55 ± 4.25 * | 54.80 ± 1.54 ♦ | 14.15 ± 2.04 * ♦,● | |
| Eosinophil | 0 | 0.33 ± 0.22 | 0.59 ± 0.07 | 0.58 ± 0.31 |
| 240 | 1.06 ± 0.44 | 0.46 ± 0.14 | 0.12 ± 0.12 | |
| 300 | 1.60 ± 0.37 * | 0.44 ± 0.21 | 0.37 ± 0.18 | |
| 360 | 2.21 ± 0.41 * | 0.55 ± 0.12 ♦ | 0.12 ± 0.12 ♦ | |
| Basophil | 0 | 0.65 ± 0.25 | 0.38 ± 0.21 | 0.27 ± 0.18 |
| 240 | 63.02 ± 4.69 * | 0.59 ± 0.26 ♦ | 0.12 ± 0.12 ♦ | |
| 300 | 58.45 ± 1.85 * | 2.13 ± 1.89 ♦ | 0 | |
| 360 | 53.54 ± 2.45 * | 0.10 ± 0.10 ♦ | 0 |
2.3. Fractionation of the Chloroform Extract
2.4. Bioassay-Guided Chemical Study (Anti-Inflammatory Effect in the Model of Ear Edema Induced by TPA)
| Group | Dose topically Administered/Ear (mg) | Edema (mg) | % of Inhibition |
|---|---|---|---|
| Control | STPA-control | 12.18 ± 0.68 | - |
| Indomethacin | 2 | 6.83 ± 0.70 * | 43.90 ± 5.81 |
| Fraction 1 | 2 | 11.46 ± 0.63 | 5.91 ± 5.42 |
| Fraction 2 | 2 | 9.18 ± 0.79 | 24.63 ± 6.55 |
| Fraction 3 | 2 | 10.10 ± 0.88 | 17.08 ± 7.29 |
| Fraction 4 | 2 | 8.66 ± 0.58 | 28.90 ± 4.82 |
| Fraction 5 | 2 | 10.16 ± 0.66 | 16.58 ± 5.45 |
| Fraction 6 | 2 | 9.51 ± 0.91 | 24.88 ± 7.51 |
| Fraction 7 | 2 | 10.05 ± 0.49 | 17.49 ± 4.05 |
| Fraction 8 | 2 | 10.98 ± 0.47 | 9.82 ± 3.93 |
| Fraction 9 | 2 | 11.13 ± 0.35 | 8.66 ± 2.94 |
| White precipitate from fractions 2–3 | 2 | 6.30 ± 0.93 * | 48.28 ± 7.64 |
2.5. Structural Elucidation (NMR, IR, MS)
| Molecular Ion | Chemical Formula | Structural Formula |
|---|---|---|
| 311.2890 | C20H40O2 | 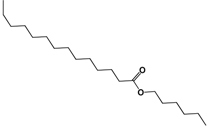 |
| 1.-Hexyl tetradecanoate | ||
| 325.3099 | C21H42O2 | 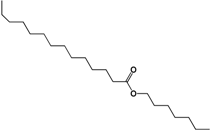 |
| 2.-Heptyl tetradecanoate | ||
| 339.3308 | C22H44O2 | 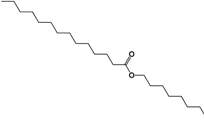 |
| 3.-Octyl tetradecanoate |
3. Experimental Section
3.1. Plant Material
3.2. Preparation of Extracts
3.3. Animal Experiments
3.4. Anti-Inflammatory Effect in the Model of Ear Edema Induced by TPA
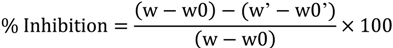
3.4.1. Differential Leukocyte Count
3.5. Fractionation of the Chloroform Extract
3.6. Purification of the Active Principles
3.7. Bioassay-Guided Chemical Study (Anti-Inflammatory Effect in the Model of Ear Edema Induced by TPA)
3.8. Structural Elucidation
3.8.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.8.2. Infrared Spectroscopy (ATR-FTIR)
3.8.3. High-Resolution Electrospray Ionization Mass Spectrometry
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar]
- Julián-Gónzalez, R.E.; Orozco-Covarrubias, L.; Durán-McKinster, C.; Palacios-Lopez, C.; Ruiz-Maldonado, R.; Sáes-de-Ocariz, M. Less common clinical manifestations of atopic dermatitis: Prevalence by age. Pediatr. Dermatol. 2012, 29, 580–583. [Google Scholar]
- Kim, H.O.; Kim, J.H.; Cho, S.I.; Chung, B.Y.; Ahn, I.S.; Lee, C.H.; Park, C.W. Improvement of atopic dermatitis severity after reducing indoor air pollutants. Ann. Dermatol. 2013, 25, 292–297. [Google Scholar]
- Wollenberg, A.; Ehmann, L.M. Long term treatment concepts and proactive therapy for atopic eczema. Ann. Dermatol. 2012, 24, 253–260. [Google Scholar]
- Guatam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar]
- Palfreeman, A.C.; McNamee, K.E.; McCann, F.E. New developments in the management of psoriasis and psoriatic arthritis: A focus on apremilast. Drug Des. Devel. Ther. 2013, 7, 201–210. [Google Scholar]
- Morgan, M.S.; Arlian, L.G.; Markey, M.P. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoSOne 2013, 8, e71143. [Google Scholar]
- Sinha, M.; Guatam, L.; Shukla, P.K.; Kaur, P.; Sharma, S.; Singh, T.P. Current perspectives in NSAID-induced gastropathy. Mediat. Inflamm 2013, 2013, 2582209:1–2582209:11. [Google Scholar]
- Irwin, H.S.; Barneby, R.C. The American Cassiinae: A Synoptical Revision of Leguminosae tribe Cassieae subtribe Cassiinae in the New World. Memoir. N. Y. Bot. Gard. 1982, 35, 1–918. [Google Scholar]
- Randell, B.R.; Barlow, B.A. Senna. In Flora of Australia; CSIRO: Melbourne, Australia, 1998; Volume 12, pp. 89–138. [Google Scholar]
- Boni, A.R.; Yapi, H.F.; Zirihi, G.N.; Bidie, A.P.; Djaman, A.J.; Niamke, L.S.; N’guessan, J.D. Comparison of antioxidant activity and total phenolic content of aqueous extracts of six Ivorian medicinal plants: Ageratum conyzoides, Alchornea cordifolia, Amaranthus spinosus, Cassia occidentalis, Chromolaena odorata and Spondias mombin. Arch. Pharm. Sci. Res. 2010, 2, 337–342. [Google Scholar]
- Iwuanyanwu, P.; Kingsley, C.; Onyeike, E.N.; Ahsana, D. Anti-inflammatory effect of crude methanolic extract and fractions of ringworm plant Senna alata (L. Roxb) leaves. Pharm. Sin. 2011, 2, 9–16. [Google Scholar]
- Rao, S.; Suresh, C. Phytochemical analysis and in vitro efficacy of two edible Cassia species on selected human pathogens. Intern. J. Pharm. Sci. Res. 2012, 3, 4982–4988. [Google Scholar]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Int. J. Nat. Prod. Res. 2012, 3, 291–319. [Google Scholar]
- Da Silva, K.A.; Manjavachi, M.N.; Paszcuk, A.F.; Pivatto, M.; Viegas, C., Jr.; Bolzani, V.S.; Calixto, J.B. Plant derived alkaloid (−)-cassine induces anti-inflammatory and anti-hyperalgesic effects in both acute and chronic inflammatory and neuropathic pain models. Neuropharmacology 2012, 62, 967–977. [Google Scholar]
- Sheeba, R.M.; Emmanuel, S.; Sreekanth, M.R. Evaluation of the antipyretic and anti-inflammatory activities of methanolic fraction and chrysophenol of Cassia occidentalis Linn. Res. J. Pharm. Tech. 2010, 3, 888–893. [Google Scholar]
- Choudhary, M.; Gulia, Y. Cassia tora: Its chemistry, medicinal uses and pharmacology. Pharmacologyonline 2011, 3, 78–96. [Google Scholar]
- Amalesh, S.; Gouranga, D.; Soma, G.; Durbadal, O. In vivo & in vitro anti-inflammatory activity of the methanolic extract and isolated compound from the leaves of Cassia tora L. (Leguminosae/caesalpinaceae). J. Pharm. Res. 2011, 4, 1999–2002. [Google Scholar]
- Sermakkani, M.; Thangapandian, V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharm. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Bhalodia, N.R.; Acharya, R.N.; Shukla, V.J. Evaluation of in vitro antioxidant activity of hydroalcoholic seed extracts of Cassia fistula Linn. Free Rad. Antiox. 2011, 1, 68–76. [Google Scholar] [CrossRef]
- Abdul, L.; Abdul, R.; Sukul, R.R.; Nazish, S. Anti-inflammatory and antihistaminic study of a Unani eye drop formulation. Ophthalmol. Eye Dis. 2010, 2, 17–22. [Google Scholar]
- Basha, S.I.; Somashekara, S.C.; Govindadas, D.; Naidu, D.C.M.; Devasankaraiah, G.; Mohato, R.; Yadav, K.C.H. Anti-inflammatory activity of Cassia occidentalis seeds in albino rats. J. Nat. Pharm. 2011, 2, 88–91. [Google Scholar] [CrossRef]
- Ekwueme, F.N.; Oje, O.A.; Nwodo, O.F.C.; Ozoemena, N.F. Anti-inflammatory capacity of the aqueous leaf extract of Senna mimosoides on inhibition of rat oedema, platelet aggregatory activity and prostaglandin synthase activity. J. Med. Plants Res. 2011, 5, 3028–3036. [Google Scholar]
- Ntandou, N.G.F.; Banzouzi, J.T.; Mbatchi, B.; Elion-Itou, R.D.; Etou-Ossibi, A.W.; Ramos, S.; Benoit-Vical, F.; Abena, A.A.; Ouamba, J.M. Analgesic and anti-inflammatory effects of Cassia siamea Lam. stem bark extracts. J. Ethnopharmacol. 2009, 127, 108–111. [Google Scholar]
- Balunas, M.J.; Kinghorn, D.A. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R.S. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar]
- Perez, R.M. Anti-inflammatory activity of compounds isolated from plants. Sci. World J. 2001, 29, 713–784. [Google Scholar] [CrossRef]
- Marín, C.J.; Nieto, A.; Cespedes, C.L. Antioxidant and anti-inflammatory activities of Pittocaulon species from Mexico. Pharm. Biol. 2013, 51, 260–266. [Google Scholar] [CrossRef]
- Bautista, A.; Amaud, M.R.; Martínez, G.A.; Sánchez, P.S.; Pacheco, P. The traditional medicinal and food uses of four plants in Oaxaca, Mexico. J. Med. Plants Res. 2011, 5, 3404–3411. [Google Scholar]
- Flora de la Península de Yucatán. Available online: http://www.cicy.mx/sitios/flora%20digital/ (accessed on 13 July 2014).
- Flores, J.S. Leguminosae, florística, etnobotánica y ecología. In Etnoflora Yucatense: Fascículo 18; Faculty of Veterninary Medicine and Animal Science-Univesity of Yucatan: Mérida, México, 2001. [Google Scholar]
- Mena, J.G.; Pech, S.G.; Brito, L. Anthraquinones from Senna villosa Mill. Rev. Latinoam. Quím 1997, 25, 128–131. [Google Scholar]
- Polanco, G.; Escalante, F.; García, K.; Acosta, V.K.; Chan, M.J.; Sagua, H.; González, J.; Osorio, L.; Moo, R.E.; Peña, L.M. In vitro and in vivo trypanocidal of native plants from the Yucatan Peninsula. Parasitol. Res. 2012, 110, 31–35. [Google Scholar] [CrossRef]
- Jiménez, M.; Guzmán, E.S.; Pérez, M.S.; Polanco, G.; Acosta, K. Antitrypanosomal activity of Senna villosa in infected BALB/c mice with Tripanosoma cruzi during the sub acute phase of infection. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 164–169. [Google Scholar]
- Jiménez, M.; Acosta, K.; Pérez, M.S.; Guzmán, E.S. In vivo activity of (8-hydroxymethylen)-treicosanyl acetate againts Trypanosoma cruzi during acute phase of the infection. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 198–207. [Google Scholar]
- Jiménez, M.; Acosta, K.; Guzmán, E.S.; Pérez, C.; Pérez, M.S. Anti-trypanosomal activity of (8-hydroxymethylen)-treicosanyl acetate against infective forms of Trypanosoma cruzy. Pharm. Biol. 2010, 48, 666–671. [Google Scholar] [CrossRef]
- Guzmán, E.S.; Pérez, C.; Zavala, M.A.; Pérez, M.S. Antiprotozoal activity of (8-hydroxymethylen)-treicosanyl acetate isolated from Senna villosa. Phytomedicine 2008, 15, 892–895. [Google Scholar] [CrossRef]
- Calzada, F.; Yépez, M.; Aguilar, A. In vivo susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J. Ethnopharmacol. 2006, 108, 367–370. [Google Scholar] [CrossRef]
- Trepicchio, W.L.; Ozawa, M.; Walters, I.B.; Kikuchi, T.; Gilleaudeau, P.; Bliss, J.L.; Schwertschlag, U.; Dorner, A.J.; Krueger, J.G. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J. Clin. Invest. 1999, 104, 1527–1537. [Google Scholar] [CrossRef]
- Van Arman, C.G. Anti-inflammatory drugs. Clin. Phamacol. Ther. 1974, 16, 900–904. [Google Scholar]
- Young, J.M.; Spires, D.A.; Bedord, C.J.; Wagner, B.; Ballaron, S.J.; de Young, L.M. The mouse ear inflammatory response to topical arachidonic acid. J. Invest. Dermatol. 1984, 82, 367–371. [Google Scholar]
- Young, J.M.; Wagner, B.M.; Spires, D.A. Tachyphylaxis in 12-O-tetradecanoylphorbol acetate- and arachidonic acid-induced ear edema. J. Invest. Dermatol. 1983, 80, 48–52. [Google Scholar] [CrossRef]
- Carlson, R.P.; O’Neill-Davis, L.; Chang, J.; Lewis, A.J. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase and inhibitors and other pharmacological agents. Agents Actions 1985, 17, 197–204. [Google Scholar]
- De Young, L.M.; Kheifets, J.B.; Ballaron, S.J.; Young, J.M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 1989, 26, 335–341. [Google Scholar] [CrossRef]
- Kotyuk, B.; Raychaudhuri, A.; DiPasquale, G. Effect of anti-inflammatory compounds on edema formation and myeloperoxidase activity in the arachidonic acid-induced ear model in the mouse. Agents Actions 1993, 39, C46–C48. [Google Scholar] [CrossRef]
- Rao, T.S.; Currie, J.L.; Schaffer, A.F.; Isakson, P.C. Comparative evaluation of arachidonic acid (aa)-and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation 1993, 17, 723–741. [Google Scholar] [CrossRef]
- Tramposch, K.M. Skin inflamation. In In Vivo Models of Inflammation; Morgan, D.W., Marshall, L.A., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 179–204. [Google Scholar]
- Crowle, A.J. Delayed hypersensitive in the mouse. Adv. Immunol. 1975, 20, 197–264. [Google Scholar] [CrossRef]
- Garrigue, J.L.; Nicolas, J.F.; Fraginals, R.; Benezra, C.; Bour, H.; Schmitt, D. Optimization of the mouse ear swelling test for in vivo and in vitro studies of weak contact sensitizers. Contact Dermat. 1994, 30, 231–237. [Google Scholar] [CrossRef]
- Saint-Mezard, P.; Krasteva, M.; Chavagnac, C.; Bosset, S.; Akiba, H.; Kehren, J.; Kanitakis, J. Afferent and efferent phases of allergic contact dermatitis (ACD) can be induced after a single skin contact with haptens: Evidence using a mouse model of primary ACD. J. Invest. Dermatol. 2003, 120, 641–647. [Google Scholar] [CrossRef]
- Gábor, M. Models of acute inflammation in the ear. Methods Mol. Biol. 2003, 225, 129–137. [Google Scholar]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunop. 2000, 88, 1–12. [Google Scholar] [CrossRef]
- Liu, W.S.; Heckman, C.A. The sevenfold way of PKC regulation. Cell. Signal. 1998, 10, 529–542. [Google Scholar] [CrossRef]
- Loret, S.; Moreno, J.J. Effects of an anti-inflammatory peptide (Antiflammin 2) on cell influx, eicosanoid biosynthesis and oedema formation by arachidonic acid and tetradecanoyl phorbol dermal application. Biochem. Pharmacol. 1995, 50, 347–353. [Google Scholar] [CrossRef]
- Bradley, P.B.; Pribat, D.A.; Christensen, R.O.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Griffiths, R.J.; Wood, B.E.; Li, S.; Blackham, A. Pharmacological modification of 12-O-tetradecanoylphorbol-13-acetate induced inflammation and epidermal cell proliferation in mouse skin. Agents Actions 1988, 25, 344–351. [Google Scholar] [CrossRef]
- Reynolds, N.J.; McCombie, S.W.; Shankar, B.B.; Bishop, W.R.; Fisher, G.J. SCH 47112, a novel staurosporine derivative, inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and epidermal hyperplasia in hairless mouse skin. Arch. Dermatol. Res. 1997, 289, 540–546. [Google Scholar] [CrossRef]
- Wang, H.Q.; Smart, R.C. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J. Cell Sci. 1999, 112, 3497–506. [Google Scholar]
- Jansen, A.P.; Dreckschmidt, N.E.; Verwiebe, E.G.; Wheeler, D.L.; Oberley, T.D.; Verma, A.K. Relation of the induction of epidermal ornithine decarboxylase and hyperplasia to the different skin tumor-promotion susceptibilities of protein kinase C α, -δ and -ε transgenic mice. Int. J. Cancer 2001, 95, 635–643. [Google Scholar]
- Hara, T.; Saito, Y.; Hirai, T.; Nakamura, K.; Nakamura, K.; Nakao, K.; Katsuki, M.; Chida, K. Deficiency of protein kinase Calpha in mice results in impairment of epidermal hyperplasia and enhancement of tumor formation in two-stage skin carcinogenesis. Cancer Res. 2005, 65, 7356–7362. [Google Scholar] [CrossRef]
- Jetten, A.M.; George, M.A.; Pettit, G.R.; Herald, C.L.; Rearick, J.I. Action of phorbol esters, bryostatins, and retinoic acid on cholesterol sulfate synthesis: Relation to the multistep process of differentiation in human epidermal keratinocytes. J. Invest. Dermatol. 1989, 93, 108–115. [Google Scholar]
- Dlugosz, A.A.; Yuspa, S.H. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J. Cell Biol. 1993, 120, 217–225. [Google Scholar] [CrossRef]
- Wilmer, J.L.; Burleson, F.G.; Kayama, F.; Kanno, J.; Luster, M.I. Cytokine induction in human epidermal keratinocytes exposed to contact irritants and its relation to chemical-induced inflammation in mouse skin. J. Invest. Dermatol. 1994, 102, 915–922. [Google Scholar] [CrossRef]
- Redondo, P.; García, J.; Espana, A.; Cuevillas, F.; Quintanilla, E. Differencial modulation of IL-8 and TNF-alpha expression in keratinocytes by buflomedil chlorhydrate and pentoxifylline. Exp. Dermatol. 1997, 6, 186–194. [Google Scholar] [CrossRef]
- Murakawa, M.; Yamaoka, K.; Tanaka, Y.; Fukuda, Y. Involvement of tumornecrosis factor (TNF)-alpha in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem. Pharmacol. 2006, 71, 1331–1336. [Google Scholar] [CrossRef]
- Chan, R.A. Búsqueda de compuestos con actividad antitripanosoma en las hojas de Senna villosa (Miller) H.S. Irwing & Barneby. Master’s Thesis, Universidad Autónoma de Yucatán, Yucatán, México, 2008. [Google Scholar]
- Baquedano, M.A. Evaluación citotóxica y determinación del perfil de los ácidos grasos del aceite extraido de las semillas de Senna villosa (Miller) H.S. Irwing & Barneby. Master’s Thesis, Universidad Autónoma de Yucatán, Yucatán, México, 1999. [Google Scholar]
- Narasimham, B.; Mourya, V.; Dhake, A. Design, synthesis, antibacterial, and QSAR studies of myristic acid derivates. Bioorg. Med. Chem. Lett. 2006, 16, 3023–3029. [Google Scholar] [CrossRef]
- NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Available online: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 13 July 2014).
- Bauer, D.J. Métodos y Diagnósticos Del Laboratorio Clínico, 8th ed.; Médica Panamericana: Buenos Aires, Argentina, 1983; pp. 581–597. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susunaga-Notario, A.D.C.; Pérez-Gutiérrez, S.; Zavala-Sánchez, M.Á.; Almanza-Pérez, J.C.; Gutiérrez-Carrillo, A.; Arrieta-Báez, D.; López-López, A.L.; Román-Ramos, R.; Flores-Sáenz, J.L.E.; Alarcón-Aguilar, F.J. Bioassay-Guided Chemical Study of the Anti-Inflammatory Effect of Senna villosa (Miller) H.S. Irwin & Barneby (Leguminosae) in TPA-Induced Ear Edema. Molecules 2014, 19, 10261-10278. https://doi.org/10.3390/molecules190710261
Susunaga-Notario ADC, Pérez-Gutiérrez S, Zavala-Sánchez MÁ, Almanza-Pérez JC, Gutiérrez-Carrillo A, Arrieta-Báez D, López-López AL, Román-Ramos R, Flores-Sáenz JLE, Alarcón-Aguilar FJ. Bioassay-Guided Chemical Study of the Anti-Inflammatory Effect of Senna villosa (Miller) H.S. Irwin & Barneby (Leguminosae) in TPA-Induced Ear Edema. Molecules. 2014; 19(7):10261-10278. https://doi.org/10.3390/molecules190710261
Chicago/Turabian StyleSusunaga-Notario, Ana Del Carmen, Salud Pérez-Gutiérrez, Miguel Ángel Zavala-Sánchez, Julio Cesar Almanza-Pérez, Atilano Gutiérrez-Carrillo, Daniel Arrieta-Báez, Ana Laura López-López, Rubén Román-Ramos, José Luis Eduardo Flores-Sáenz, and Francisco Javier Alarcón-Aguilar. 2014. "Bioassay-Guided Chemical Study of the Anti-Inflammatory Effect of Senna villosa (Miller) H.S. Irwin & Barneby (Leguminosae) in TPA-Induced Ear Edema" Molecules 19, no. 7: 10261-10278. https://doi.org/10.3390/molecules190710261
APA StyleSusunaga-Notario, A. D. C., Pérez-Gutiérrez, S., Zavala-Sánchez, M. Á., Almanza-Pérez, J. C., Gutiérrez-Carrillo, A., Arrieta-Báez, D., López-López, A. L., Román-Ramos, R., Flores-Sáenz, J. L. E., & Alarcón-Aguilar, F. J. (2014). Bioassay-Guided Chemical Study of the Anti-Inflammatory Effect of Senna villosa (Miller) H.S. Irwin & Barneby (Leguminosae) in TPA-Induced Ear Edema. Molecules, 19(7), 10261-10278. https://doi.org/10.3390/molecules190710261