Jasmonic Acid Effect on the Fatty Acid and Terpenoid Indole Alkaloid Accumulation in Cell Suspension Cultures of Catharanthus roseus
Abstract
:1. Introduction
| Systematic Name | Common Name | Formula |
|---|---|---|
| Decanoic acid | Capric acid | C10:0 |
| Dodecanoic acid | Lauric acid | C12:0 |
| Tetradecanoic acid | Myristic acid | C14:0 |
| ∆-9-Tetradecanoic acid | Myristoleic acid | C14:1 |
| Pentadecanoic acid | Pentadecanoic acid | C15:0 |
| Hexadecanoic acid | Palmitic acid | C16:0 |
| ∆-9-Hexadecanoic acid | Palmitoleic acid | C16:1 |
| Heptadecanoic acid | Heptadecanoic acid | C17:0 |
| Octadecanoic acid | Stearic acid | C18:0 |
| ∆-9-Octadecanoic acid | Oleic acid | C18:1n9c |
| ∆-9,12-Octadecadienoic acid | Linoleic acid | C18:2 |
| ∆-9,12,15-Octadecatrienoic acid | α-Linolenic acid | C18:3n3 |
| Eicosanoic acid | Arachidic acid | C20:0 |
| Heneicosanoic acid | Heneicosanoic acid | C21:0 |
| Docosanoic acid | Behenic acid | C22:0 |
| Tetracosanoic acid | Lignoceric acid | C24:0 |
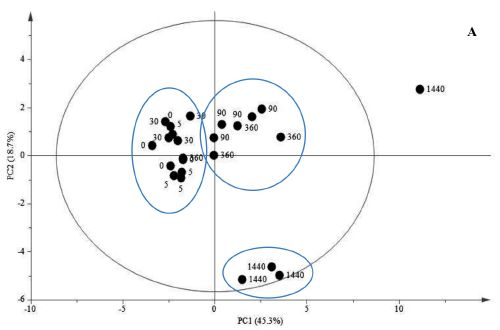
2. Results and Discussion
2.1. JA Induces the Biosynthesis of Its Own Precursor, C18:3
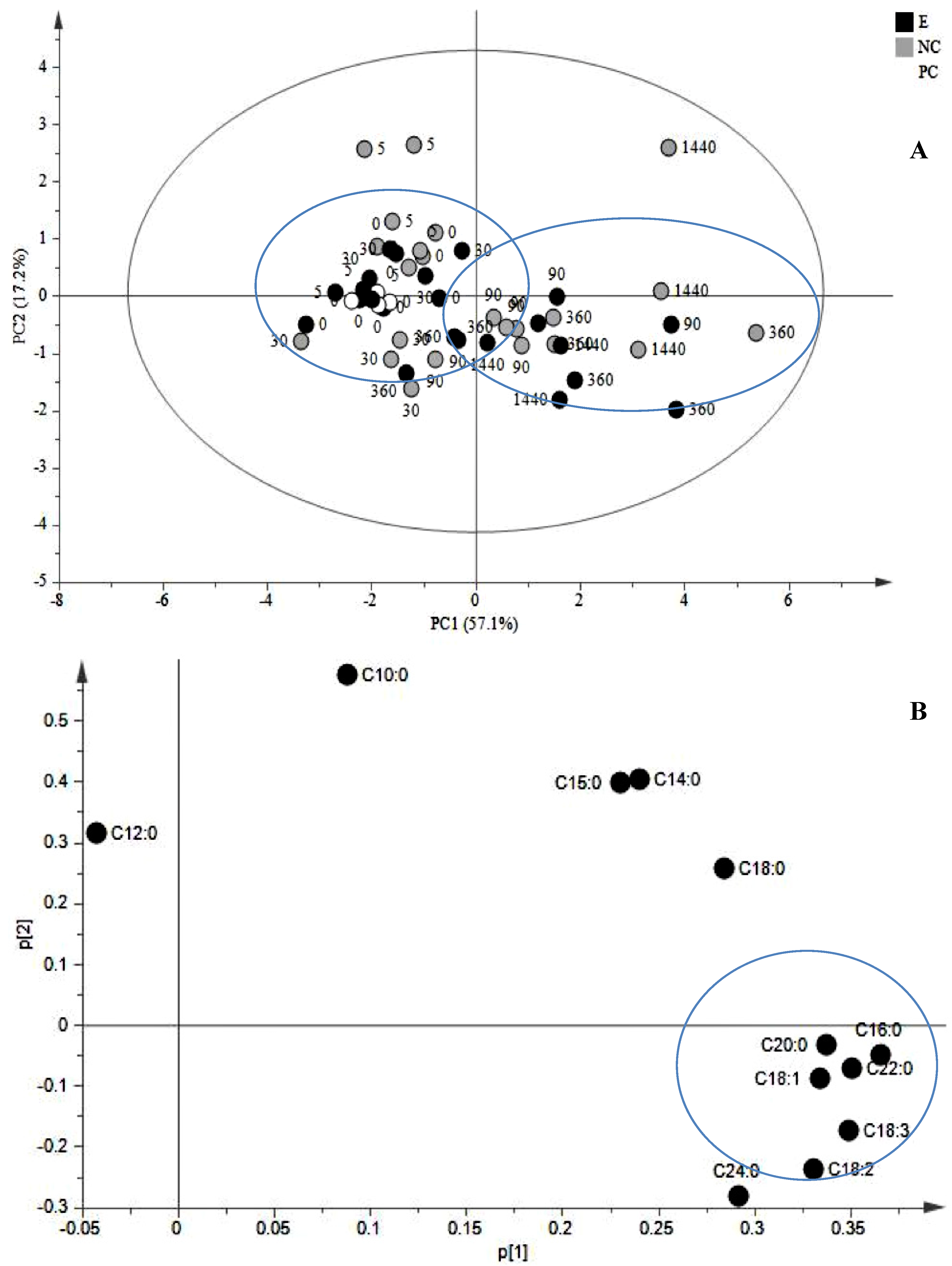
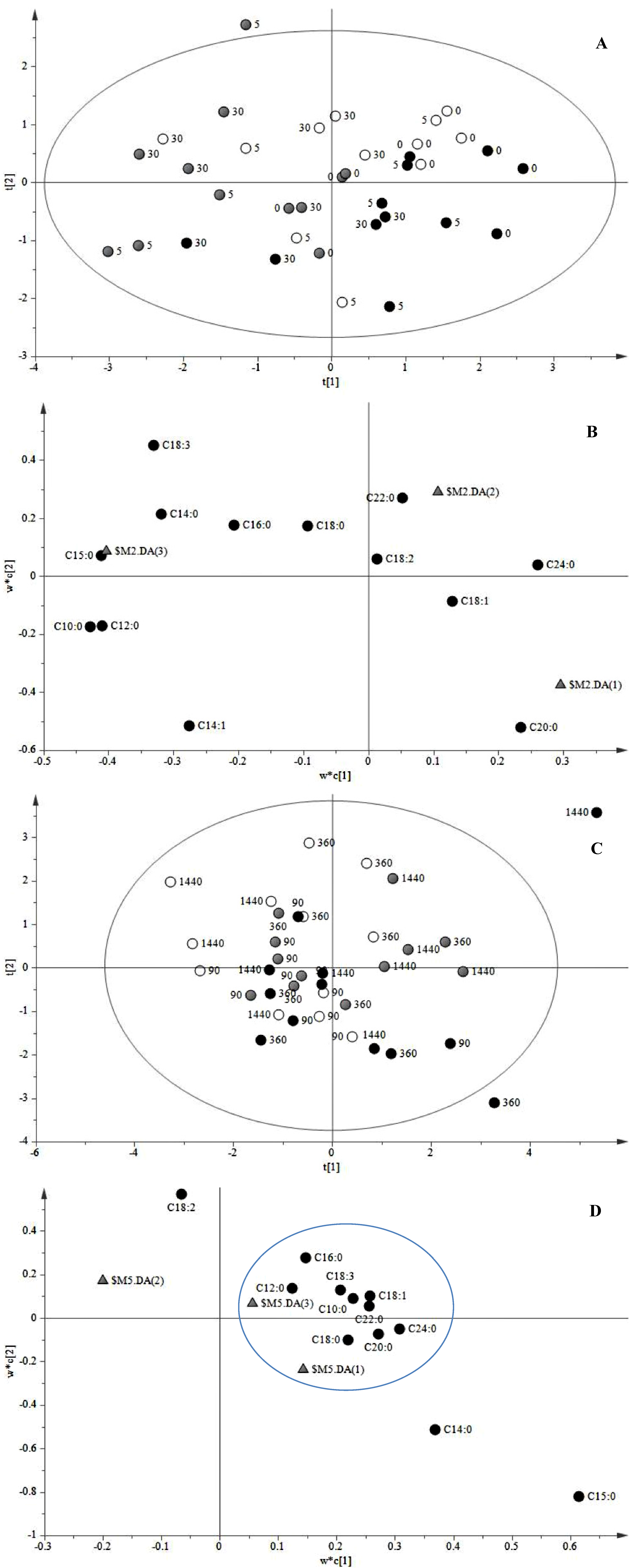
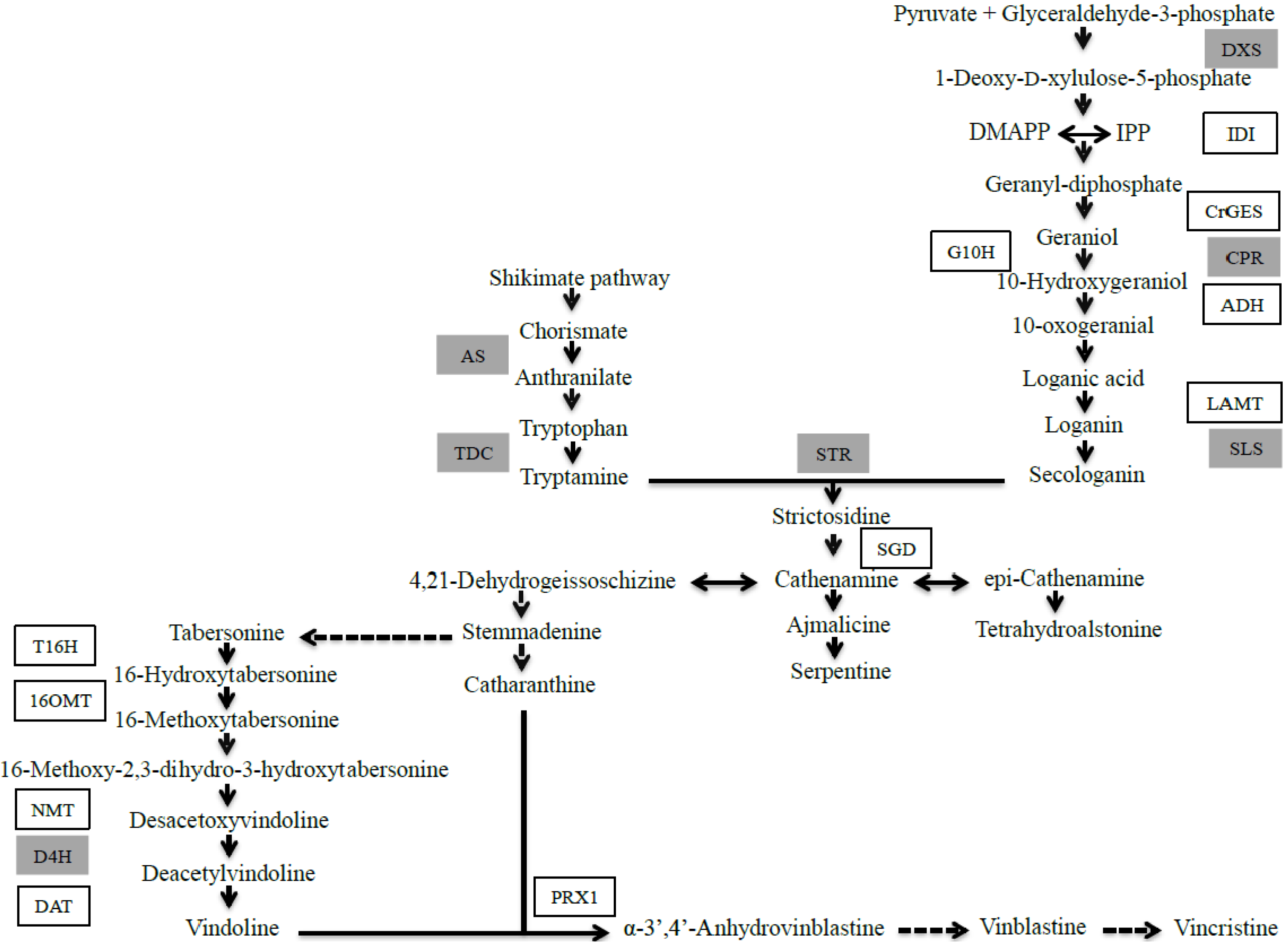
2.1.1. Effect of JA on TIA Accumulation in Cell Suspensions of C. roseus
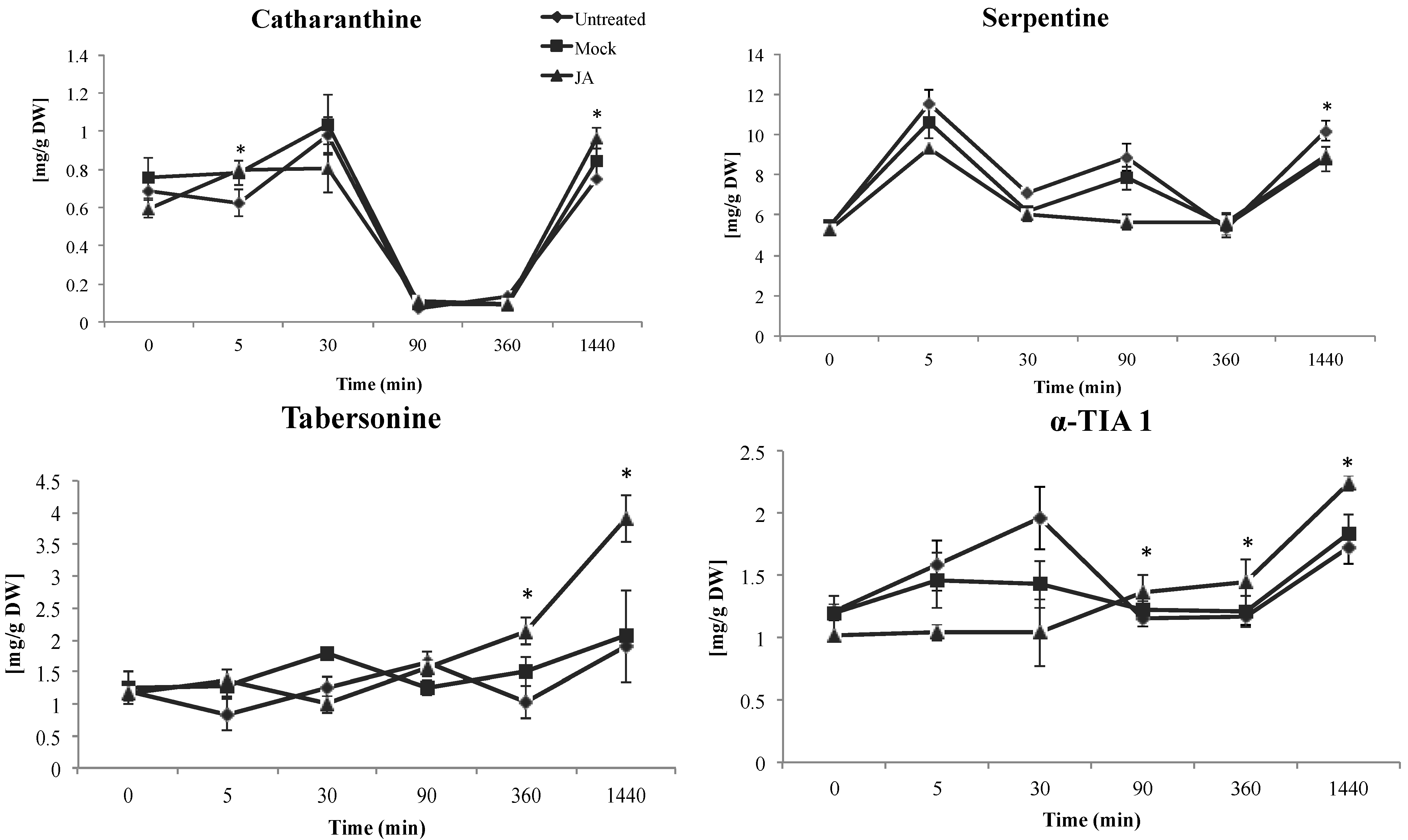
2.1.2. Late Events in the JA Stress Response
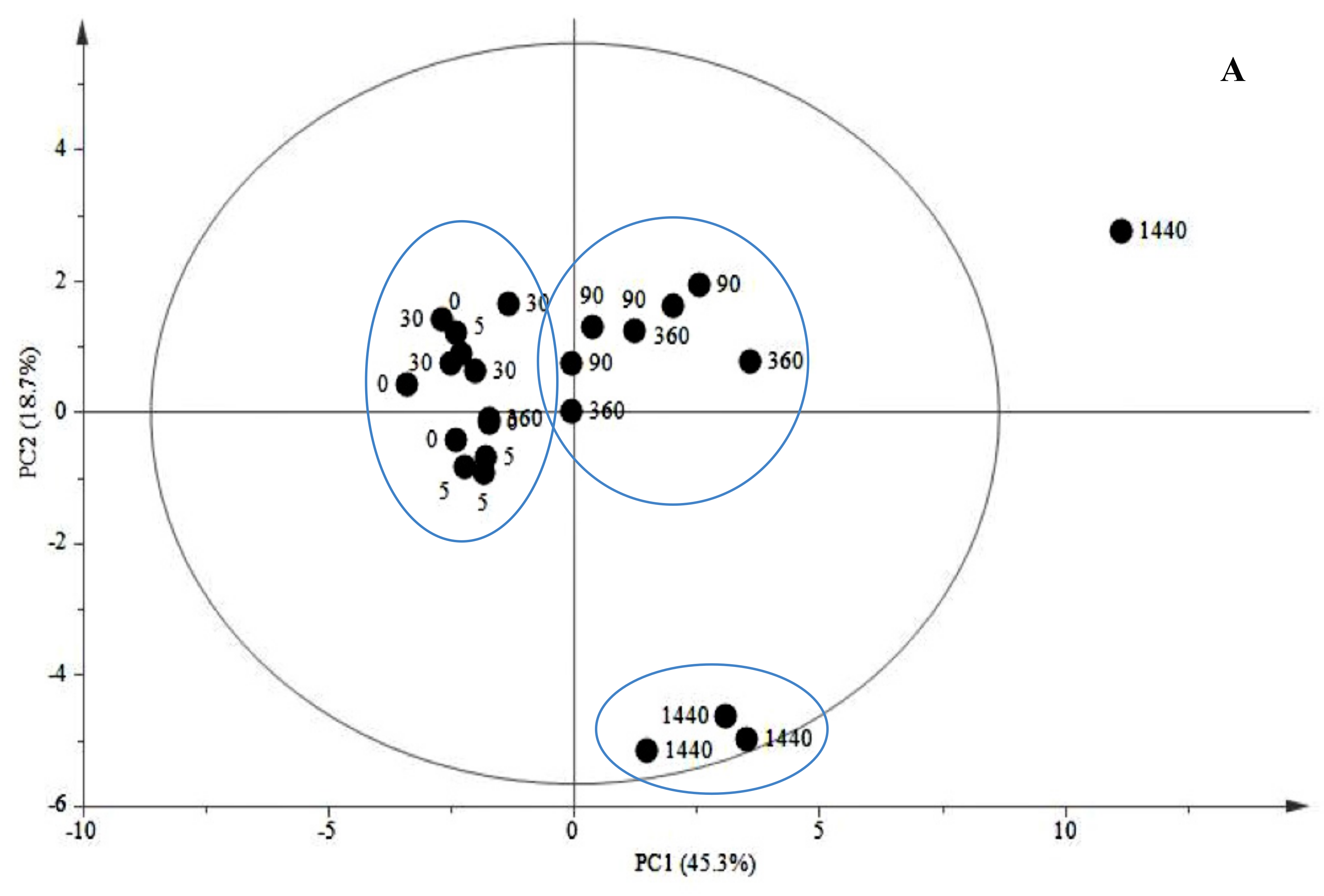
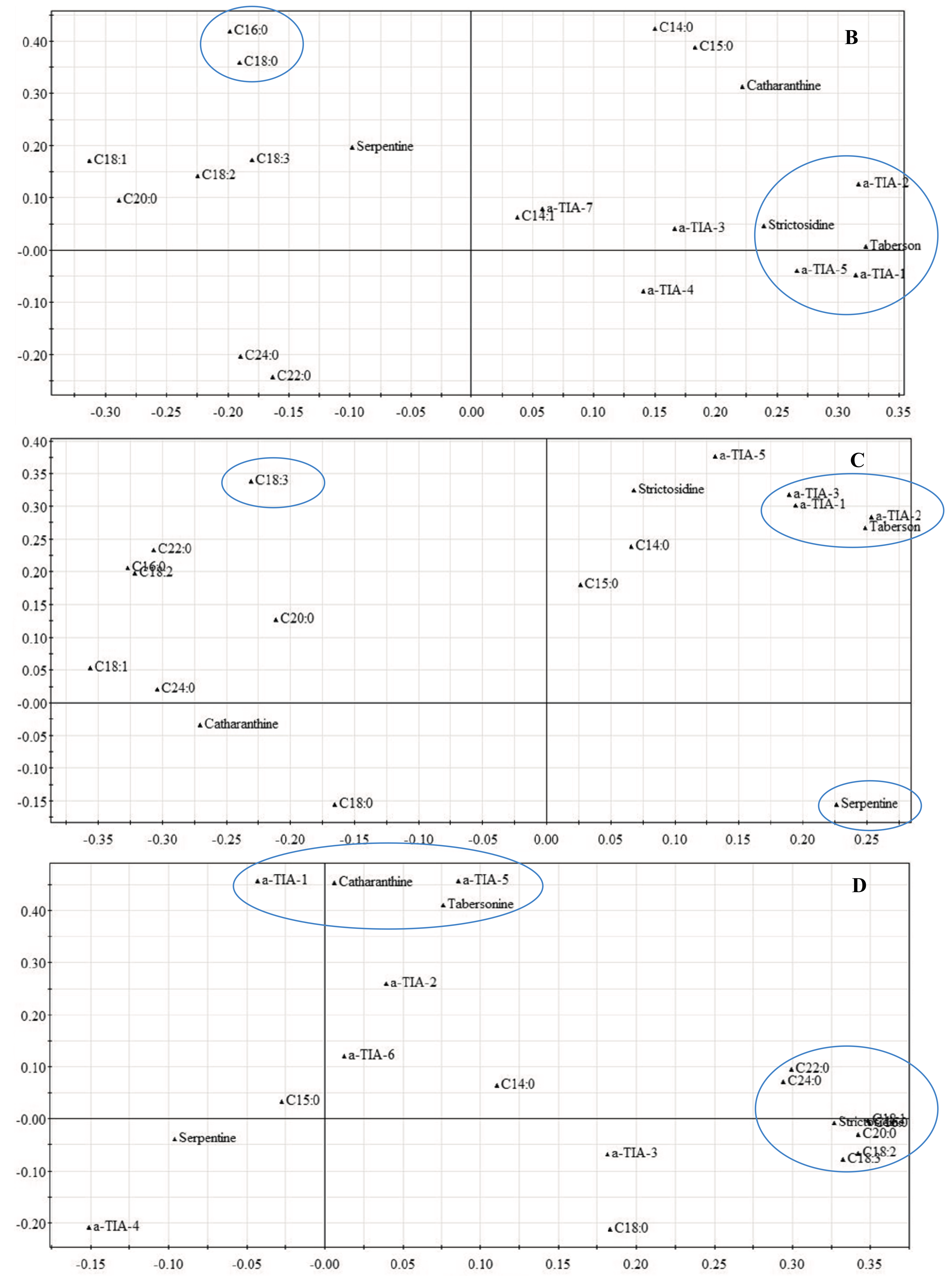
3. Experimental Section
3.1. Cell Cultures and Elicitation with Jasmonic Acid
3.2. Chemicals Used for Cell Suspension Cultures
3.3. Chemicals Used for Fatty Acid Determination and Alkaloid Standards
3.4. Fatty Acid Extraction
3.5. Gas Chromatography-Mass Spectrometry
3.6. Alkaloid and Precursor Extraction for HPLC
3.7. HPLC Analysis
3.8. Data Handling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Millar, A.A.; Smith, M.A.; Kunst, L. All fatty acids are not equal: Discrimination in plant membrane lipids. Trends Plant. Sci. 2000, 5, 95–101. [Google Scholar] [CrossRef]
- Kachroo, A.; Kachroo, P. Fatty acid-derived signals in plant defense. Ann. Rev. Phytopathol. 2009, 47, 153–176. [Google Scholar] [CrossRef]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionary conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3191–3205. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Ann. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- MacCarthy, J.J.; Stumpf, P.K. The effect of different temperatures on fatty-acid synthesis and polyunsaturation in cell suspension cultures. Planta 1980, 147, 389–395. [Google Scholar] [CrossRef]
- Kazemi Shahandashti, S.S.; Maali Amiri, R.; Zeinali, H.; Sanaz Ramezanpour, S.S. Change in membrane fatty acid compositions and cold-induced responses in chickpea. Mol. Biol. Rep. 2013, 40, 893–903. [Google Scholar] [CrossRef]
- Elkahoui, S.; Smaoui, A.; Zarrouk, M.; Ghir, R.; Limam, F. Salt-induced lipid changes in Catharanthus roseus cultured cell suspensions. Phytochemistry 2004, 65, 1911–1917. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Ann. Rev. Plant Physiol. Plant. Mol. Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates and octadecanoids: Signals in plant stress responses and development. Prog. Nucleic Acid. Res. Mol. Biol. 2002, 72, 165–221. [Google Scholar] [CrossRef]
- Wasternack, C.; Forner, S.; Strnad, M.; Hause, B. Jasmonates in flower and seed development. Biochimie 2013, 95, 79–85. [Google Scholar] [CrossRef]
- De Domenico, S.; Bonsegna, S.; Horres, R.; Pastor, V.; Taurino, M.; Poltronieri, P.; Imtiaz, M.; Kahl, G.; Flors, V.; Winter, P.; et al. Transcriptomic analysis of oxylipins biosynthesis genes and chemical profiling reveal early induction of jasmonates in chickpea roots under drought stress. Plant. Physiol. Biochem. 2012, 61, 115–122. [Google Scholar] [CrossRef]
- Collu, G.; Unver, N.; Peltenburg-Looman, A.M.G.; van der Heijden, R.; Verpoorte, R.; Memelink, J. Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett. 2001, 508, 215–220. [Google Scholar] [CrossRef]
- Lee-Parsons, C.W.T.; Ertürk, S.; Tengtrakool, J. Enhancement of ajmalicine production in Catharanthus roseus cell cultures with methyl jasmonate is dependent on timing and dosage of elicitation. Biotechnol. Lett. 2004, 0, 1595–1599. [Google Scholar]
- Li, C.Y.; Leopold, A.L.; Sander, G.W.; Shanks, J.V.; Zhao, L.; Gibson, S.I. The ORCA2 transcription factor plays a key role in regulation of the terpenoid indole alkaloid pathway. BMC Plant. Biol. 2013, 13, 155. [Google Scholar] [CrossRef]
- Memelink, J.; Verpoorte, R.; Kijne, J.W. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant. Sci. 2001, 6, 212–219. [Google Scholar] [CrossRef]
- Wei, S. Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthus roseus seedlings. Plant Growth Reg. 2010, 61, 243–251. [Google Scholar]
- Moreno, P.R.H.; Poulsen, C.; van der Heijden, R.; Verpoorte, R. Effects of elicitation on different metabolic pathways in Catharanthus roseus (L.) G. Don cell suspension cultures. Enz. Microb. Technol. 1996, 18, 99–107. [Google Scholar] [CrossRef]
- Vázquez-Flota, F.; Hernández-Domínguez, E.; Miranda-Ham, M.L.; Monforte-González, M. A differential response to chemical elicitors in Catharanthus roseus in vitro cultures. Biotechnol. Lett. 2009, 31, 591–595. [Google Scholar]
- Glauser, G.; Dubugnon, L.; Mousavi, S.A.R.; Rudaz, S.; Wolfender, J.L.; Farmer, E.E. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J. Biol. Chem. 2009, 284, 34506–34513. [Google Scholar]
- Simkin, A.J.; Miettinen, K.; Claudel, P.; Burlat, V.; Guirimand, G.; Courdavault, V.; Papon, N.; Meyer, S.; Godet, S.; St-Pierre, B.; et al. Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 2013, 85, 36–43. [Google Scholar] [CrossRef]
- Toivonen, L.; Laakso, S.; Rosenqvist, H. The effect of temperature on growth, indole alkaloid accumulation and lipid composition of Catharanthus roseus cell suspension cultures. Plant Cell Rep. 1992, 11, 390–394. [Google Scholar]
- López, M.G.; Sánchez-Mendoza, I.R.; Ochoa-Alejo, N. Comparative study of volatile components and fatty acid of plants and in vitro cultures of parsley (Petroselinum crispum (Mill) Nym ex Hill. J. Agric. Food Chem. 1999, 47, 3292–3296. [Google Scholar] [CrossRef]
- MacCarthy, J.J.; Stumpf, P.K. Tissue culture of plants for studies of lipid metabolism. Methods Enzymol. 1981, 72, 754–768. [Google Scholar] [CrossRef]
- Radwan, S.S.; Mangold, H.K.; Spener, F. Lipids in plant tissue cultures. III. Very long-chain fatty acids in the lipids of callus cultures and suspension cultures. Chem. Phys. Lipids 1974, 13, 103–107. [Google Scholar] [CrossRef]
- Leathers, R.R.; Scragg, A.H. The effect of different temperatures on the growth, lipid content and fatty acid composition of Theobroma cacao cell suspension cultures. Plant Sci. 1989, 62, 217–227. [Google Scholar] [CrossRef]
- Pandey-Rai, S.; Rao Mallavarapu, G.; Naqvi, A.A.; Yadav, A.N.; Kumar Rai, S.; Srivastava, S.; Singh., D.; Mishra, R.; Kumar, S. Volatile components of leaves and flowers of periwinkle Catharanthus roseus (L.) Don from New Delhi. Flav. Fragr. J. 2006, 21, 427–430. [Google Scholar] [CrossRef]
- Brun, G.; Bessière, J.M.; Dijoux-Franca, M.G.; David, B.; Mariotte, A.M. Volatile components of Catharanthus roseus (L.) Don (Apocynaceae). Flav. Fragr. J. 2001, 16, 116–119. [Google Scholar] [CrossRef]
- Guedes De Pinho, P.; Gonçalves, R.F.; Valentão, P.; Pereira, D.M.; Seabra, R.M.; Andrade, P.B.; Sotomayor, M. Volatile composition of Catharanthus roseus (L.) Don using solid-phase microextraction and gas chromatography/mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 674–685. [Google Scholar] [CrossRef]
- Rezanka, T.; Sigler, K. Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog. Lipid Res. 2009, 48, 206–238. [Google Scholar] [CrossRef]
- Carriere, F.; Chagvardieff, P.; Gil, G.; Pean, M.; Sigoillot, J.C.; Tapie, P. Fatty acid patterns of neutral lipids from seeds, leaves and cell suspension cultures of Euphorbia characias. Phytochemistry 1992, 31, 2351–2353. [Google Scholar]
- Gemmrich, A.R.; Schraudolf, H. Fatty acid composition of lipids from differentiated tissues and cell cultures of Euonymus europaeus. Chem. Phys. Lipids 1980, 26, 259–264. [Google Scholar] [CrossRef]
- Hansen, C.E.; Rossi, P. Effects of culture condition on accumulation of arachidonic and eicosapentaenoic acids cultured cells of Rhytidiadelphus squarrosus and Eurhynchium striatum. Phytochemistry 1991, 30, 1837–1841. [Google Scholar] [CrossRef]
- Chiou, S.Y.; Su, W.W.; Su, Y.C. Optimizing production of polyunsaturated fatty acids in Marchantia polymorpha cell suspension culture. J. Biotechnol. 2001, 85, 247–257. [Google Scholar] [CrossRef]
- Miersch, O.; Neumerkel, J.; Dippe, M.; Stenzel, I.; Wasternack, C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 2008, 177, 114–127. [Google Scholar]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar]
- Conconi, A.; Miquel, M.; Browse, J.A.; Ryan, C.A. Intracellular levels of free linolenic and linoleic acids increase in tomato leaves in response to wounding. Plant. Physiol. 1996, 111, 797–803. [Google Scholar]
- Mueller, M.J.; Brodschelm, W.; Spannagl, E.; Zenk, M.H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. USA 1993, 90, 7490–7494. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Chakrabarty, D.; Wu, C.H.; Hahn, E.J.; Jeon, W.K.; Paek, K.Y. Influences of polyunsaturated fatty acids (PUFAs) on growth and secondary metabolite accumulation in Panax ginseng C.A. Meyer adventitious roots cultures in air-lift bioreactors. South Afr. J. Bot. 2010, 76, 354–358. [Google Scholar]
- Wu, C.H.; Popova, E.V.; Hahn, E.J.; Paek, K.Y. Linolenic and α-linolenic fatty acids affect biomass and secondary metabolite production and nutritive properties of Panax. ginseng adventitious roots cultured in bioreactors. Biochem. Eng. J. 2009, 47, 109–115. [Google Scholar] [CrossRef]
- Gundlach, H.; Zenk, M.H. Biological activity and biosynthesis of pentacyclic oxylipins: The linoleic acid pathway. Phytochemistry 1998, 47, 527–537. [Google Scholar] [CrossRef]
- Véronési, C.; Pouénat, M.L.; Rickauer, M.; Esquerrê-Tugayé, M.T. Regulation of tobacco lipoxygenase by methyl jasmonate and fatty acids. Comp. Rend Acad. Sci. Series III Sci. Vie 1999, 322, 491–497. [Google Scholar]
- El-Sayed, M.; Verpoorte, R. Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tis. Org. Cult. 2002, 68, 265–270. [Google Scholar]
- Saiman, M.Z.; Mustafa, R.N.; Pomahacova, B.; Verbenne, M.; Verpoorte, R.; Choi, Y.H.; Schulte, A.E. Analysis of metabolites in the terpenoid pathway of Catharanthus roseus cell suspensions. Plant Cell Tissue Organ. Cult. 2014, 117, 225–239. [Google Scholar] [CrossRef]
- Endo, T.; Goodbody, A.; Misawa, M. Alkaloid production in root and shoot cultures of Catharanthus roseus. Planta Med. 1987, 53, 479–482. [Google Scholar] [CrossRef]
- Vázquez-Flota, F.; de Luca, V.; Carrillo-Pech, M.; Canto-Flick, A.; Miranda-Ham, M.L. Vindoline biosynthesis is transcriptionally blocked in Catharanthus roseus cell suspension cultures. Mol. Biotechnol. 2002, 22, 1–8. [Google Scholar] [CrossRef]
- Shukla, A.K.; Shasany, A.K.; Verma, R.K.; Gupta, M.M.; Mathur, A.K.; Khanuja, S.P.S. Influence of cellular differentiation and elicitation on intermediate and late steps of terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Protoplasma 2010, 242, 35–47. [Google Scholar] [CrossRef]
- Besseau, S.; Kellner, F.; Lanoue, A.; Thamm, A.M.K.; Salim, V.; Schneider, B.; Geu-Flores, F.; Höfer, R.; Guirimand, G.; Guihur, A.; et al. A pair of tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol. 2013, 163, 1792–1803. [Google Scholar] [CrossRef]
- Verpoorte, R.; van der Heijden, R.; Moreno, P.R.H. Biosynthesis of terpenoid indole alkaloids in Catharanthus roseus cells. In The Alkaloids; Cordell, G.A., Ed.; Academic Press: Waltham, MA, USA, 1997; Volume 49, pp. 221–299. [Google Scholar]
- Schröder, G.; Unterbusch, E.; Kaltenbach, M.; Schmidt, J.; Strack, D.; de Luca, V.; Schröder, J. Light-induced cytochrome P450-dependent enzyme in indole alkaloid biosynthesis: Tabersonine 16-hydroxylase. FEBS Lett. 1999, 458, 97–102. [Google Scholar]
- St-Pierre, B.; de Luca, V. A cytochrome P-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol. 1995, 109, 131–139. [Google Scholar]
- Gundlach, H.; Müeller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 1992, 89, 2389–2393. [Google Scholar]
- Bell, E.; Creelman, R.A.; Mullet, J.E. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1995, 92, 8675–8679. [Google Scholar]
- Chauvin, A.; Caldelari, D.; Wolfender, J.L.; Farmer, E.E. Four lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: A role for lipoxygenase 6 in response to long-distance wound signals. New Phytol. 2013, 197, 566–575. [Google Scholar] [CrossRef]
- Peebles, C.A.M.; Shanks, J.V.; San, K.Y. The role of the octadecanoid pathway in the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots under normal and UV-B stress conditions. Biotechnol. Bioeng. 2009, 103, 1248–1254. [Google Scholar]
- Lee-Parsons, C.W.T.; Ertürk, S. Ajmalicine production in methyl jasmonate-induced Catharanthus roseus cell cultures depends on Ca2+ level. Plant Cell Rep. 2005, 24, 677–682. [Google Scholar] [CrossRef]
- Van der Fits, L.; Zhang, H.; Menke, F.L.H.; Deneka, M.; Memelink, J. A Catharanthus roseus BPF-1 homologue interacts with an elicitor-responsive region of the secondary metabolite biosynthetic gene Str. and is induced by elicitor via a JA-dependent signal transduction pathway. Plant Mol. Biol. 2000, 44, 675–685. [Google Scholar] [CrossRef]
- Hisamatsu, Y.; Goto, N.; Hasegawa, K.; Shigemori, H. Arabidopsides A and B, tow new oxylipins from Arabidopsis thaliana. Tehtrah. Lett. 2003, 44, 5553–5556. [Google Scholar] [CrossRef]
- Hisamatsu, Y.; Goto, N.; Sekiguchi, M.; Hasegawa, K.; Shigemori, H. Oxylipins arabidopsides C and D from Arabidopsis thaliana. J. Nat. Prod. 2005, 68, 600–603. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Moreno, P.R.H.; van de Heijden, R.; Verpoorte, R. Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures or Catharanthus roseus. Plant Cell Rep. 1993, 12, 702–705. [Google Scholar]
- Tikhomiroff, C.; Jolicoeur, M. Screening of Catharanthus roseus secondary metabolites by high-performance liquid chromatography. J. Chromatogr. A 2002, 955, 87–93. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldhaber-Pasillas, G.D.; Mustafa, N.R.; Verpoorte, R. Jasmonic Acid Effect on the Fatty Acid and Terpenoid Indole Alkaloid Accumulation in Cell Suspension Cultures of Catharanthus roseus. Molecules 2014, 19, 10242-10260. https://doi.org/10.3390/molecules190710242
Goldhaber-Pasillas GD, Mustafa NR, Verpoorte R. Jasmonic Acid Effect on the Fatty Acid and Terpenoid Indole Alkaloid Accumulation in Cell Suspension Cultures of Catharanthus roseus. Molecules. 2014; 19(7):10242-10260. https://doi.org/10.3390/molecules190710242
Chicago/Turabian StyleGoldhaber-Pasillas, Guitele Dalia, Natali Rianika Mustafa, and Robert Verpoorte. 2014. "Jasmonic Acid Effect on the Fatty Acid and Terpenoid Indole Alkaloid Accumulation in Cell Suspension Cultures of Catharanthus roseus" Molecules 19, no. 7: 10242-10260. https://doi.org/10.3390/molecules190710242
APA StyleGoldhaber-Pasillas, G. D., Mustafa, N. R., & Verpoorte, R. (2014). Jasmonic Acid Effect on the Fatty Acid and Terpenoid Indole Alkaloid Accumulation in Cell Suspension Cultures of Catharanthus roseus. Molecules, 19(7), 10242-10260. https://doi.org/10.3390/molecules190710242