Design, Synthesis and Biological Evaluation of C(6)-Modified Celastrol Derivatives as Potential Antitumor Agents
Abstract
:1. Introduction
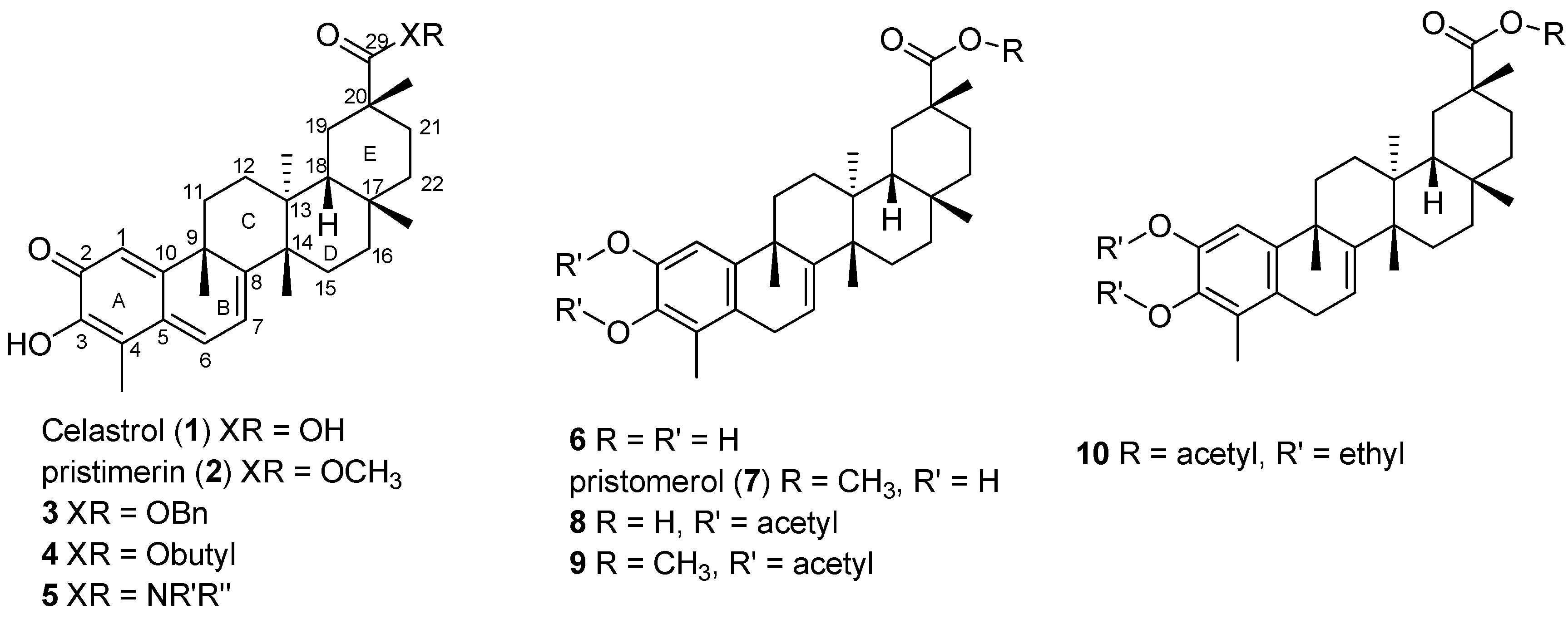
2. Results and Discussion
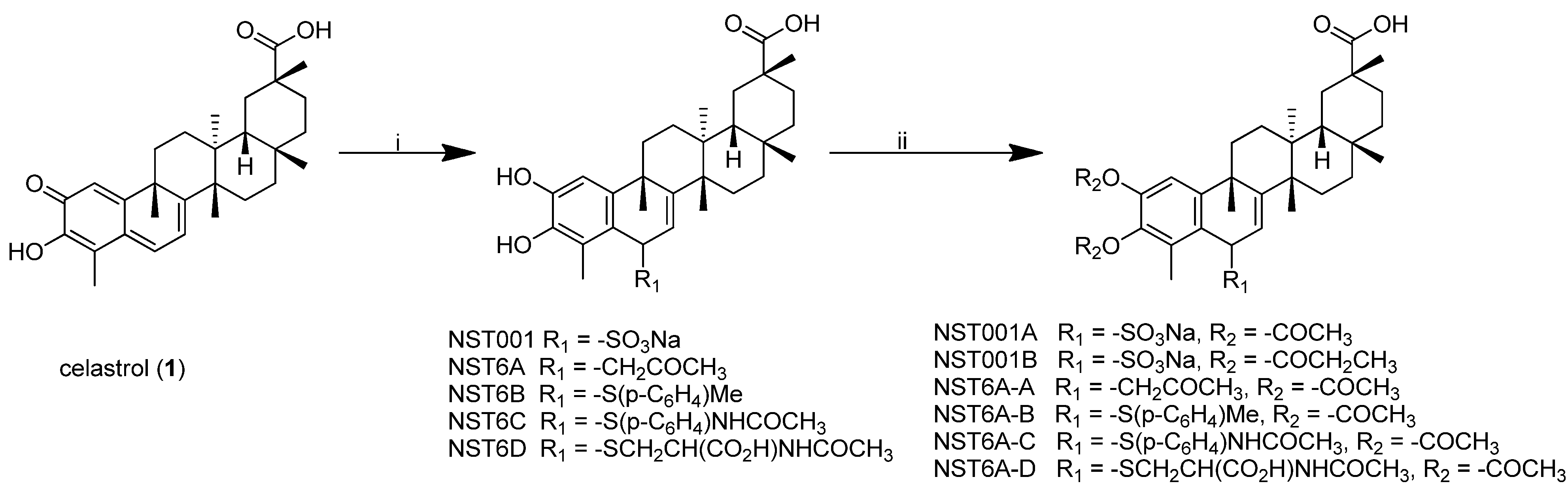
2.1. Synthesis of Celastrol C-6 Analogues (Scheme 1)
2.2. Activity of Celastrol Analogues against Human Cancer Cell Lines
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| BGC823 | H4 | Bel7402 | |
| Celastrol (1) | 3.73 | 2.09 | 1.55 |
| NST001 | 0.77 | 0.91 | 1.17 |
| NST001A | 0.49 | 1.37 | 1.73 |
| NST001B | 0.74 | 1.12 | 2.39 |
| NST6A-A | >150 | >150 | >150 |
| NST6A-B | 0.47 | 0.37 | 0.45 |
| NST6A-C | 0.42 | 0.35 | 0.46 |
| NST6A-D | 0.50 | 0.46 | 0.54 |
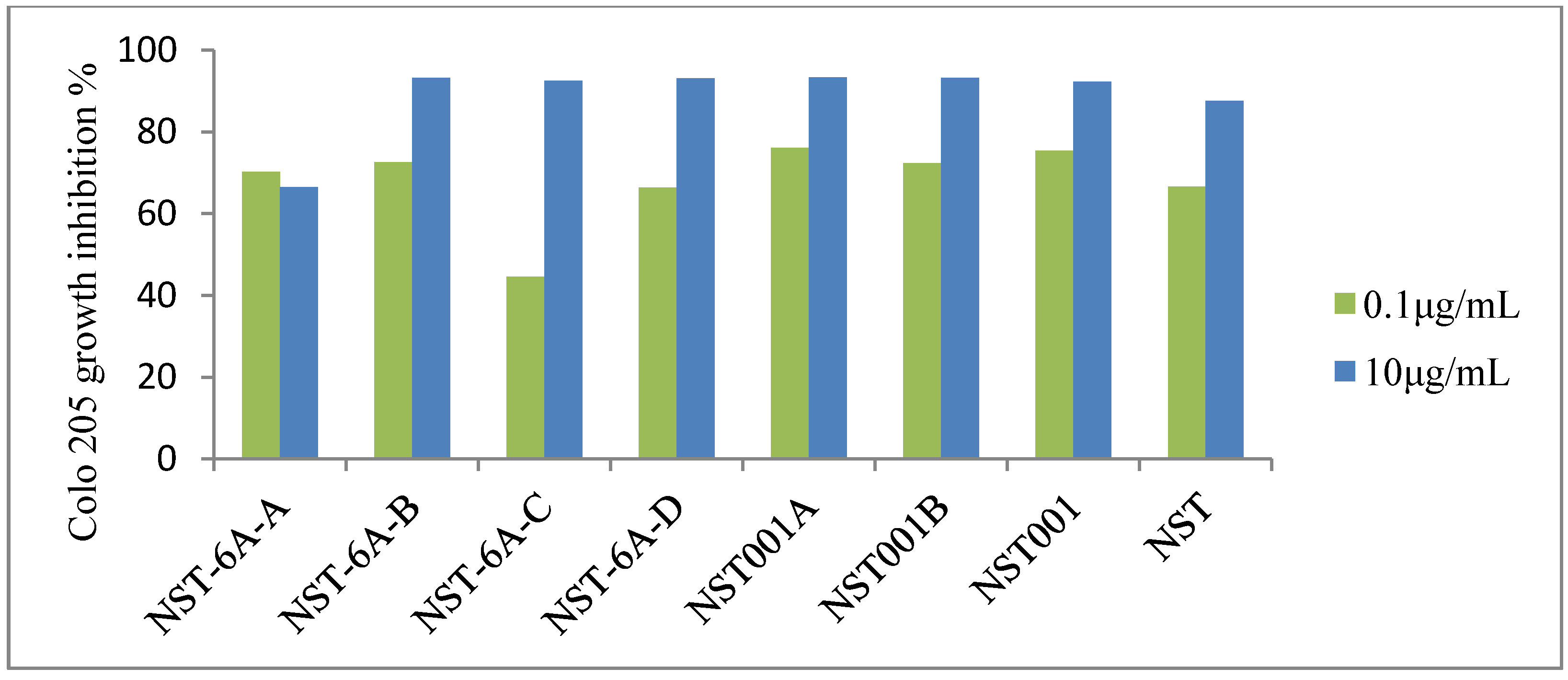
| Compounds | IC50 (μM) for 72 h | ||||
|---|---|---|---|---|---|
| H522 | Colo 205 | HepG2 | MDA-MB-468 | BGC823 | |
| NST001A | 0.39 | 0.06 | 0.29 | 0.33 | 1.53 |
| NST001 | 0.47 | 0.51 | 0.68 | 0.89 | 0.77 |
2.3. In Vivo Anti-Tumor Activity Evaluation
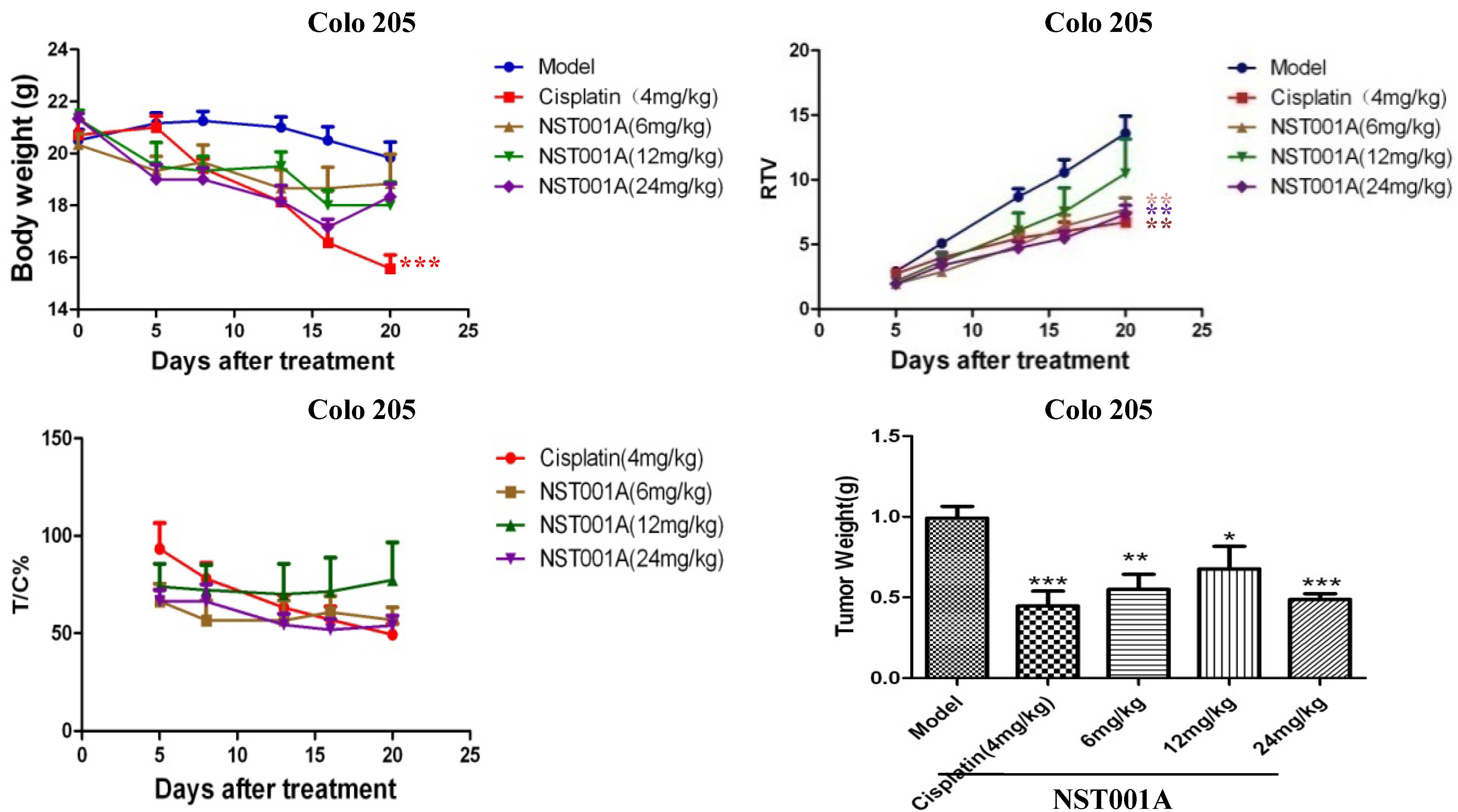
2.4. Preliminary Non-Clinical Safety Evaluation
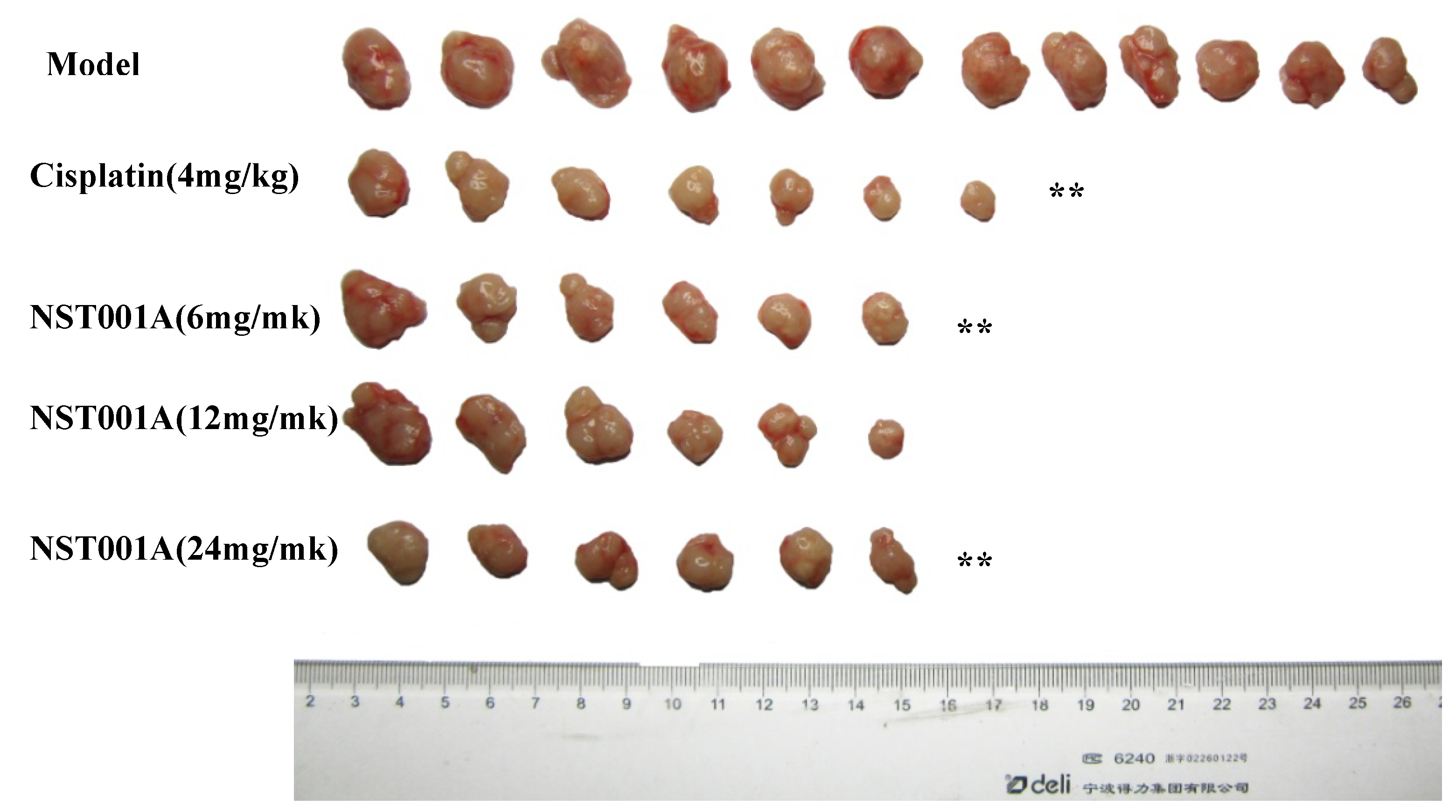
3. Experimental Section
3.1. General Information
3.2. Synthesis
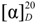 −94.76° (MeOH, c = 0.2). 1H-NMR (DMSO-d6): δ 7.01 (s, 1H), 5.83 (d, J = 6.1 Hz, 1H), 4.60 (d, J = 6.0 Hz, 1H), 2.50 (s, 1H), 2.29 (s, 2H), 2.24 (d, J = 6.7 Hz, 5H), 2.08 (s, 1H), 1.99 (dd, J = 23.8, 12.5 Hz, 3H), 1.83 (dd, J = 21.7, 15.3 Hz, 1H), 1.67 (s, 2H), 1.63–1.36 (m, 8H), 1.28 (s, 1H), 1.20 (s, 3H), 1.09 (s, 3H), 1.05 (s, 2H), 0.85 (d, J = 13.8 Hz, 1H), 0.61 (s, 2H). 13C-NMR (DMSO-d6): δ 179.9, 168.9, 168.7, 149.0, 148.6, 141.3, 138.5, 130.5, 129.8, 118.5, 117.3, 60.1, 56.5, 44.3, 44.1, 38.8, 37.8, 36.9, 36.6, 35.1, 34.4, 32.9, 31.9, 30.6, 30.5, 29.9, 29.1, 21.6, 20.9, 20.6, 19.0, 18.6, 13.8. IR (KBr) 3447.9, 2944.5, 2873.1, 1768.8, 1466.0, 1374.3, 1228.7, 1194.0, 1158.3, 1039.7 cm−1. LC-ESI-MS (−) m/z (%) = 615.41 [M−1Na+]− (100%), 1231.86 [2M−2Na+]2− (10%).
−94.76° (MeOH, c = 0.2). 1H-NMR (DMSO-d6): δ 7.01 (s, 1H), 5.83 (d, J = 6.1 Hz, 1H), 4.60 (d, J = 6.0 Hz, 1H), 2.50 (s, 1H), 2.29 (s, 2H), 2.24 (d, J = 6.7 Hz, 5H), 2.08 (s, 1H), 1.99 (dd, J = 23.8, 12.5 Hz, 3H), 1.83 (dd, J = 21.7, 15.3 Hz, 1H), 1.67 (s, 2H), 1.63–1.36 (m, 8H), 1.28 (s, 1H), 1.20 (s, 3H), 1.09 (s, 3H), 1.05 (s, 2H), 0.85 (d, J = 13.8 Hz, 1H), 0.61 (s, 2H). 13C-NMR (DMSO-d6): δ 179.9, 168.9, 168.7, 149.0, 148.6, 141.3, 138.5, 130.5, 129.8, 118.5, 117.3, 60.1, 56.5, 44.3, 44.1, 38.8, 37.8, 36.9, 36.6, 35.1, 34.4, 32.9, 31.9, 30.6, 30.5, 29.9, 29.1, 21.6, 20.9, 20.6, 19.0, 18.6, 13.8. IR (KBr) 3447.9, 2944.5, 2873.1, 1768.8, 1466.0, 1374.3, 1228.7, 1194.0, 1158.3, 1039.7 cm−1. LC-ESI-MS (−) m/z (%) = 615.41 [M−1Na+]− (100%), 1231.86 [2M−2Na+]2− (10%).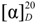 −98.21° (MeOH, c = 0.2). 1H-NMR (DMSO-d6): δ 7.01 (s, 1H), 5.83 (d, J = 6.2 Hz, 1H), 4.60 (d, J = 6.1 Hz, 1H), 2.66–2.45 (m, 3H), 2.36 (d, J = 14.7 Hz, 1H), 2.24 (s, 1H), 2.10 (d, J = 12.8 Hz, 1H), 2.06–1.93 (m, 1H), 1.82 (d, J = 5.7 Hz, 1H), 1.68 (s, 2H), 1.40 (d, J = 15.0 Hz, 4H), 1.22 (d, J = 14.1 Hz, 2H), 1.17–1.07 (m, 4H), 1.05 (s, 3H), 0.83 (d, J = 11.8 Hz, 1H), 0.64 (s, 2H). 13C-NMR (DMSO-d6): δ 180.6, 172.1, 171.9, 149.0, 148.7, 141.3, 138.3, 130.4, 129.7, 118.3, 117.2, 60.0, 56.3, 44.3, 44.0, 38.7, 37.8, 37.0, 36.7, 35.2, 34.5, 33.2, 32.6, 31.9, 30.6, 30.4, 30.2, 30.0, 29.1, 27.1, 26.9, 21.6, 18.5, 13.8, 9.6, 9.4. IR (KBr): 2969.5, 2944.6, 2873.1, 1762.0, 1559.5, 1457.3, 1155.4, 1040.6 cm−1. LC-ESI-MS (−) m/z (%) = 643.45 [M−1Na+]− (100%), 1288.09 [2M−2Na+]2− (5%).
−98.21° (MeOH, c = 0.2). 1H-NMR (DMSO-d6): δ 7.01 (s, 1H), 5.83 (d, J = 6.2 Hz, 1H), 4.60 (d, J = 6.1 Hz, 1H), 2.66–2.45 (m, 3H), 2.36 (d, J = 14.7 Hz, 1H), 2.24 (s, 1H), 2.10 (d, J = 12.8 Hz, 1H), 2.06–1.93 (m, 1H), 1.82 (d, J = 5.7 Hz, 1H), 1.68 (s, 2H), 1.40 (d, J = 15.0 Hz, 4H), 1.22 (d, J = 14.1 Hz, 2H), 1.17–1.07 (m, 4H), 1.05 (s, 3H), 0.83 (d, J = 11.8 Hz, 1H), 0.64 (s, 2H). 13C-NMR (DMSO-d6): δ 180.6, 172.1, 171.9, 149.0, 148.7, 141.3, 138.3, 130.4, 129.7, 118.3, 117.2, 60.0, 56.3, 44.3, 44.0, 38.7, 37.8, 37.0, 36.7, 35.2, 34.5, 33.2, 32.6, 31.9, 30.6, 30.4, 30.2, 30.0, 29.1, 27.1, 26.9, 21.6, 18.5, 13.8, 9.6, 9.4. IR (KBr): 2969.5, 2944.6, 2873.1, 1762.0, 1559.5, 1457.3, 1155.4, 1040.6 cm−1. LC-ESI-MS (−) m/z (%) = 643.45 [M−1Na+]− (100%), 1288.09 [2M−2Na+]2− (5%).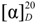 −92.16° (MeOH, c = 0.2); 1H-NMR (CDCl3): δ 7.00 (s, 1H), 5.85 (d, J = 6.0 Hz, 1H), 4.25–3.86 (m, 1H), 2.36 (t, J = 11.1 Hz, 1H), 2.32 (s, 3H), 2.29 (s, 3H), 2.15 (s, 3H), 2.09 (m, 4H), 1.88–1.57 (m, 5H), 1.53 (d, J = 4.3 Hz, 1H), 1.50 (s, 3H), 1.42 (dd, J = 16.3, 5.1 Hz, 3H), 1.27 (s, 3H), 1.20 (s, 3H), 1.10 (s, 3H), 1.06 (s, 3H), 0.93–0.83 (m, 4H), 0.66 (s, 3H); 13C-NMR (CDCl3): δ 207.2, 184.1, 168.5, 168.2, 149.7, 148.2, 140.8, 138.7, 134.1, 127.4, 121.3, 117.3, 53.7, 51.4, 44.2, 43.8, 40.1, 37.6, 36.6, 35.4, 34.5, 33.0, 32.6, 31.5, 30.8, 30.4, 30.1, 29.4, 28.8, 22.6, 22.4, 20.7, 20.4, 18.6, 12.1; IR (KBr) 2941.6, 2872.1, 1768.8, 1465.0, 1369.5, 1213.3, 1188.2 cm−1; LC-ESI-MS (−) m/z (%) = 591.3 [M−1H+]− (5%), 1183.3 [2M−1H+]− (100%), 1775.8 [3M−1H+]− (60%).
−92.16° (MeOH, c = 0.2); 1H-NMR (CDCl3): δ 7.00 (s, 1H), 5.85 (d, J = 6.0 Hz, 1H), 4.25–3.86 (m, 1H), 2.36 (t, J = 11.1 Hz, 1H), 2.32 (s, 3H), 2.29 (s, 3H), 2.15 (s, 3H), 2.09 (m, 4H), 1.88–1.57 (m, 5H), 1.53 (d, J = 4.3 Hz, 1H), 1.50 (s, 3H), 1.42 (dd, J = 16.3, 5.1 Hz, 3H), 1.27 (s, 3H), 1.20 (s, 3H), 1.10 (s, 3H), 1.06 (s, 3H), 0.93–0.83 (m, 4H), 0.66 (s, 3H); 13C-NMR (CDCl3): δ 207.2, 184.1, 168.5, 168.2, 149.7, 148.2, 140.8, 138.7, 134.1, 127.4, 121.3, 117.3, 53.7, 51.4, 44.2, 43.8, 40.1, 37.6, 36.6, 35.4, 34.5, 33.0, 32.6, 31.5, 30.8, 30.4, 30.1, 29.4, 28.8, 22.6, 22.4, 20.7, 20.4, 18.6, 12.1; IR (KBr) 2941.6, 2872.1, 1768.8, 1465.0, 1369.5, 1213.3, 1188.2 cm−1; LC-ESI-MS (−) m/z (%) = 591.3 [M−1H+]− (5%), 1183.3 [2M−1H+]− (100%), 1775.8 [3M−1H+]− (60%). 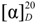 −69.82° (MeOH, c = 0.2). 1H-NMR (CDCl3) δ 7.34 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 7.8 Hz, 1H), 7.01 (s, 1H), 5.70 (d, J = 6.1 Hz, 1H), 4.75 (d, J = 6.0 Hz, 1H), 2.34 (s, 2H), 2.32 (s, 1H), 2.30 (s, 1H), 2.28 (s, 1H), 1.47 (s, 1H), 1.32 (d, J = 11.9 Hz, 3H), 1.25 (s, 3H), 1.22 (s, 1H), 1.09 (s, 2H), 1.04 (s, 1H), 0.96 (t, J = 7.4 Hz, 1H), 0.87 (d, J = 7.1 Hz, 1H), 0.60 (s, 3H); 13C-NMR (DMSO-d6): δ 179.8, 168.9, 168.7, 152.0, 149.7, 142.1, 139.1, 138.0, 133.7, 130.9, 130.2, 130.1, 129.6, 119.9, 117.8, 44.8, 44.1, 44.1, 38.2, 37.6, 36.7, 35.0, 34.8, 34.1, 32.7, 31.8, 30.6, 30.4, 29.9, 29.8, 29.5, 28.8, 22.3, 21.2, 20.9, 20.5, 18.6, 12.3; IR (KBr) 2924.2, 2870.2, 1772.7, 1696.5, 1490.1, 1369.5, 1210.4, 1188.2 cm−1. LC-ESI-MS (−) m/z (%) = 657.1 [M−1H+]− (6%), 1315.2 [2M−1H+]− (100%), 1927.8 [3M−1H+]− (65%).
−69.82° (MeOH, c = 0.2). 1H-NMR (CDCl3) δ 7.34 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 7.8 Hz, 1H), 7.01 (s, 1H), 5.70 (d, J = 6.1 Hz, 1H), 4.75 (d, J = 6.0 Hz, 1H), 2.34 (s, 2H), 2.32 (s, 1H), 2.30 (s, 1H), 2.28 (s, 1H), 1.47 (s, 1H), 1.32 (d, J = 11.9 Hz, 3H), 1.25 (s, 3H), 1.22 (s, 1H), 1.09 (s, 2H), 1.04 (s, 1H), 0.96 (t, J = 7.4 Hz, 1H), 0.87 (d, J = 7.1 Hz, 1H), 0.60 (s, 3H); 13C-NMR (DMSO-d6): δ 179.8, 168.9, 168.7, 152.0, 149.7, 142.1, 139.1, 138.0, 133.7, 130.9, 130.2, 130.1, 129.6, 119.9, 117.8, 44.8, 44.1, 44.1, 38.2, 37.6, 36.7, 35.0, 34.8, 34.1, 32.7, 31.8, 30.6, 30.4, 29.9, 29.8, 29.5, 28.8, 22.3, 21.2, 20.9, 20.5, 18.6, 12.3; IR (KBr) 2924.2, 2870.2, 1772.7, 1696.5, 1490.1, 1369.5, 1210.4, 1188.2 cm−1. LC-ESI-MS (−) m/z (%) = 657.1 [M−1H+]− (6%), 1315.2 [2M−1H+]− (100%), 1927.8 [3M−1H+]− (65%).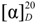 −66.23° (MeOH, c = 0.2). 1H-NMR (CDCl3) δ 7.76 (d, J = 22.2 Hz, 1H), 7.37–7.21 (m, 4H), 7.01 (s, 1H), 5.59 (d, J = 6.1 Hz, 1H), 4.51 (d, J = 6.0 Hz, 1H), 3.47 (d, J = 2.9 Hz, 1H), 2.39 (s, 1H), 2.34 (d, J = 7.0 Hz, 7H), 2.30 (s, 3H), 2.12 (t, J = 7.4 Hz, 4H), 2.02 (d, J = 13.1 Hz, 2H), 1.88–1.54 (m, 6H), 1.50 (s, 1H), 1.47 (s, 3H), 1.43–1.29 (m, 4H), 1.25 (s, 2H), 1.21 (s, 3H), 1.14 (s, 3H), 1.05 (s, 3H), 0.94–0.82 (m, 1H), 0.53 (s, 3H); 13C-NMR (DMSO-d6): δ 180.6, 179.8, 168.9, 168.7, 151.9, 149.7, 142.0, 139.9, 139.1, 135.0, 130.1, 129.6, 127.4, 119.9, 119.7, 117.8, 60.0, 45.2, 44.1, 44.1, 38.2, 37.7, 36.7, 35.0, 34.8, 34.1, 32.7, 31.8, 30.6, 30.4, 30.0, 29.8, 28.8, 24.5, 22.3, 20.9, 20.5, 18.6, 12.4; IR (KBr) 2940.6, 2871.2, 1775.5, 1700.2, 1590.3, 1524.8, 1369.5, 1360.8, 1213.3 cm−1. LC-ESI-MS (−) m/z (%) = 700.9 [M−1H+]− (7%), 746.0 [M+2Na+−1H+]+ (55%), 1402.1 [2M−1H+]− (15%), 1447.5 [2M+2Na+−1H+]+ (100%).
−66.23° (MeOH, c = 0.2). 1H-NMR (CDCl3) δ 7.76 (d, J = 22.2 Hz, 1H), 7.37–7.21 (m, 4H), 7.01 (s, 1H), 5.59 (d, J = 6.1 Hz, 1H), 4.51 (d, J = 6.0 Hz, 1H), 3.47 (d, J = 2.9 Hz, 1H), 2.39 (s, 1H), 2.34 (d, J = 7.0 Hz, 7H), 2.30 (s, 3H), 2.12 (t, J = 7.4 Hz, 4H), 2.02 (d, J = 13.1 Hz, 2H), 1.88–1.54 (m, 6H), 1.50 (s, 1H), 1.47 (s, 3H), 1.43–1.29 (m, 4H), 1.25 (s, 2H), 1.21 (s, 3H), 1.14 (s, 3H), 1.05 (s, 3H), 0.94–0.82 (m, 1H), 0.53 (s, 3H); 13C-NMR (DMSO-d6): δ 180.6, 179.8, 168.9, 168.7, 151.9, 149.7, 142.0, 139.9, 139.1, 135.0, 130.1, 129.6, 127.4, 119.9, 119.7, 117.8, 60.0, 45.2, 44.1, 44.1, 38.2, 37.7, 36.7, 35.0, 34.8, 34.1, 32.7, 31.8, 30.6, 30.4, 30.0, 29.8, 28.8, 24.5, 22.3, 20.9, 20.5, 18.6, 12.4; IR (KBr) 2940.6, 2871.2, 1775.5, 1700.2, 1590.3, 1524.8, 1369.5, 1360.8, 1213.3 cm−1. LC-ESI-MS (−) m/z (%) = 700.9 [M−1H+]− (7%), 746.0 [M+2Na+−1H+]+ (55%), 1402.1 [2M−1H+]− (15%), 1447.5 [2M+2Na+−1H+]+ (100%).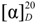 −111.01° (MeOH, c = 0.2); 1H-NMR (DMSO-d6) δ 8.14–7.78 (m, 1H), 7.09 (s, 1H), 6.09 (dd, J = 24.2, 5.7 Hz, 1H), 4.71 (d, J = 5.4 Hz, 1H), 4.38 (s, 1H), 4.27 (s, 1H), 3.56–3.53 (m, 1H), 3.44 (dd, J = 13.8, 6.9 Hz, 1H), 3.24 (d, J = 9.5 Hz, 1H), 3.06 (d, J = 5.5 Hz, 1H), 2.76 (dd, J = 13.3, 8.2 Hz, 1H), 2.50 (s, 1H), 2.33 (d, J = 16.1 Hz, 2H), 2.24 (s, 1H), 2.19 (d, J = 9.8 Hz, 1H), 2.16–1.92 (m, 2H), 1.86 (s, 1H), 1.83 (s, 1H), 1.63 (d, J = 9.2 Hz, 2H), 1.55–1.36 (m, 3H), 1.25 (d, J = 24.4 Hz, 3H), 1.10 (s, 2H), 1.05 (d, J = 9.1 Hz, 2H), 0.87 (d, J = 11.2 Hz, 1H), 0.64 (s, 2H); 13C-NMR (DMSO-d6): δ 179.9, 172.8, 169.2, 169.0, 168.9, 168.7, 152.0, 151.6, 149.5, 149.4, 141.8, 141.7, 138.9, 131.4, 131.2, 129.4, 129.4, 120.3, 119.8, 117.5, 117.4, 56.4, 54.6, 53.8, 45.7, 44.3, 44.2, 38.1, 38.1, 37.6, 36.8, 35.0, 34.8, 34.2, 34.2, 34.0, 33.9, 32.8, 31.8, 31.7, 30.6, 30.4, 29.9, 29.9, 28.6, 23.2, 23.2, 22.6, 22.5, 22.4, 22.3, 21.7, 20.9, 20.5, 19.0, 18.8, 12.3, 12.1; IR (KBr) 2939.6, 2871.2, 1771.69, 1374.3, 1213.3 cm−1; LC-ESI-MS (−) m/z (%) = 696.4 [M−1H+]− (8%), 1393.5 [2M−1H+]− (100%).
−111.01° (MeOH, c = 0.2); 1H-NMR (DMSO-d6) δ 8.14–7.78 (m, 1H), 7.09 (s, 1H), 6.09 (dd, J = 24.2, 5.7 Hz, 1H), 4.71 (d, J = 5.4 Hz, 1H), 4.38 (s, 1H), 4.27 (s, 1H), 3.56–3.53 (m, 1H), 3.44 (dd, J = 13.8, 6.9 Hz, 1H), 3.24 (d, J = 9.5 Hz, 1H), 3.06 (d, J = 5.5 Hz, 1H), 2.76 (dd, J = 13.3, 8.2 Hz, 1H), 2.50 (s, 1H), 2.33 (d, J = 16.1 Hz, 2H), 2.24 (s, 1H), 2.19 (d, J = 9.8 Hz, 1H), 2.16–1.92 (m, 2H), 1.86 (s, 1H), 1.83 (s, 1H), 1.63 (d, J = 9.2 Hz, 2H), 1.55–1.36 (m, 3H), 1.25 (d, J = 24.4 Hz, 3H), 1.10 (s, 2H), 1.05 (d, J = 9.1 Hz, 2H), 0.87 (d, J = 11.2 Hz, 1H), 0.64 (s, 2H); 13C-NMR (DMSO-d6): δ 179.9, 172.8, 169.2, 169.0, 168.9, 168.7, 152.0, 151.6, 149.5, 149.4, 141.8, 141.7, 138.9, 131.4, 131.2, 129.4, 129.4, 120.3, 119.8, 117.5, 117.4, 56.4, 54.6, 53.8, 45.7, 44.3, 44.2, 38.1, 38.1, 37.6, 36.8, 35.0, 34.8, 34.2, 34.2, 34.0, 33.9, 32.8, 31.8, 31.7, 30.6, 30.4, 29.9, 29.9, 28.6, 23.2, 23.2, 22.6, 22.5, 22.4, 22.3, 21.7, 20.9, 20.5, 19.0, 18.8, 12.3, 12.1; IR (KBr) 2939.6, 2871.2, 1771.69, 1374.3, 1213.3 cm−1; LC-ESI-MS (−) m/z (%) = 696.4 [M−1H+]− (8%), 1393.5 [2M−1H+]− (100%).3.3. Activity of Celastrol Analogues against Human Cancer Cell Lines
3.4. Anti-Tumor Activity Evaluation and Preliminary Non-Clinical Safety Evaluation in Vivo
| Groups | N.O. of Mice | Dosage | Route of Administration | Defined Daily Dose | |
|---|---|---|---|---|---|
| (mg/kg) | (mL/10 g) | ||||
| Model | 12 | 0.2 mL | 0.1 | i.p. | qd × 20 |
| Cisplatin (4 mg/kg) | 6 | 4 | i.p. | 3 times/week | |
| NST001A (6 mg/kg) | 6 | 6 | i.p. | qd × 20 | |
| NST001A (12 mg/kg) | 6 | 12 | i.p. | qd × 20 | |
| NST001A (24 mg/kg) | 6 | 24 | i.p. | qd × 20 | |
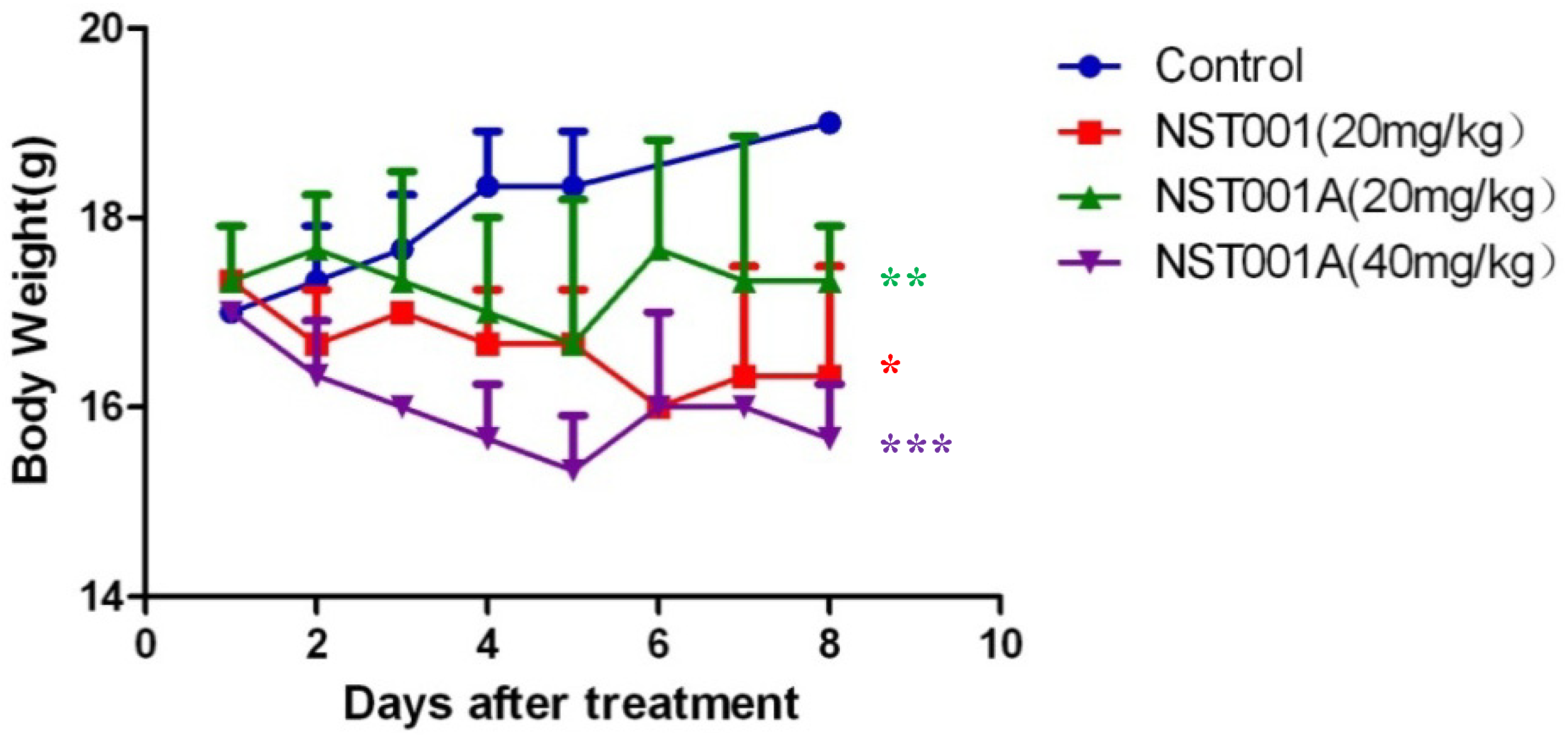
| Group | N.O. of Mice | Body Weight (g) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| control | 3 | 3 | 17.0 ± 0.00 | 19.00 ± 0.00 |
| NST001 (20 mg/kg) | 3 | 3 | 17.33 ± 0.58 | 16.33 ± 1.15 * |
| NST001A (20 mg/kg) | 3 | 3 | 17.33 ± 0.58 | 17.33 ± 0.58 ** |
| NST001A (40 mg/kg) | 3 | 3 | 17.0 ± 0.00 | 15.67 ± 0.58 *** |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sreeramulu, S.; Gande, S.L.; Gobel, M.; Schwalbe, H. Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37, a kinome chaperone-cochaperone, by triterpene celastrol. Angew. Chem. Int. Ed. Engl. 2009, 48, 5853–5855. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Shanmugam, M.K.; Sethi, G. Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011, 303, 9–20. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Huang, Y.L. Antiangiogenic effect of celastrol on the growth of human glioma: An in vitro and in vivo study. Chin. Med. J. (Engl.) 2009, 122, 1666–1673. [Google Scholar]
- Jiang, H.L.; Jin, J.Z.; Wu, D.; Xu, D.; Lin, G.F.; Yu, H.; Ma, D.Y.; Liang, J. Celastrol exerts synergistic effects with PHA-665752 and inhibits tumor growth of c-Met-deficient hepatocellular carcinoma in vivo. Mol. Biol. Rep. 2013, 40, 4203–4209. [Google Scholar] [CrossRef]
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Dou, Q.P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765. [Google Scholar] [CrossRef]
- Zhang, T.; Hamza, A.; Cao, X.; Wang, B.; Yu, S.; Zhan, C.G.; Sun, D. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 162–170. [Google Scholar] [CrossRef]
- Trott, A.; West, J.D.; Klaic, L.; Westerheide, S.D.; Silverman, R.B.; Morimoto, R.I.; Morano, K.A. Activation of heat shock and antioxidant responses by the natural product celastrol: Transcriptional signatures of a thiol-targeted molecule. Mol. Biol. Cell 2008, 19, 1104–1112. [Google Scholar]
- Westerheide, S.D.; Bosman, J.D.; Mbadugha, B.N.; Kawahara, T.L.; Matsumoto, G.; Kim, S.; Gu, W.; Devlin, J.P.; Silverman, R.B.; Morimoto, R.I. Celastrols as inducers of the heat shock response and cytoprotection. J. Biol. Chem. 2004, 279, 56053–56060. [Google Scholar] [CrossRef]
- Klaic, L.; Morimoto, R.I.; Silverman, R.B. Celastrol analogues as inducers of the heat shock response. Design and synthesis of affinity probes for the identification of protein targets. ACS Chem. Biol. 2012, 7, 928–937. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.; Yu, P.; Jiang, J.; Zhang, G.; Wang, Y. Synthesis and preliminary evaluation of neuroprotection of celastrol analogues in PC12 cells. Bioorg. Med. Chem. Lett. 2010, 20, 3844–3847. [Google Scholar] [CrossRef]
- Zeng, J.F.; Pan, J.F.; Li, B.Y.; Zhu, Q.; Fang, T.; Ni, H.Y. Inventor A Water-Soluble Triterpenephenol Compound Having Antitumor Activities and the Preparation Method Thereof. WIPO Patent Application WO/2009/067891, 4 June 2009. [Google Scholar]
- Sample Availability: Samples of the compounds NST001, NST001A, NST001B, NST6A-A are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.; Huang, Q.; Zeng, J.; Wu, G.; Huang, J.; Pan, J.; Lu, W. Design, Synthesis and Biological Evaluation of C(6)-Modified Celastrol Derivatives as Potential Antitumor Agents. Molecules 2014, 19, 10177-10188. https://doi.org/10.3390/molecules190710177
Tang K, Huang Q, Zeng J, Wu G, Huang J, Pan J, Lu W. Design, Synthesis and Biological Evaluation of C(6)-Modified Celastrol Derivatives as Potential Antitumor Agents. Molecules. 2014; 19(7):10177-10188. https://doi.org/10.3390/molecules190710177
Chicago/Turabian StyleTang, Kaiyong, Qingqing Huang, Jafeng Zeng, Guangming Wu, Jinwen Huang, Junfang Pan, and Wei Lu. 2014. "Design, Synthesis and Biological Evaluation of C(6)-Modified Celastrol Derivatives as Potential Antitumor Agents" Molecules 19, no. 7: 10177-10188. https://doi.org/10.3390/molecules190710177
APA StyleTang, K., Huang, Q., Zeng, J., Wu, G., Huang, J., Pan, J., & Lu, W. (2014). Design, Synthesis and Biological Evaluation of C(6)-Modified Celastrol Derivatives as Potential Antitumor Agents. Molecules, 19(7), 10177-10188. https://doi.org/10.3390/molecules190710177




