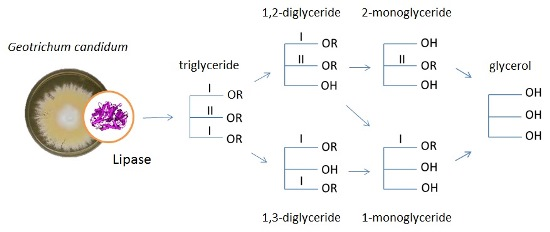
Mycelium-Bound Lipase from a Locally Isolated Strain of Geotrichum candidum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Cultivation Scale and Time Course on MBL Activity
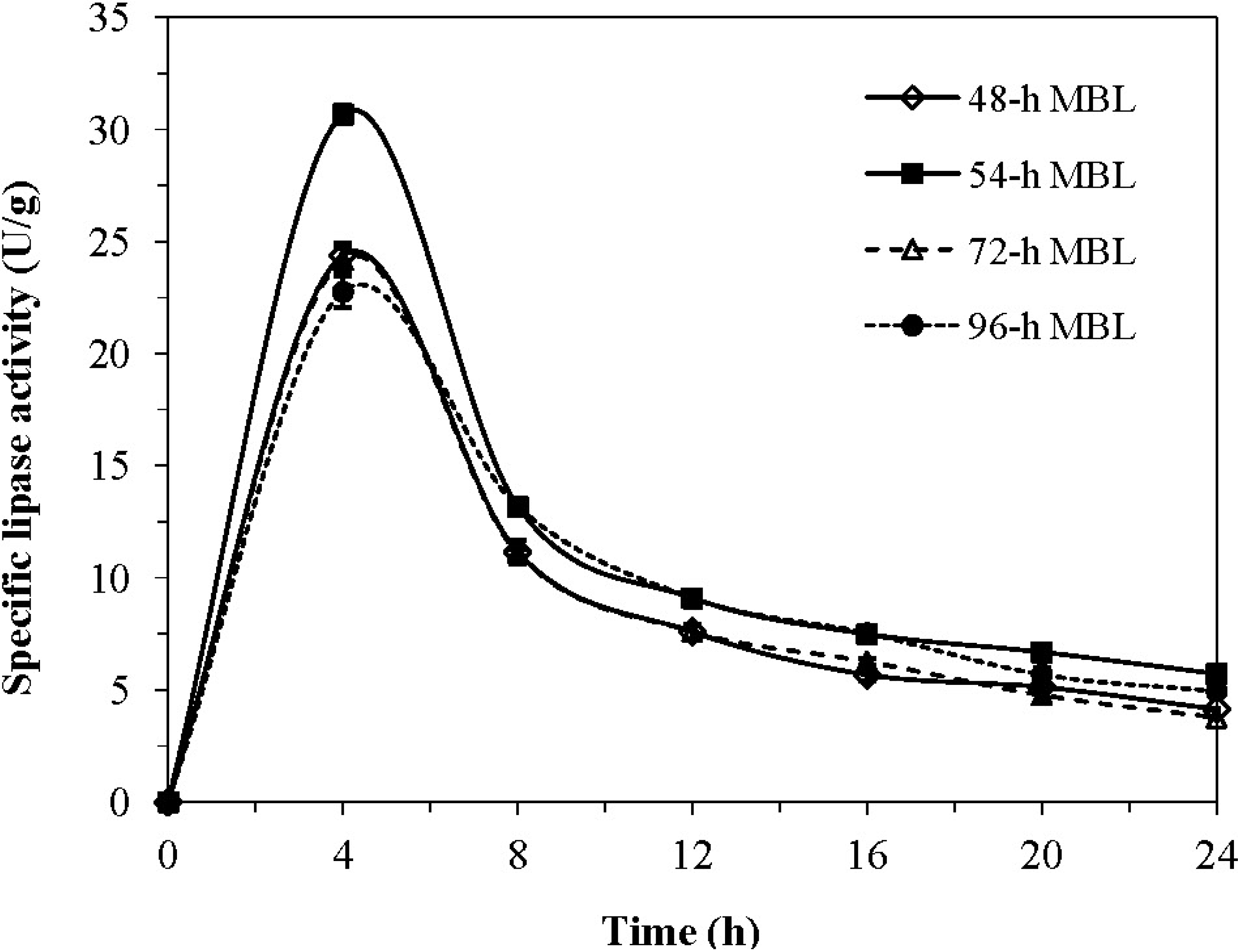
2.2. Characteristics and Properties of MBL
| Replicate | Yield (g/L Dry Weight) | * Moisture Content (%) | * Protein Content (mg/g) | * Lipase Activity (U/g) | * Specific Lipase Activity (U/g Protein) |
|---|---|---|---|---|---|
| 1 | 2.6 | 3.7 ± 0.0 a | 28.2 ± 1.8 a | 16.5 ± 0.6 a | 582.6 ± 35.8 d |
| 2 | 3.0 | 3.9 ± 0.0 bc | 38.8 ± 1.2 a | 17.5 ± 1.1 a | 450.7 ± 29.1 ad |
| 3 | 4.6 | 4.0 ± 0.0 c | 39.2 ± 3.9 a | 15.7 ± 0.2 a | 400.6 ± 29.2 a |
| 4 | 5.0 | 3.8 ± 0.1 b | 46.0 ± 1.8 a | 22.5 ± 1.2 b | 489.1 ± 23.1 bc |
| 5 | 2.3 | 4.0 ± 0.0 c | 55.4 ± 1.4 a | 30.4 ± 0.5 c | 548.2 ± 4.6 cd |
| 6 | 4.4 | 3.8 ± 0.0 b | 57.0 ± 1.4 b | 33.0 ± 2.5 c | 578.8 ± 8.6 d |
| Mean | 3.9 ± 1.1 | 3.8 ± 0.1 | 44.1 ± 11.0 | 22.6 ± 7.5 | 508.3 ± 74.0 |
2.3. Effect of Storage at 4 °C on the Activity of Mycelium-Bound Lipase
| Storage Time (Month) | Lipase Activity a (U/g) | Remaining Activity (%) | Specific Lipase Activity a (U/g) | Remaining Specific Activity (%) |
|---|---|---|---|---|
| 0 | 20.8 ± 0.4 | 100.0 | 550.7 ± 3.7 | 100.0 |
| 1 | 19.2 ± 0.4 | 95.7 | 519.5 ± 3.6 | 94.3 |
| 2 | 18.1 ± 0.3 | 88.9 | 470.5 ± 8.6 | 85.4 |
| 4 | 17.0 ± 0.5 | 83.4 | 447.5 ± 3.2 | 81.3 |
| 8 | 14.6 ± 0.4 | 70.0 | 410.5 ± 3.8 | 74.6 |
2.4. Characteristics and Properties of Free or Extracellular Lipase
| Replicate | Lipase Activity (U/mL) | Protein Content (μg/mL) | Specific Lipase Activity (U/g protein) |
|---|---|---|---|
| 1 | 0.1 ± 0.0 c | 39.3 ± 4.8 b | 2812.7 ± 195.1 a |
| 2 | 0.1 ± 0.0 a | 26.1 ± 0.8 a | 2981.8 ± 214.1 a |
| 3 | 0.2 ± 0.0 d | 55.6 ± 1.7 c | 3072.9 ± 135.2 ab |
| 4 | 0.1 ± 0.0 c | 28.7 ± 3.5 a | 3739.5 ± 306.0 c |
| 5 | 0.1 ± 0.0 b | 23.7 ± 3.0 a | 3933.7 ± 174.4 d |
| 6 | 0.2 ± 0.1 d | 44.5 ± 3.4 b | 3318.3 ± 235.4 bc |
| Mean | 0.1 ± 0.0 | 36.3 ± 12.4 | 3309.8 ± 443.8 |
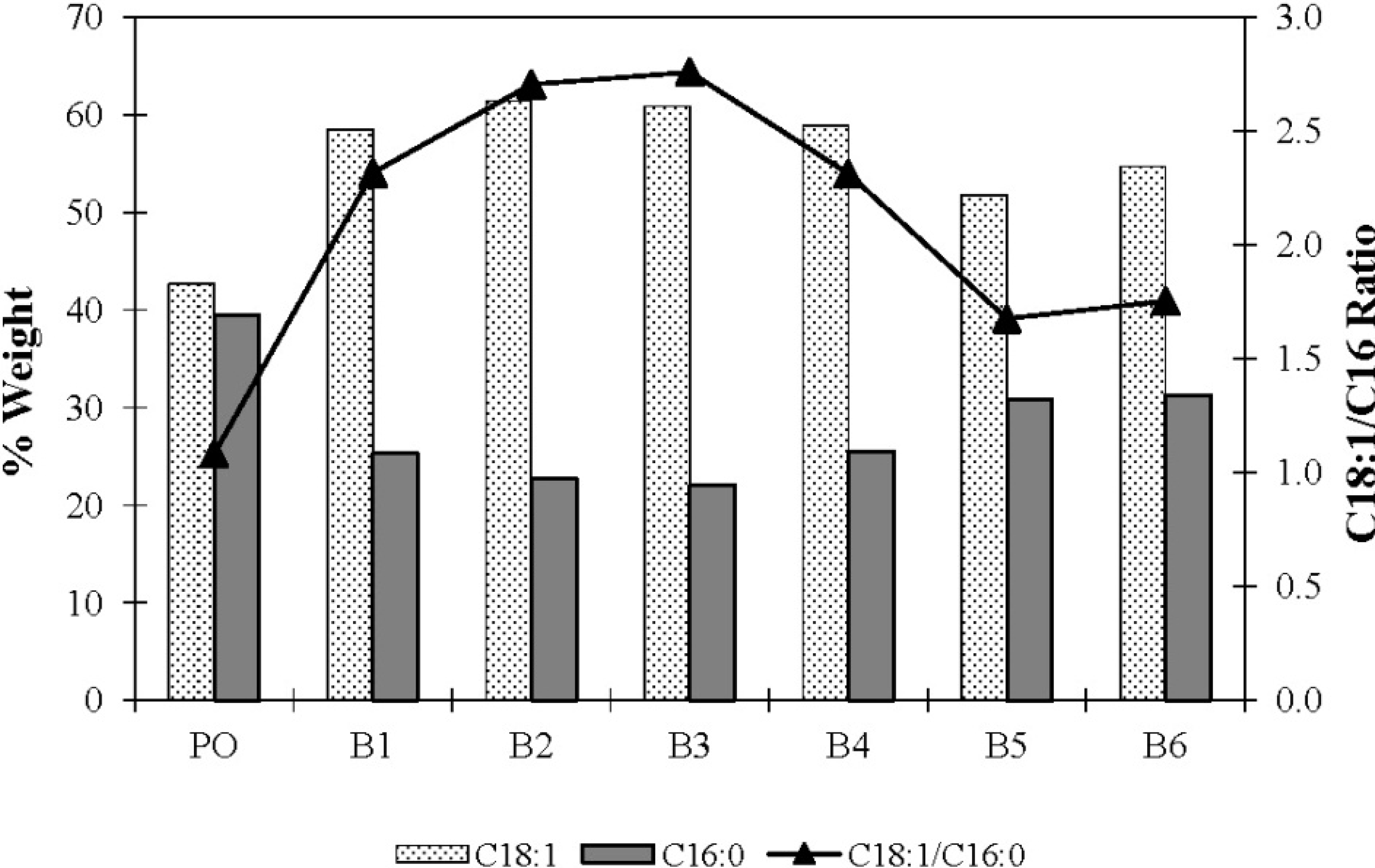
2.5. Properties and Composition of Extracted Lipid Materials
| Replicate | FFA (%) | MAG (%) | DAG (%) | TAG (%) |
|---|---|---|---|---|
| 1 | 48.6 ± 1.5 | 1.5 ± 0.0 | 19.6 ± 0.6 | 30.3 ± 1.0 |
| 2 | 40.8 ± 2.0 | 2.0 ± 0.1 | 23.1 ± 1.1 | 34.1 ± 3.2 |
| 3 | 46.4 ± 0.5 | 3.1 ± 0.1 | 23.4 ± 0.6 | 27.1 ± 0.0 |
| 4 | 26.4 ± 0.5 | 1.1 ± 0.1 | 25.2 ± 2.0 | 47.3 ± 2.4 |
| 5 | 36.5 ± 1.2 | 2.9 ± 1.0 | 18.0 ± 0.9 | 43.7 ± 0.7 |
| 6 | 27.8 ± 0.4 | 1.3 ± 0.1 | 24.8 ± 0.7 | 46.1 ± 0.2 |
| Mean | 37.7 ± 9.3 | 2.0 ± 0.8 | 22.2 ± 3.2 | 38.1 ± 8.7 |
| Replicate | Relative Amount (% Weight) | |||||
|---|---|---|---|---|---|---|
| C16 | C18 | C18:1 | C18:2 | C18:3 | C20 | |
| b PO | 39.4 | 3.5 | 42.7 | 11.8 | 0.3 | 0.2 |
| 1 | 25.3 | 1.7 | 58.5 | 12.4 | 0.3 | 0.3 |
| 2 | 22.7 | 2.1 | 61.4 | 10.1 | 0.5 | 0.2 |
| 3 | 22.1 | 2.2 | 60.9 | 12.6 | 0.4 | 0.2 |
| 4 | 25.5 | 2.1 | 58.9 | 11.9 | 0.3 | 0.2 |
| 5 | 30.9 | 1.9 | 51.8 | 11.2 | 0.2 | 0.1 |
| 6 | 31.2 | 1.7 | 54.7 | 11.3 | 0.2 | 0.1 |
| Mean | 26.3 ± 3.9 | 2.0 ± 0.2 | 57.7 ± 3.8 | 11.6 ± 0.9 | 0.3 ± 0.1 | 0.2 ± 0.1 |
3. Experimental
3.1. Materials
3.2. Preparation of Inoculum
3.3. Production of Mycelium-Bound Lipase
3.4. Lyophilisation of Mycelium-Bound Lipase
3.5. Determination of Moisture Content
3.6. Lipase Activity Assay
3.7. Protein Determination
3.8. Determination of MBL Activity towards RBD Palm Olein (Lipolysis)
3.9. Extraction of Lipid Materials
3.10. Neutralisation and Fractionation of Free Fatty Acids from Extracted Lipid Materials
3.11. Determination of Total Free Fatty Acids
3.12. Analysis of Fatty Acids Composition
3.13. Analysis of Free Fatty Acid Composition
3.14. Quantification of Lipid Class
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar]
- Gandolfi, R.; Converti, A.; Pirozzi, D.; Molinari, F. Efficient and selective microbial esterification with dry mycelium of Rhizopus oryzae. J. Biotechnol. 2001, 92, 21–26. [Google Scholar] [CrossRef]
- Converti, A.; del Borgi, A.; Gandolfi, R.; Lodi, A.; Molinari, F.; Palazzi, E. Reactivity and stability of mycelium-bound carboxylesterase from Aspergillus oryzae. Biotechnol. Bioeng. 2002, 77, 232–237. [Google Scholar] [CrossRef]
- Mustranta, A.; Forssell, P.; Poutanen, K. Applications of immobilized lipases to tranesterification and esterification reactions in nonaqueous system. Enzyme Microb. Technol. 1993, 15, 133–139. [Google Scholar] [CrossRef]
- Romero, C.M.; Baigori, M.D.; Pera, L.M. Catalytic properties of mycelium-bound lipases from Aspergillus niger MYA 135. Appl. Microb. Biotech. 2007, 76, 861–866. [Google Scholar] [CrossRef]
- Ghazali, H.M. Production and Characterization of Cell-bound Lipases Secreted by a Newly Isolated Strain of Geotrichum Candidum. Ph.D. Thesis, Faculty of Food Science and Biotechnology, University Putra Malaysia, Selangor, Malaysia, 1990. [Google Scholar]
- Long, K.; Ghazali, M.G.; Ariff, A.; Ampon, K.; Bucke, C. Mycelium-bound lipase from a locally isolated strain of Aspergillus flavus link: Pattern and factors involved in its production. J. Chem. Technol. Biotechnol. 1996, 67, 157–163. [Google Scholar] [CrossRef]
- Long, K.; Ghazali, M.G.; Ariff, A.; Ampon, K.; Bucke, C. In-situ crosslinking of Aspergillus flavus lipase: Improvement of activity, stability and properties. Biotechnol. Lett. 1996, 18, 1169–1174. [Google Scholar] [CrossRef]
- Liew, M.Y.B.; Ghazali, H.M.; Long, K.; Lai, O.M.; Yazid, A.M. Physical properties of palm kernel olein-anhydrous milk fat mixtures transesterified using mycelium-bound lipase from Rhizomucor miehei. Food Chem. 2001, 72, 447–454. [Google Scholar] [CrossRef]
- Long, K.; Ghazali, H.M.; Ariff, A.; Bucke, C. Acidolysis of several vegetable oils by mycelium-bound lipase of Aspergillus flavus Link. J. Am. Oil Chem. Soc. 1997, 74, 1121–1128. [Google Scholar] [CrossRef]
- Liew, M.Y.B.; Ghazali, H.M.; Long, K.; Yazid, A.M.; Lai, O.M. Production and transesterification activity of mycelium-bound lipase from Rhizomucor miehei. Asia Pac. J. Mol. Biol. Biotechnol. 2000, 8, 57–66. [Google Scholar]
- Charton, E.; Macrae, A.R. Specificities of immobilized Geotrichum candidum CMICC 335426 lipases A and B in hydrolysis and ester synthesis reactions in organic solvents. Enzym. Microb. Technol. 1993, 15, 489–493. [Google Scholar] [CrossRef]
- Sonnet, P.E.; Foglia, T.A.; Baillargeon, M.W. Fatty acid selectivity: The selectivity of lipases of Geotrichum candidum. J. Am. Oil Chem. Soc. 1993, 70, 1043–1045. [Google Scholar] [CrossRef]
- Shimada, Y.; Maruyama, K.; Nakamura, M.; Nakayama, S.; Sughihara, A.; Tominaga, Y. Selective hydrolysis of polyunsaturated fatty acid-containing oil with Geotrichum candidum lipase. J. Am. Oil Chem. Soc. 1995, 72, 1577–1581. [Google Scholar] [CrossRef]
- Loo, J.L.; Lai, O.M.; Long, K.; Ghazali, H.M. Identification and characterization of a locally isolated lipolytic microfungus-Geotrichum candidum. Malays. J. Microbiol. 2006, 2, 22–29. [Google Scholar]
- Jacobsen, T.; Jensen, N.; Olsen, J.; Allerman, K. Extracellular and cell bound lipase activity in relation to growth of Geotrichum candidum. Appl. Microbiol. Biotechnol. 1989, 32, 256–261. [Google Scholar]
- Zaks, A.; Klibanov, A.M. The effect of water on enzyme action in organic media. J. Biol. Chem. 1988, 263, 8017–8021. [Google Scholar]
- Tsujisaka, Y.; Iwai, M.; Fukumoto, J.; Okamoto, Y. Induced formation of lipase by Geotrichum candidum Link. Agric. Biol. Chem. 1973, 37, 837. [Google Scholar] [CrossRef]
- Hiol, A.; Jonzo, M.D.; Rugani, N.; Druet, D.; Sarda, L.; Comeau, L.C. Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzym. Microb. Technol. 2000, 26, 421–30. [Google Scholar] [CrossRef]
- Van Tilbeurgh, H.; Esloff, M.P.; Martinez, C.; Rugani, N.; Verger, R.; Combillau, C. Interfacial activation of lipase procolipase complex by mixed micelles revealed by x-ray crystallography. Nature 1993, 362, 814–820. [Google Scholar] [CrossRef]
- Molinari, F.; Gandolfi, R.; Aragozzini, F. Microbial catalyzed esterification of primary and secondary alcohols in organic solvent. Biotechnol. Tech. 1996, 10, 103–108. [Google Scholar] [CrossRef]
- Molinari, F.; Villa, R.; Aragozzini, F. Production of geranyl acetate and other acetates by direct esterification catalyzed by mycelium of Rhizopus delemar in organic solvent. Biotechnol. Lett. 1998, 20, 41–44. [Google Scholar]
- Baillargeon, B.W.; McCarthy, S.G. Geotrichum candidum NRRLY-553 lipase: Purification, characterization and fatty acid specificity. Lipids 1991, 26, 831–836. [Google Scholar] [CrossRef]
- PORIM Test Methods; Siew, W.L.; Tang, T.S.; Tan, Y.A. (Eds.) Palm Oil Research Institute of Malaysia: Kajang, Selangor, Malaysia, 1995.
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kosugi, Y.; Suzuki, H.; Funada, T. Hydrolysis of beef tallow by lipase from Pseudomonas sp. Biotechnol. Bioeng. 1988, 31, 349–356. [Google Scholar] [CrossRef]
- Wang, C.; Ning, J.; Krishnan, P.G.; Matthees, D.P. Effects of steeping conditions during wet-millingon the retentions of tocopherols and tocotrienols in corn. J. Am. Oil Chem. Soc. 1998, 75, 609–613. [Google Scholar] [CrossRef]
- Freeman, B.; Pryde, E.H.; Mounts, J.L. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Verleyen, T.; Verhe, R.; Gracia, L.; Dewettinck, K.; Huyghebaert, A.; de Greyt, W. Gas chromatographic characterization of vegetable oil deodorization distillate. J. Chromatogr. A 2001, 921, 277–285. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Loo, J.L.; Khoramnia, A.; Lai, O.M.; Long, K.; Ghazali, H.M. Mycelium-Bound Lipase from a Locally Isolated Strain of Geotrichum candidum. Molecules 2014, 19, 8556-8570. https://doi.org/10.3390/molecules19068556
Loo JL, Khoramnia A, Lai OM, Long K, Ghazali HM. Mycelium-Bound Lipase from a Locally Isolated Strain of Geotrichum candidum. Molecules. 2014; 19(6):8556-8570. https://doi.org/10.3390/molecules19068556
Chicago/Turabian StyleLoo, Joo Ling, Anahita Khoramnia, Oi Ming Lai, Kamariah Long, and Hasanah Mohd Ghazali. 2014. "Mycelium-Bound Lipase from a Locally Isolated Strain of Geotrichum candidum" Molecules 19, no. 6: 8556-8570. https://doi.org/10.3390/molecules19068556
APA StyleLoo, J. L., Khoramnia, A., Lai, O. M., Long, K., & Ghazali, H. M. (2014). Mycelium-Bound Lipase from a Locally Isolated Strain of Geotrichum candidum. Molecules, 19(6), 8556-8570. https://doi.org/10.3390/molecules19068556