Inhibitory Activity of the Flower Buds of Lonicera japonica Thunb. against Histamine Production and L-Histidine Decarboxylase in Human Keratinocytes
Abstract
:1. Introduction
2. Results and Discussion
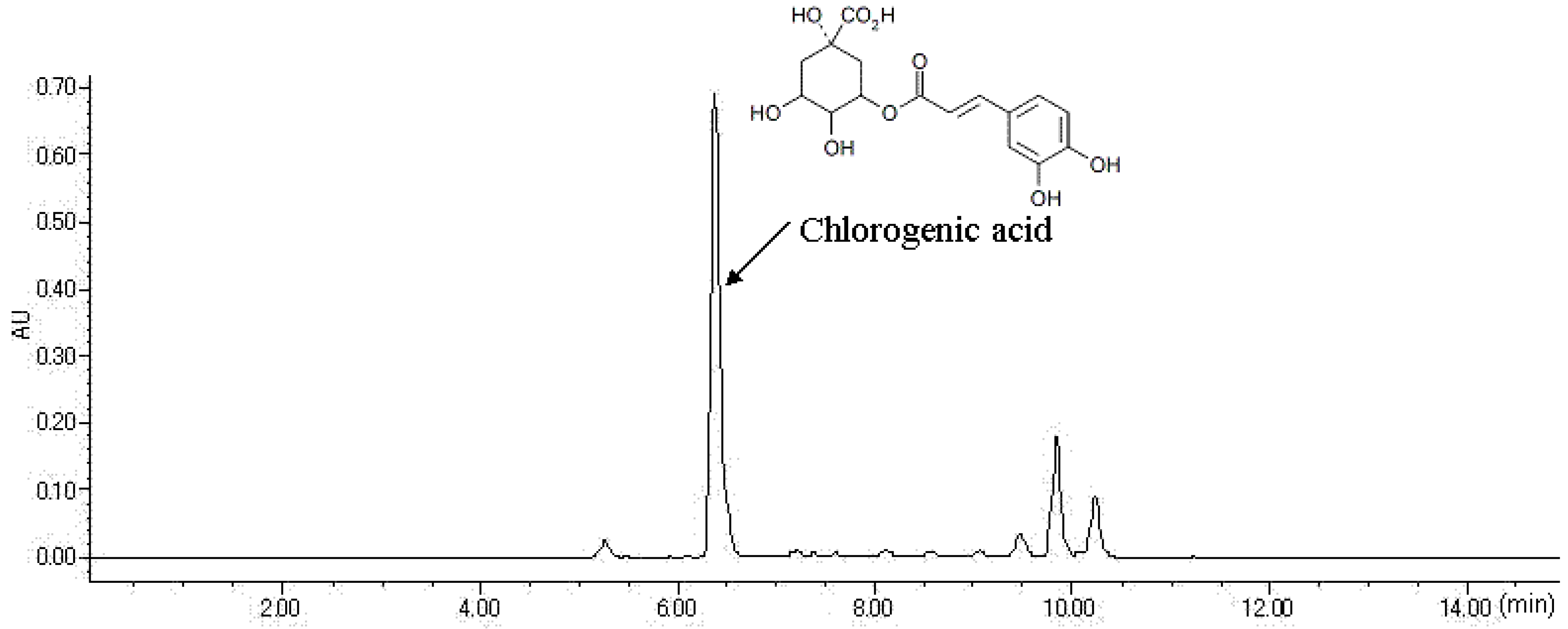
2.1. HPLC Analysis of FLJ Extract
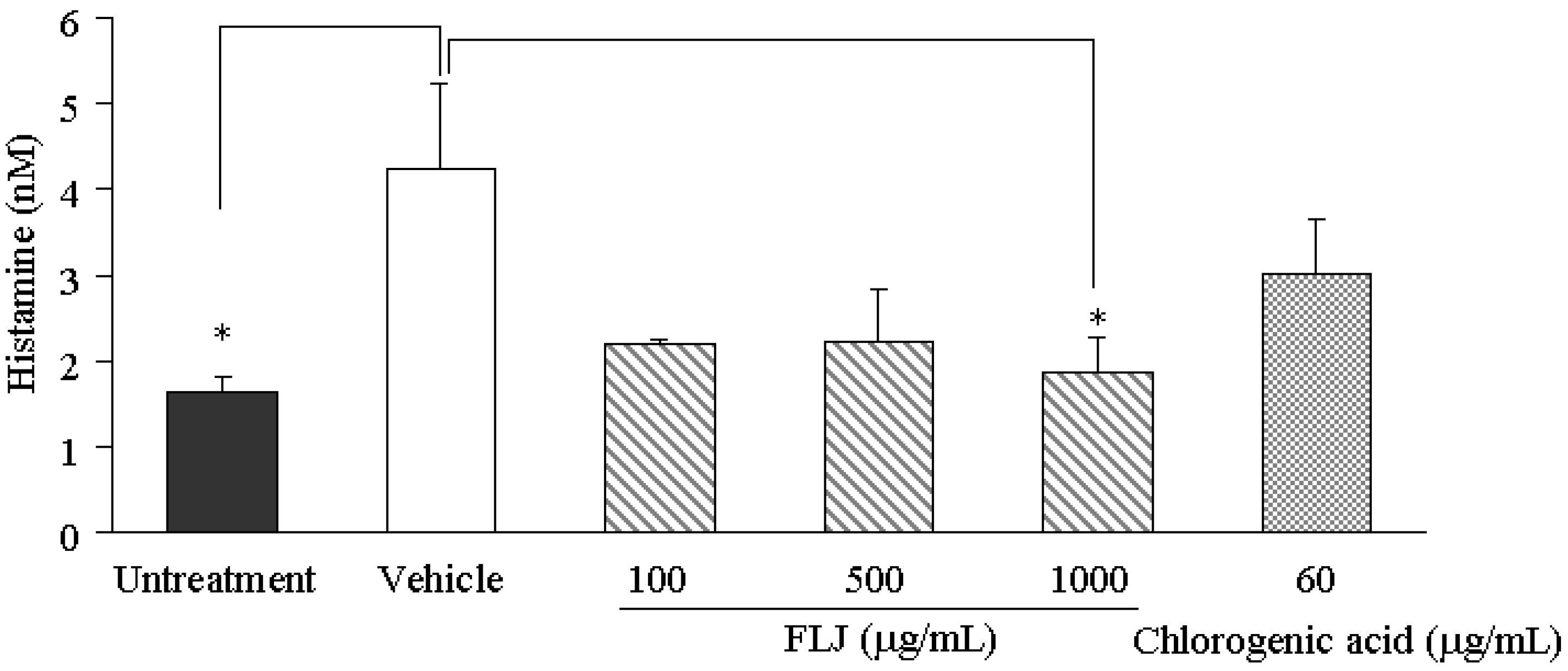
2.2. Effects of the FLJ Extract and Chlorogenic Acid on SL-Enhanced Histamine Production
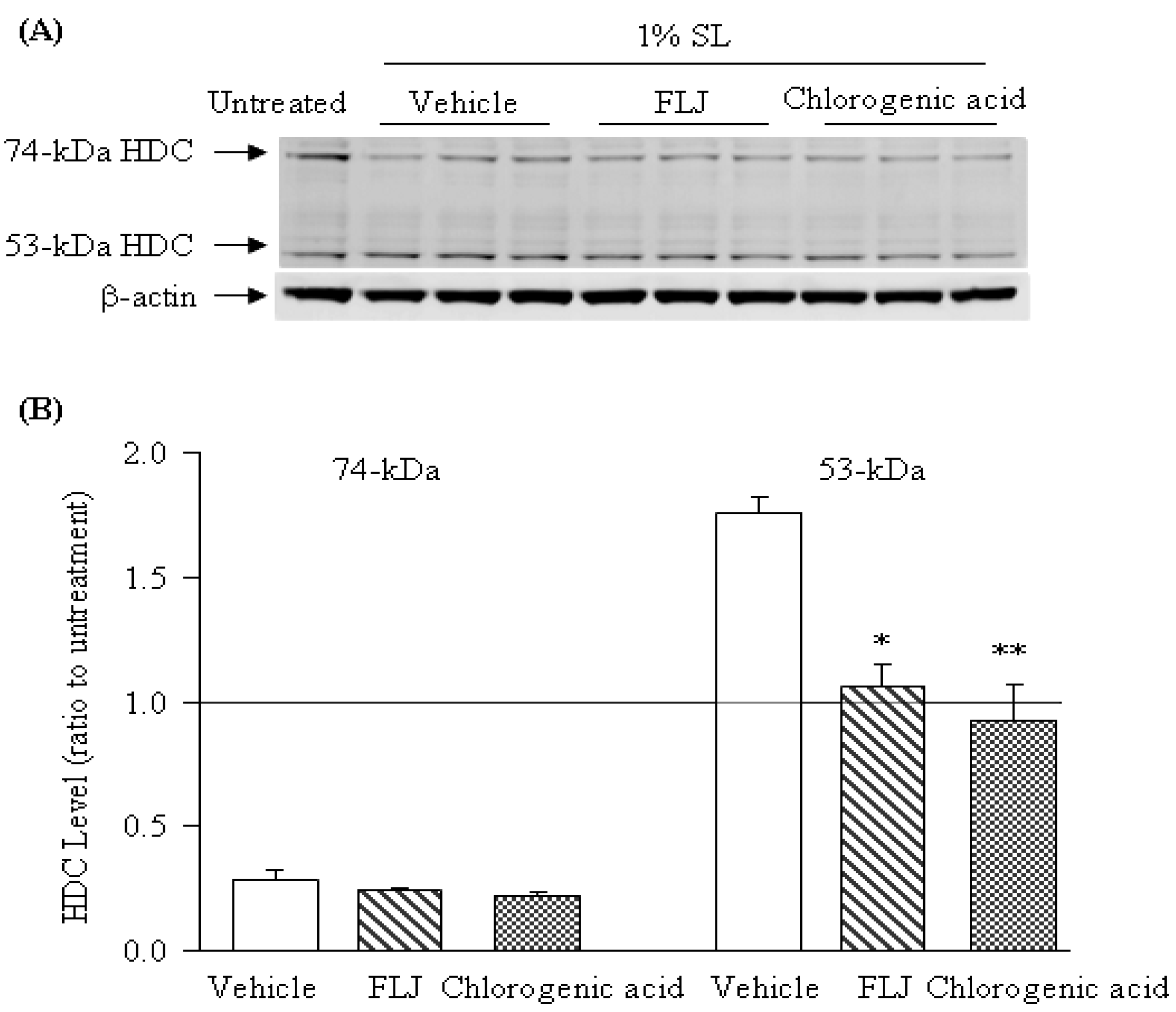
2.3. Effects of the FLJ Extract and Chlorogenic Acid on the Expression of HDC Induced by SL Treatment
3. Experimental Section
3.1. Sample Preparation and HPLC Analysis
3.2. Three-Dimensional Keratinocyte Culture and Treatment Methods
3.3. Western Blotting
3.4. Enzyme Immunoassay for Histamine Production
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Groot, A.C.; Nater, J.P.; Lender, R.; Rijcken, B. Adverse effects of cosmetics and toiletries: A retrospective study in the general population. Int. J. Cosmet. Sci. 1987, 9, 255–259. [Google Scholar] [CrossRef]
- Berne, B.; Boström, A.; Grahnén, A.F.; Tammela, M. Adverse effects of cosmetics and toiletries reported to the Swedish Medical Products Agency 1989–1994. Contact Dermatitis 1996, 34, 359–362. [Google Scholar] [CrossRef]
- Agner, T.; Serup, J. Sodium lauryl sulphate for irritant patch testing-a dose-response study using bioengineering methods for determination of skin irritation. J. Invest. Dermatol. 1990, 95, 543–547. [Google Scholar]
- Effendy, I.; Maibach, H.I. Surfactant and experiment irritant contact dermatitis. Contact Dermatitis 1995, 33, 217–225. [Google Scholar] [CrossRef]
- Baba, H.; Masuyama, A.; Yoshimura, C.; Aoyama, Y.; Takano, T.; Ohki, K. Oral intake of Lactobacillus helveticus-fermented milk whey decreased transepidermal water loss and prevented the onset of sodium dodecylsulfate-induced dermatitis in mice. Biosci. Biotechnol. Biochem. 2010, 74, 18–23. [Google Scholar] [CrossRef]
- Inami, Y.; Andoh, T.; Sasaki, A.; Kuraishi, Y. Topical surfactant-induced pruritus: Involvement of histamine released from epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2013a, 344, 459–466. [Google Scholar]
- Inami, Y.; Sasaki, A.; Andoh, T.; Kuraishi, Y. Surfactant-induced chronic pruritus: Role of l-histidine decarboxylase expression and histamine production in epidermis. Acta Derm. Venereol. 2014. [Google Scholar] [CrossRef]
- Ichikawa, A.; Sugimoto, Y.; Tanaka, S. Molecular biology of histidine decarboxylase and prostaglandin receptors. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 848–866. [Google Scholar] [CrossRef]
- Directive 2003/15/EC of the European Parliament and the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Members States relating to cosmetic products. Off. J. Eur. Union 2003, L66, 26–35.
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Wu, L. Effect of chlorogenic acid on antioxidant activity of Flos Lonicerae extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef]
- Wong, R.W.; Hägg, U.; Samaranayake, L.; Yuen, M.K.; Seneviratne, C.J.; Kao, R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study. Int. J. Oral Maxillofac. Surg. 2010, 39, 599–605. [Google Scholar] [CrossRef]
- Liao, Y.; Dong, S.; Kiyama, R.; Cai, P.; Liu, L.; Shen, H. Flos lonicerae extracts and chlorogenic acid protect human umbilical vein endothelial cells from the toxic damage of perfluorooctane sulphonate. Inflammation 2013, 36, 767–779. [Google Scholar] [CrossRef]
- Inami, Y.; Andoh, T.; Kuraishi, Y. Prevention of topical surfactant-induced itch-related responses by chlorogenic acid through the inhibition of increased histamine production in the epidermis. J. Pharmacol. Sci. 2013b, 121, 242–245. [Google Scholar]
- Nitta, Y.; Kikuzaki, H.; Ueno, H. Food components inhibiting recombinant human histidine decarboxylase activity. J. Agric. Food Chem. 2007, 55, 299–304. [Google Scholar]
- Sample Availability: Samples of the extracts of flower buds of Lonicera. japonica Thunb. are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inami, Y.; Matsui, Y.; Hoshino, T.; Murayama, C.; Norimoto, H. Inhibitory Activity of the Flower Buds of Lonicera japonica Thunb. against Histamine Production and L-Histidine Decarboxylase in Human Keratinocytes. Molecules 2014, 19, 8212-8219. https://doi.org/10.3390/molecules19068212
Inami Y, Matsui Y, Hoshino T, Murayama C, Norimoto H. Inhibitory Activity of the Flower Buds of Lonicera japonica Thunb. against Histamine Production and L-Histidine Decarboxylase in Human Keratinocytes. Molecules. 2014; 19(6):8212-8219. https://doi.org/10.3390/molecules19068212
Chicago/Turabian StyleInami, Yoshihiro, Yuko Matsui, Tomoko Hoshino, Chiaki Murayama, and Hisayoshi Norimoto. 2014. "Inhibitory Activity of the Flower Buds of Lonicera japonica Thunb. against Histamine Production and L-Histidine Decarboxylase in Human Keratinocytes" Molecules 19, no. 6: 8212-8219. https://doi.org/10.3390/molecules19068212
APA StyleInami, Y., Matsui, Y., Hoshino, T., Murayama, C., & Norimoto, H. (2014). Inhibitory Activity of the Flower Buds of Lonicera japonica Thunb. against Histamine Production and L-Histidine Decarboxylase in Human Keratinocytes. Molecules, 19(6), 8212-8219. https://doi.org/10.3390/molecules19068212



