Effect of Two Anti-Fungal Treatments (Metrafenone and Boscalid Plus Kresoxim-methyl) Applied to Vines on the Color and Phenol Profile of Different Red Wines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of New Generation Fungicides Residues on the Color
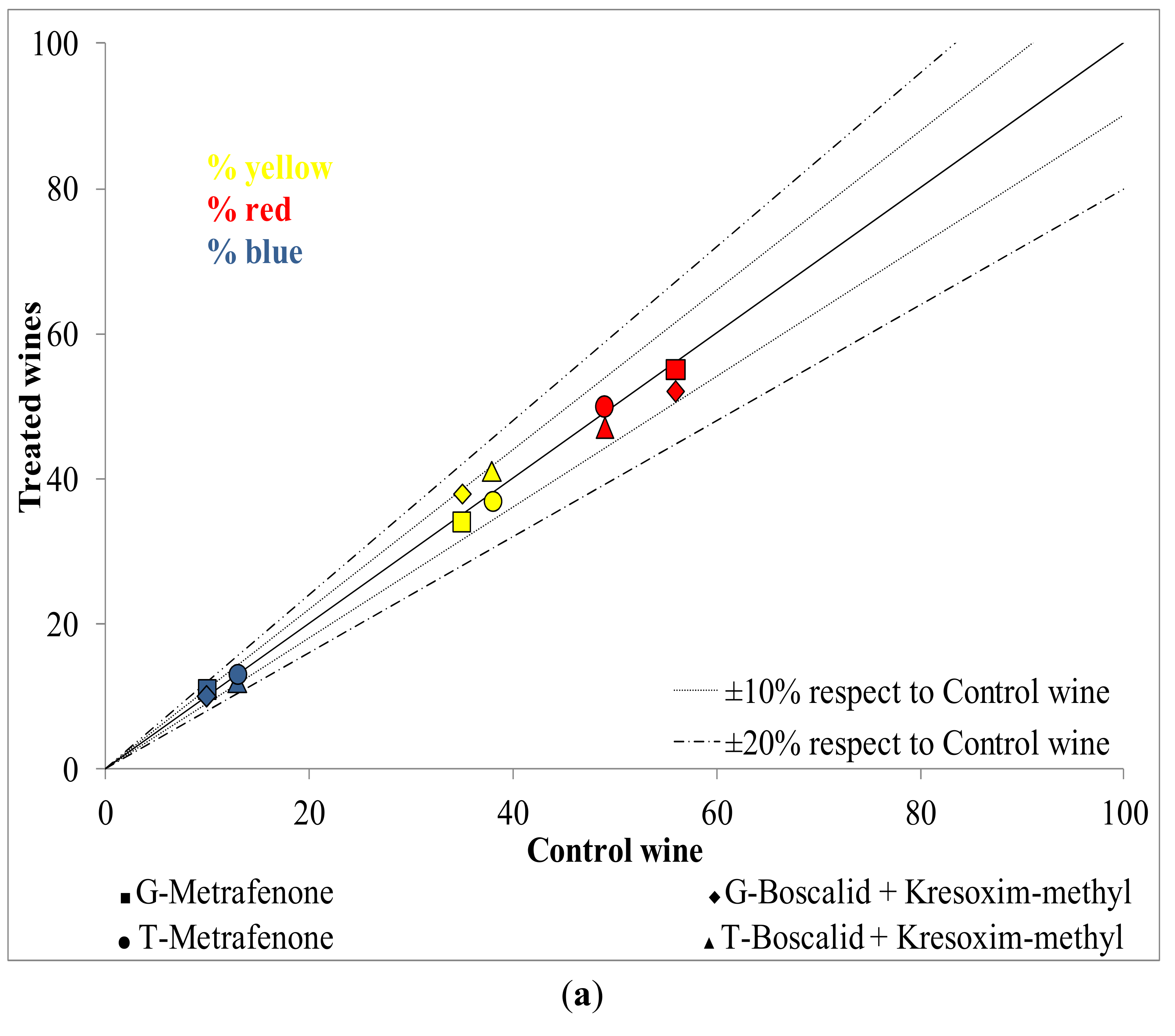
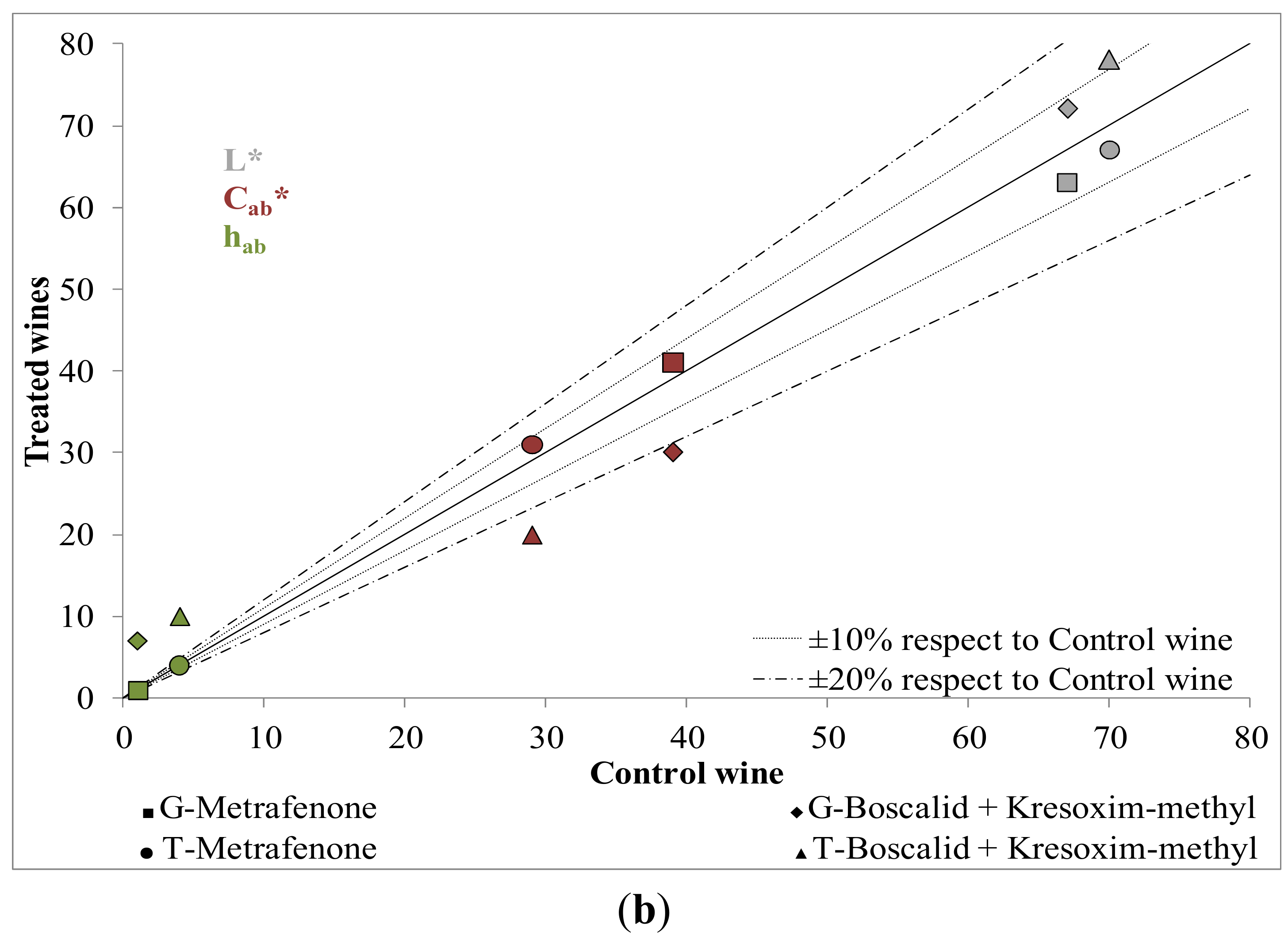
2.2. Influence of New Generation Fungicides Residues on the Phenolic Profile of Wines
2.2.1. Anthocyanins
| Wines | T-Control | T-Metrafenone | T-Boscalid-Kresoxim-methyl | G-Control | G-Metrafenone | G-Boscalid-Kresoxim-methyl | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthocyanins by UV/Vis | ||||||||||||
| Monomeric (%) | 34.68 ± 0.949 | 38.91 ± 1.753 37.37 ± 1.594 23.71 ± 0.159 3.880 ± 0.014 | 28.83 * ± 1.689 53.66 * ± 0.740 17.51 * ± 0.949 4.100 * ± 0.057 | 29.58 ± 1.244 43.05 ± 1.054 27.37 ± 0.190 5.180 ± 0.028 | 38.20 * ± 2.645 39.48 ± 1.983 22.33 * ± 0.662 4.345 * ± 0.021 | 34.36 * ± 1.060 46.37 ± 0.994 19.27 * ± 0.066 4.270 * ± ≤0.001 | ||||||
| Copigmented (%) | 41.87 ± 0.678 | |||||||||||
| Polymeric (%) | 23.46 ± 0.271 | |||||||||||
| TOTAL Anthocyanins (absorbance units) | 3.905 ± 0.021 | |||||||||||
| Monomeric anthocyanins by HPLC | ||||||||||||
| Malvidin derivatives | ||||||||||||
| malvidin-3-O-glucoside | 161.59 ± 4.204 13.25 ± 0.886 5.79 ± 0.592 0.85 ± 0.021 0.54 ± 0.032 0.25 ± 0.005 | 152.12 ± 2.326 11.26 ± 0.018 5.05 ± 0.088 0.91 ± 0.039 0.56 ± ≤0.001 0.37 * ± 0.020 | 158.57 ± 4.068 13.46 ± 0.445 6.62 ± 0.058 1.55 * ± 0.023 0.47 ± 0.055 0.06 * ± 0.006 | 163.75 ± 1.684 8.87 ± 0.723 11.44 ± 0.382 1.40 ± 0.131 1.37 ± 0.049 0.46 ± 0.043 | 128.79 * ± 3.121 9.63 ± 0.783 10.93 ±1.116 1.62 ± 0.168 2.10 * ± 0.204 0.36 ± 0.004 | 121.94 * ± 4.149 7.82 ± 0.202 10.30 * ± 0.300 1.85 * ± 0.100 1.93 * ± 0.100 0.47± 0.040 | ||||||
| malvidin-3-O-(6-O-p-coumaroyl)glucoside | ||||||||||||
| malvidin-3-O-(6-O-acetyl)glucoside | ||||||||||||
| malvidin-3-O-(6-O-caffeoyl)glucoside | ||||||||||||
| vitisin A | ||||||||||||
| vitisin B | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 182.12 (78.5) | 170.27 (79.9) | 180.73 (79.6) | 187.29 (80.8) | 153.44 * (86.7) | 144.31 * (85.6) | ||||||
| Petunidin derivatives | ||||||||||||
| petunidin-3-O-glucoside | 25.38 ± 1.947 3.00 ± 0.125 1.20 ± 0.117 | 22.82 ± 0.834 2.34 * ± 0.144 1.12 ± 0.059 | 25.32 ± 0.395 2.80 ± 0.059 1.00 ± 0.054 | 10.59 ± 0.095 0.39 ± 0.005 0.60 ± 0.045 | 6.35 * ± 0.074 0.03 * ± ≤0.001 0.60 ± 0.047 | 4.70 * ± 0.176 0.41 ± 0.020 0.27 * ± 0.016 | ||||||
| petunidin-3-O-(6-O-p-coumaroyl)glucoside | ||||||||||||
| petunidin-3-O-(6-O-acetyl)glucoside | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 29.58 (12.7) | 26.28 (12.3) | 29.12 (12.7) | 11.58 (5.0) | 6.98 * (3.9) | 5.38 * (5.2) | ||||||
| Delphinidin derivatives | ||||||||||||
| delphinidin-3-O-glucoside | 10.06 ± 0.735 2.99 ± 0.111 0.66 ± 0.041 | 8.24 * ± 0.284 0.95 * ± 0.001 0.68 ± 0.017 | 11.06 ± 0.070 1.20 * ± 0.007 0.70 ± 0.015 | 5.08 ± 0.071 n.d. 0.37 ± 0.009 | 1.99 * ± 0.219 n.d. 0.32 ± 0.035 | 1.36 * ± 0.064 n.d. 1.36 * ± 0.156 | ||||||
| delphinidin-3-O-(6-O-p-coumaroyl)glucoside | ||||||||||||
| delphinidin-3-O-(6-O-acetyl)glucoside | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 13.71 (5.9) | 9.87 * (4.6) | 12.96 (5.6) | 5.45 (2.4) | 2.31 * (1.3) | 2.72 * (1.6) | ||||||
| Peonidin derivatives | ||||||||||||
| peonidin-3-O-glucoside | 4.53 ± 0.123 0.82 ± 0.023 0.06 ± 0.004n.d. | 3.98 * ± 0.186 1.00 ± 0.010 0.37 * ± 0.004n.d. | 4.69 ± 0.027 0.84 ± 0.082 0.10 * ± 0.009n.d. | 18.76 ±0.385 4.39 ± 0.173 2.95 ± 0.053 0.16 ± 0.012 | 8.04 * ± 0.749 2.90 * ± 0.259 2.17 * ± 0.232 0.17 ± 0.008 | 10.39 * ± 0.290 2.49 * ± 0.060 2.30 * ± 0.222 0.25 * ± ≤0.001 | ||||||
| peonidin-3-O-(6-O-p-coumaroyl)glucoside | ||||||||||||
| peonidin-3-O-(6-O-acetyl)glucoside | ||||||||||||
| peonidin-3-O-(6-O-caffeoyl)glucoside | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 5.41 (2.3) | 5.37 (2.5) | 5.63 * (2.4) | 26.25 (11.3) | 13.28 * (7.5) | 15.43 * (9.1) | ||||||
| Cyanidin derivatives | ||||||||||||
| cyanidin-3-O-glucoside | 0.44 ± 0.003 0.56 ± 0.033 0.34 ± 0.017 | 0.48 ± 0.024 0.51 ± 0.002 0.41 * ± 0.011 | 0.46 ± 0.028 0.61 ± 0.031 0.38 * ± 0.004 | 0.43 ± 0.024 0.43 ± 0.006 0.22 ± 0.012 | 0.25 * ± 0.032 0.41 ± 0.021 0.34 * ± 0.001 | 0.25 * ± 0.017 0.10 * ± 0.009 0.49 * ± 0.007 | ||||||
| cyanidin-3-O-(6-O-p-coumaroyl)glucoside | ||||||||||||
| cyanidin-3-O-(6-O-acetyl)glucoside | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 1.34 (0.6) | 1.40 (0.7) | 1.45 (0.6) | 1.09 (0.5) | 1.00 (0.6) | 0.84 * (0.5) | ||||||
| TOTAL monomeric anthocyanins (mg∙L−1) | 232.16 | 213.17 | 229.89 | 231.65 | 177.01 * | 168.68 * | ||||||
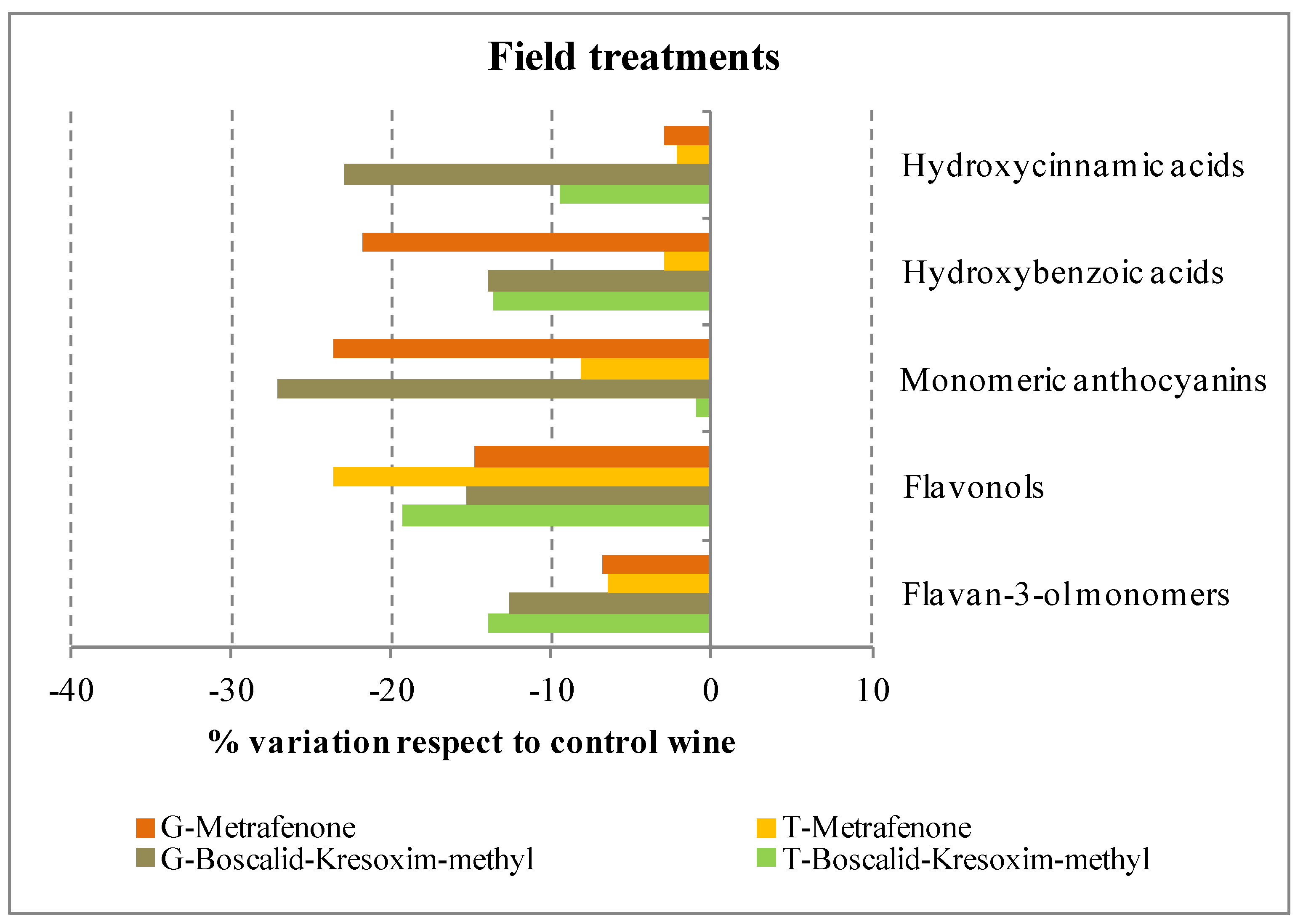
2.2.2. Flavan-3-ol Monomers and Proanthocyanidins
| Wines | T-Control | T-Metrafenone | T-Boscalid-Kresoxim-methyl | G-Control | G-Metrafenone | G-Boscalid-Kresoxim-methyl | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavan-3-ol monomers | ||||||||||||
| catechin (C) | 19.49 ± 0.013 7.93 ± 0.035 1.14 ± 0.023 | 19.26 ± 0.036 7.43 ± 0.239 n.d. | 18.32 * ± 0.054 6.27 * ± 0.003 n.d. | 24.39 ± 0.419 23.77 ± 0.270 1.48 ± 0.004 | 23.39 ± 0.276 21.70 ± 0.721 1.42 * ± 0.004 | 20.94 * ± 0.232 20.98 * ± 0.167 1.44 * ± 0.001 | ||||||
| epicatechin (EC) | ||||||||||||
| galocatechin (GC) | ||||||||||||
| subTOTAL (mg∙L−1) | 28.56 | 26.69 | 24.59 * | 49.64 | 46.29 | 43.36 * | ||||||
| Proanthocyanidins | ||||||||||||
| aDP (%) | 2.6 765.4 59 40 0.5 | 2.7 811.5 60 40 0.5 | 2.6 778.1 68 3 10.6 | 1.8 526.4 79 20 1.3 | 1.8 516.9 81 18 1.2 | 1.8 521.9 81 18 0.8 | ||||||
| aMW (%) | ||||||||||||
| procyanidins (%PC) | ||||||||||||
| prodelphinidins (% PD) | ||||||||||||
| galloylated (% G) | ||||||||||||
| subTOTAL (mg∙L−1) | 241.94 ± 0.11 | 228.05 ± 1.83 | 200.80 * ± 1.11 | 219.18 ± 1.46 | 207.51 ± 0.35 | 191.40 * ± 2.23 | ||||||
2.2.3. Flavonols
| Wines | T-Control | T-Metrafenone | T-Boscalid-Kresoxim-methyl | G-Control | G-Metrafenone | G-Boscalid-Kresoxim-methyl | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-O-glucoside derivatives | ||||||||||||
| myricetin-3-O-glucoside | 3.46 ± 0.279 0.32 ± 0.021 1.01 ± 0.030 0.12 ± 0.001 0.15 ± 0.005 0.95 ± 0.025 | 2.48 * ± 0.072 0.36 * ± 0.021 0.78 * ± 0.035 0.09 * ± 0.002 0.13 * ± 0.004 0.90 ±0.078 | 2.50 * ± 0.215 0.26 * ± 0.012 0.86 * ± 0.028 0.14 * ± 0.009 0.14 ± 0.006 0.86 ± 0.057 | 0.61 ± 0.001 0.13 ± 0.002 0.74 ± 0.035 n.d. 0.25 ± 0.016 2.55 ± 0.161 | 0.37 * ± ≤0.001 n.d. 0.61 * ± 0.002 n.d. 0.24 ± 0.005 2.39 ± 0.072 | 0.37 * ± 0.001 0.13 ± 0.002 0.60 * ± 0.048 n.d. 0.44 * ± 0.022 2.36 ± 0.171 | ||||||
| quercetin-3-O-glucoside | ||||||||||||
| laricitrin-3-O-glucoside | ||||||||||||
| kaempherol-3-O-glucoside | ||||||||||||
| isohamnetin-3-O-glucoside | ||||||||||||
| syringetin-3-O-glucoside | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 6.01 (73.3) | 4.74 * (75.7) | 4.76 * (71.9) | 4.28 (66.2) | 3.62 * (65.8) | 3.90 (71.3) | ||||||
| 3-O-glucuronide derivatives | ||||||||||||
| myricetin-3-O-glucuronide | 0.55 ± 0.006 0.63 ± 0.037 0.19 ± 0.017 | 0.43 * ± 0.040 0.50 * ± 0.029 0.14 * ± 0.001 | 0.41 * ± 0.024 0.63 ± 0.048 0.17 ± 0.001 | 0.23 ± 0.017 0.93 ± 0.038 0.23 ± ≤0.001 | 0.21 ± 0.007 0.95 ± 0.011 0.28 * ± 0.004 | 0.15 * ± 0.003 0.72 * ± 0.022 0.25 ± 0.001 | ||||||
| quercetin-3-O-glucuronide | ||||||||||||
| kaempherol-3-O-glucuronide | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 1.37 (16.7) | 1.07 * (17.1) | 1.21 (18.3) | 1.39 (21.5) | 1.43 (26.0) | 1.12 * (20.5) | ||||||
| 3-O-galactoside derivatives | ||||||||||||
| quercetin-3-O-galactoside | 0.14 ± 0.001 0.07 ± 0.001 | 0.10 * ± 0.001 n.d. | 0.14 ± 0.003 0.08 ± 0.001 | 0.07 ± 0.002 n.d. | n.d. n.d. | 0.06 * ± ≤0.001 n.d. | ||||||
| kaempherol-3-O-galactoside | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 0.21 (2.6) | 0.10 * (1.6) | 0.22 (3.3) | 0.07 (1.1) | 0.06 * (1.1) | |||||||
| Aglycons | ||||||||||||
| myricetin | 0.27 ± 0.025 0.21 ± 0.011 0.06 ± ≤0.001 0.07 ± ≤0.001 | 0.15 * ± 0.005 0.14 * ± ≤0.001 n.d. 0.06 * ± 0.002 | 0.23 ± 0.006 0.14 * ± 0.004 n.d. 0.06 * ±≤0.001 | 0.24 ± 0.019 0.37 ± 0.028 0.05 ± ≤0.001 0.06 ± 0.001 | 0.18 * ± ≤0.001 0.27 * ± ≤0.001 n.d. n.d. | 0.11 * ± ≤0.001 0.17 * ± ≤0.001 0.05 ± ≤0.001 0.05 * ± ≤0.001 | ||||||
| quercetin | ||||||||||||
| kaempherol | ||||||||||||
| laricitrin | ||||||||||||
| subTOTAL (mg∙L−1) (%) | 0.60 (7.3) | 0.35 * (5.6) | 0.43 * (6.5) | 0.72 (11.1) | 0.45 * (8.2) | 0.39 * (7.2) | ||||||
| TOTAL flavonols (mg∙L−1) | 8.20 | 6.26 * | 6.62 * | 6.46 | 5.50 * | 5.47 * | ||||||
| Wines | T-Control | T-Metrafenone | T-Boscalid-Kresoxim-methyl | G-Control | G-Metrafenone | G-Boscalid-Kresoxim-methyl | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||||||||||
| gallic acid | 17.00 ± 0.788 6.01 ± 0.252 2.54 ± 0.098 | 17.25 ± 0.160 4.93 * ± 0.226 2.45 ± 0.124 | 13.88 * ± 0.754 5.69 ± 0.189 2.17 * ± 0.151 | 10.55 ± 0.046 3.42 ± 0.070 1.72 ± 0.106 | 5.41 * ± 0.110 2.42 * ± 0.120 1.58 ± 0.008 | 7.29 * ± 0.152 2.21 * ± 0.048 1.97 ± 0.003 | |||||||
| 3,5-dihydroxibenzoic acid | |||||||||||||
| protocatechuic acid | |||||||||||||
| Hydroxybenzoic acids | |||||||||||||
| vanillic acid | 2.58 ± 0.005 4.07 ± 0.007 | 3.19 ± 0.002 3.42 * ± 0.231 | 2.21 * ± 0.047 3.86 ± 0.297 | 6.25 ± 0.348 3.52 ± 0.355 | 6.23 ± 0.200 4.26 ± 0.134 | 5.93 ± 0.588 4.52 ± 0.393 | |||||||
| syringic acid | |||||||||||||
| subTOTAL (mg∙L−1) (%) | 32.20 (51.4) | 31.24 (51.2) | 27.82 * (50.2) | 25.46 (65.2) | 19.90 * (60.1) | 21.91 * (67.9) | |||||||
| Hydroxycinnamic acids and their derivatives | |||||||||||||
| caftaric acid | 16.44 ± 1.022 0.46 ± 0.012 2.36 ± 0.141 10.08 ± 0.824 1.10 ± 0.112 | 16.48 ± 0.948 0.50 ± ≤0.001 2.14 ± 0.080 9.69 ± 0.631 0.98 ± 0.060 | 14.74 ± 0.478 0.32 * ± 0.001 2.13 ± 0.132 9.40 ± 0.645 0.98 ± 0.044 | 7.31 ± 0.130 0.12 ± 0.007 1.08 ± 0.003 3.17 ± 0.017 1.58 ± 0.127 | 7.37 ± 0.340 0.07 * ± 0.001 0.94 * ± 0.009 3.30 ± 0.192 1.20 * ± 0.017 | 5.77 * ± 0.487 0.01 * ± ≤0.001 0.78 * ± 0.034 2.37 * ± 0.228 1.30 * ± 0.073 | |||||||
| caffeic acid | |||||||||||||
| c-coutaric acid | |||||||||||||
| t-coutaric acid | |||||||||||||
| p-coumaric acid | |||||||||||||
| subTOTAL (mg∙L−1) (%) | 30.43 (48.6) | 29.78 (48.8) | 27.57 (49.8) | 13.27 (34.0) | 12.88 (38.9) | 10.23 * (31.7) | |||||||
| Stylbene | |||||||||||||
| resveratrol | n.d. | n.d. | n.d. | 0.30 ± 0.002 | 0.32 ± 0.011 | 0.10 * ± 0.002 | |||||||
| TOTAL phenolic acids (mg∙L−1) | 62.63 | 61.02 | 55.39 * | 39.03 | 33.10 * | 32.24 * | |||||||
2.2.4. Acids
3. Experimental
3.1. Fungicide Experiments
3.2. Winemaking Process and Wine Samples
3.3. Analytical Standards, Reagents and Materials
3.4. Characterization of the Color Fraction and Phenolic Content
- -
- Aacet: 20 μL of 10% (v/v) acetaldehyde was added to 2 mL of prepared wine and the sample was allowed to sit for 45 min at room temperature before measuring A520 nm;
- -
- A20: to another 100 µL of prepared wine, 1,900 µL hydroalcoholic solution was added and absorbance A520 nm was also measured;
- -
- ASO2: 160 μL of 5% (w/v) SO2 was added to 2 mL of prepared wine and absorbance A520 nm was measured.
- copigmented anthocyanins = Aacet − A20
- monomeric anthocyanins = A20 − ASO2
- polymeric anthocyanins = ASO2
- total anthocyanins = Aacet
- % copigmented = [(Aacet − A20)/Aacet] × 100
- % monomeric = [(A20 − ASO2)/Aacet] × 100
- % polymeric = [ASO2/Aacet] × 100
3.5. Determination of Phenolic Compounds
3.5.1. Extraction Procedures
3.5.2. HPLC/DAD–ESI/MS Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Russell, P.E. Fungicide resistance: Occurrence and management. J. Agric. Sci. 1995, 124, 317–323. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Pirisi, F.P.; Farris, G.A.; Madau, G.; Emonti, G. Pesticides in fermentative processes of wine. J. Agric. Food Chem. 1999, 47, 3854–3857. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Simal-Gándara, J. Efficacy of new commercial formulations to control downy mildew and dissipation of their active fungicides in wine after good agricultural practices. J. Sci. Food Agric. 2009, 89, 2625–2635. [Google Scholar] [CrossRef]
- Barba, A.; Oliva, J.; Payá, P. Influence of fungicide residues in wine quality. In Fungicides; Carisse, O., Ed.; InTech Europe: Rijeka, Croatia, 2010; pp. 421–440. [Google Scholar]
- Oliva, J.; Navarro, S.; Barba, A.; Navarro, G.; Salinas, M.R. Effect of pesticide residues on the aromatic composition of red wines. J. Agric. Food Chem. 1999, 47, 2830–2836. [Google Scholar]
- García, M.A. Influencia de los Residuos de Fungicidas en la Cinética Fermentativa y Calidad de Vinos Blancos de la D.O. Jumilla. Ph.D. Thesis, Universidad de Murcia, Facultad de Química, Jumilla, Murcia, Spain, 2002. [Google Scholar]
- Noguerol-Pato, R.; González-Rodríguez, R.M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Influence of tebuconazole residues on the aroma composition of Mencía red wines. Food Chem. 2011, 124, 1525–1532. [Google Scholar] [CrossRef]
- Oliva, J.; Barba, A.; San Nicolás, F.T.; Payá, P. Efectos de residuos de fungicidas en la composición fenólica de vinos tintos (var. Monastrell). Tecnología Del Vino 2005, 23, 37–40. [Google Scholar]
- García-Marino, M.; Escudero-Gilete, M.L.; Heredia, F.J.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C. Color-copigmentation study by tristimulus colorimetry (CIELAB) in red wines obtained from Tempranillo and Graciano varieties. Food Res. Int. 2013, 51, 123–131. [Google Scholar] [CrossRef]
- García-Marino, M.; Hernández-Hierro, J.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Multivariate analysis of the polyphenol composition of Tempranillo and Graciano red wines. Talanta 2011, 85, 2060–2066. [Google Scholar] [CrossRef]
- Boulton, R.B. A method for the assessment of copigmentation in red wines. In Proceedings of the Forty-seventh Annual Meeting of the American Society for Enology and Viticulture; Reno, NV, USA: 26–28 June 1996.
- Hermosín-Gutiérrez, I. Copigmentación y piranoantocianos: el papel de los flavonoles y los ácidos hidroxicinámicos en el color del vino tinto. ACE Revista de Enología. 2007, 81. Available online: http://www.acenologia.com/ciencia81_2.htm (accessed on 4 June 2014).
- Mateus, N.; de Freitas, V. Evolution and stability of anthocyanin-derived pigments during port wine aging. J. Agric. Food Chem. 2001, 49, 5217–5222. [Google Scholar] [CrossRef]
- Tsanova-Savova, S.; Dimov, S.; Ribarova, F. Anthocyanins and color variables of Bulgarian aged red wines. J. Food Comp. Anal. 2002, 15, 647–654. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailon, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Identification of dimeric anthocyanins and new oligomeric pigments in red wine by means of HPLC-DAD-ESI/MS. J. Mass Spectrom. 2007, 42, 735–748. [Google Scholar] [CrossRef]
- Monagas, M.; Núñez, V.; Bartolomé, B.; Laureano, O.; Ricardo da Silva, J.M. Monomeric, oligomeric and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. cv. Graciano, Tempranillo and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the copigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, R.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The chemistry of wine Stabilization and treatments. In Handbook of Enology; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 2, pp. 141–203. [Google Scholar]
- Cheynier, V.; Sarni-Manchado, P. Wine taste and mouthfeel. In Managing Wine Quality, Viticulture and Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; Volume 1, pp. 29–58. [Google Scholar]
- Quijada-Morín, N.; Regueiro, J.; Simal-Gándara, J.; Tomás, E.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between the sensory-determined astringency and the flavanolic composition of red wines. J. Agric. Food Chem. 2012, 60, 12355–12361. [Google Scholar] [CrossRef]
- González-Manzano, S.; Santos-Buelga, C.; Pérez-Alonso, J.J.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterization of the mean degree of polymerization of proanthocyanidins in red wines using liquid chromatography–mass spectrometry (LC-MS). J. Agric. Food Chem. 2006, 54, 4326–4332. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. 1-ère partie. Les equilibres des anthocyanes et des tanins. Connaissance de la Vigne et du Vin 1984, 18, 195–217. [Google Scholar]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2000. [Google Scholar]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Separation of pyranoanthocyanins from red wine by column chromatography. Anal. Chim. Acta 2004, 513, 305–318. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago-Blanco, J.L.; Martínez-Rodríguez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol fingerprints, with correlations between their biosynthesis pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Briz-Cid, N.; Figueiredo-González, M.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Effect of Two Anti-Fungal Treatments (Metrafenone and Boscalid Plus Kresoxim-methyl) Applied to Vines on the Color and Phenol Profile of Different Red Wines. Molecules 2014, 19, 8093-8111. https://doi.org/10.3390/molecules19068093
Briz-Cid N, Figueiredo-González M, Rial-Otero R, Cancho-Grande B, Simal-Gándara J. Effect of Two Anti-Fungal Treatments (Metrafenone and Boscalid Plus Kresoxim-methyl) Applied to Vines on the Color and Phenol Profile of Different Red Wines. Molecules. 2014; 19(6):8093-8111. https://doi.org/10.3390/molecules19068093
Chicago/Turabian StyleBriz-Cid, Noelia, María Figueiredo-González, Raquel Rial-Otero, Beatriz Cancho-Grande, and Jesús Simal-Gándara. 2014. "Effect of Two Anti-Fungal Treatments (Metrafenone and Boscalid Plus Kresoxim-methyl) Applied to Vines on the Color and Phenol Profile of Different Red Wines" Molecules 19, no. 6: 8093-8111. https://doi.org/10.3390/molecules19068093
APA StyleBriz-Cid, N., Figueiredo-González, M., Rial-Otero, R., Cancho-Grande, B., & Simal-Gándara, J. (2014). Effect of Two Anti-Fungal Treatments (Metrafenone and Boscalid Plus Kresoxim-methyl) Applied to Vines on the Color and Phenol Profile of Different Red Wines. Molecules, 19(6), 8093-8111. https://doi.org/10.3390/molecules19068093








