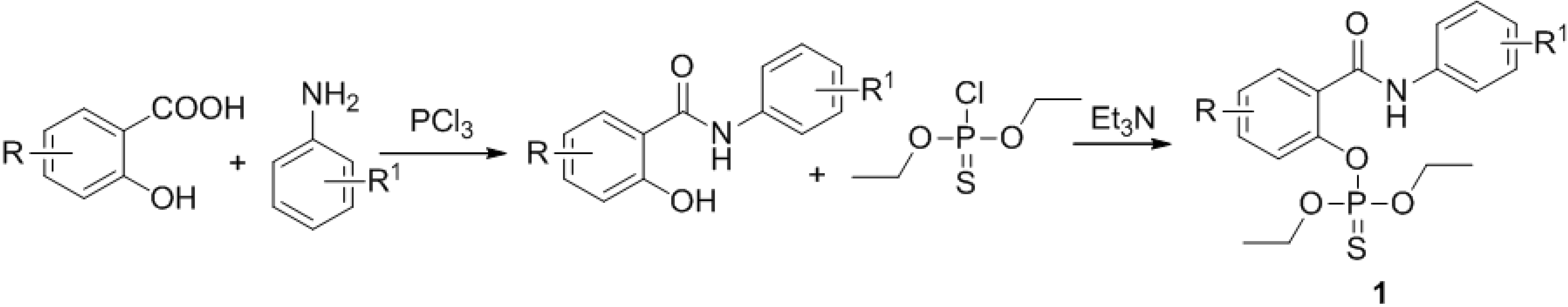
3.2. Chemistry
General Procedure for the Synthesis of Diethyl (2-phenylcarbamoyl)thiophosphates
Appropriate salicylanilide (2.0 mmol) was suspended at 20 °C in dichloromethane (10 mL) and then 1.5 equivalents of triethylamine (0.418 mL; 3.0 mmol) were added under vigorous stirring. After 5 min, 1.2 equivalents of O,O-diethyl phosphorochloridothioate (0.347 mL) was added to the mixture. The mixture was stirred at the room temperature for 2 h; the reaction was monitored by TLC using a 4:1 toluene/ethyl acetate mixture as eluent. After this time, the solution was added to a chromatography column using chloroform or chloroform/hexane 9:1 as eluent. The solvent was removed under reduced pressure to obtain the product, which was recrystallized from acetone-hexane, if necessary.
O-{4-Bromo-2-[(3-chlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1a). Yield: 86%, white solid; m.p. 55–56 °C; IR: 1678 (amide I), 1593 (ν CCaromatic), 1529 (amide II), 1478 (ν CCaromatic) cm−1; 1H-NMR: δ 9.06 (1H, bs, NH), 8.15 (1H, dd, J = 2.5 Hz, J = 1.0 Hz, H3), 7.86 (1H, t, J = 2.0 Hz, H2'), 7.61–7.57 (2H, m, H5, H6'), 7.30–7.26 (2H, m, H6, H5'), 7.14–7.11 (1H, m, H4'), 4.28–4.16 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 0.9 Hz, CH3); 13C-NMR: δ 161.4, 146.7 (1C, d, J = 8.1 Hz), 139.0, 135.4 (1C, d, J = 1.5 Hz), 134.6, 134.5, 130.0, 128.5 (1C, d, J = 6.1 Hz), 124.6, 122.9 (1C, d, J = 3.0 Hz), 120.1, 119.1 (1C, d, J = 2.0 Hz), 118.0, 66.0 (2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd. for C17H18BrClNO4PS (478.72): C, 42.65; H, 3.79; N, 2.93. Found: C, 42.45; H, 4.00; N, 2.96.
O-{4-Bromo-2-[(4-chlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1b). Yield: 89%, white solid; m.p. 75–77 °C; IR: 1666 (amide I), 1592 (ν CCaromatic), 1530 (amide II), 1492, 1478 (ν CCaromatic) cm−1; 1H-NMR: δ 9.05 (1H, bs, NH), 8.14 (1H, dd, J = 2.5 Hz, 1.0 Hz, H3), 7.72–7.69 (2H, m, H2', H6'), 7.58 (1H, dd, J = 8.5 Hz, 2.5 Hz, H5), 7.34–7.31 (2H, m, H3', H5'), 7.27 (1H, dd, J = 8.5 Hz, 1.0 Hz, H6), 4.28–4.15 (4H, m, CHAHB), 1.31 (6H, dt, J = 7.0 Hz, 0.7 Hz, CH3); 13C-NMR: δ 161.4, 146.6 (1C, d, J = 8.5 Hz), 136.4, 135.3 (1C, d, J = 1.4 Hz), 134.5, 129.6, 129.0, 128.7 (1C, d, J = 6.3 Hz), 122.9 (1C, d, J = 2.8 Hz), 121.3, 119.0 (1C, d, J = 2.0 Hz), 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C17H18BrClNO4PS (478.72): C, 42.65; H, 3.79; N, 2.93. Found: C, 42.76; H, 3.70; N, 2.99.
O-{4-Bromo-2-[(3,4-dichlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1c). Yield: 69%, white solid; m.p. 80–81 °C; IR: 1678 (amide I), 1585 (ν CCaromatic), 1525 (amide II), 1476 (ν CCaromatic) cm−1; 1H-NMR: δ 9.13 (1H, bs, NH), 8.15 (1H, dd, J = 2.5 Hz, 1.0 Hz, H3), 8.00 (1H, d, J = 2.5 Hz, H2'), 7.61–7.57 (2H, m, H5, H6'), 7.41 (1H, d, J = 8.5 Hz, H5'), 7.28 (1H, dd, J = 8.5 Hz, 1.0 Hz, H6), 4.29–4.16 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.4, 146.7 (1C, d, J = 8.4 Hz), 137.3, 135.6 (1C, d, J = 1.5 Hz), 134.6, 132.8, 130.5, 128.1 (1C, d, J = 6.1 Hz), 127.8, 122.9 (1C, d, J = 2.9 Hz), 121.7, 119.3, 119.1 (1C, d, J = 1.9 Hz), 66.1 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.3 Hz, CH3); 31P-NMR: δ 62.5. Anal. Calcd for C17H17BrCl2NO4PS (513.17): C, 39.79; H, 3.34; N, 2.73. Found: C, 39.88; H, 3.46; N, 2.85.
O-{4-Bromo-2-[(3-bromophenyl)carbamoyl]phenyl} diethyl phosphate (1d). Yield: 63%, white solid; m.p. 67–68 °C (acetone-hexane); IR: 1677 (amide I), 1591 (ν CCaromatic), 1530 (amide II), 1476 (ν CCaromatic) cm−1; 1H-NMR: δ 9.05 (1H, bs, NH), 8.15 (1H, dd, J = 2.5 Hz, 1.0 Hz, H3), 8.00 (1H, t, J = 1.8 Hz, H2'), 7.67–7.64 (1H, m, H6'), 7.59 (1H, dd, J = 8.5 Hz, 2.5 Hz, H5), 7.30–7.21 (3H, m, H6, H4', H5'), 4.29–4.16 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.4, 146.7 (1C, d, J = 8.3 Hz), 139.1, 135.4 (1C, d, J = 1.5 Hz), 134.6, 130.3, 128.5 (1C, d, J = 6.3 Hz), 127.6, 122.9, 122.9 (1C, d, J = 2.9 Hz), 122.6, 119.1 (1C, d, J = 1.9 Hz), 118.5, 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C17H18Br2NO4PS (523.18): C, 39.03; H, 3.47; N, 2.68. Found: C, 39.14; H, 3.34; N, 2.55.
O-{4-Bromo-2-[(4-bromophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1e). Yield: 69%, white solid; m.p. 82–83 °C (acetone-hexane); IR: 1666 (amide I), 1589 (ν CCaromatic), 1527 (amide II), 1488, 1441 (ν CCaromatic) cm−1; 1H-NMR: δ 9.05 (1H, bs, NH), 8.14 (1H, dd, J = 2.5 Hz, 1.0 Hz, H3), 7.67–7.64 (2H, m, H2', H6'), 7.58 (1H, dd, J = 8.5 Hz, J = 2.5 Hz, H5), 7.49–7.45 (2H, m, H3', H5'), 7.27 (1H, dd, J = 8.5 Hz, 1.3 Hz, H6), 4.27–4.15 (4H, m, CHAHB), 1.31 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.4, 146.6 (1C, d, J = 8.4 Hz), 136.9, 135.3 (1C, d, J = 1.4 Hz), 134.5, 132.0, 128.7 (1C, d, J = 6.0 Hz), 122.9 (1C, d, J = 2.9 Hz), 121.6, 119.1 (1C, d, J = 1.9 Hz), 117.2, 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.5. Anal. Calcd for C17H18Br2NO4PS (523.18): C, 39.03; H, 3.47; N, 2.68. Found: C, 39.18; H, 3.34; N, 2.71.
O-{4-Bromo-2-[(3-fluorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1f). Yield: 73%, oily liquid; IR: 1680 (amide I), 1606 (ν CCaromatic), 1540 (amide II), 1492, 1473 (ν CCaromatic) cm−1; 1H-NMR: δ 9.09 (1H, bs, NH), 8.15 (1H, d, J = 2.5 Hz, H3), 7.70 (1H, d, J = 11.0 Hz, H6'), 7.58 (1H, dd, J = 8.5 Hz, 2.5 Hz, H5), 7.41–7.25 (2H, m, H6, H2', H5'), 6.85 (1H, td, J = 8.5 Hz, 1.8 Hz, H4'), 4.26–4.17 (4H, m, CHAHB), 1.31 (6H, t, J = 7.0 Hz, CH3); 13C-NMR: δ 162.9 (1C, d, J = 243.3 Hz, C3'), 161.5, 146.6 (1C, d, J = 8.4 Hz), 139.3 (1C, d, J = 10.8 Hz), 135.3 (1C, d, J = 1.5 Hz), 134.5, 130.0 (1C, d, J = 9.3 Hz), 128.6 (1C, d, J = 6.1 Hz), 122.9 (1C, d, J = 3.0 Hz), 119.0 (1C, d, J = 2.0 Hz), 115.3 (1C, d, J = 2.9 Hz), 111.3 (1C, d, J = 21.4 Hz), 107.5 (1C, d, J = 26.5 Hz), 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C17H18BrFNO4PS (462.27): C, 44.17; H, 3.92; N, 3.03. Found: C, 44.03; H, 4.05; N, 3.20.
O-{4-Bromo-2-[(4-fluorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1g). Yield: 49%, oily liquid; IR: 1664 (amide I), 1614 (ν CCaromatic), 1541 (amide II), 1509, 1470 (ν CCaromatic) cm−1; 1H-NMR: δ 9.00 (1H, bs, NH), 8.14 (1H, dd, J = 2.5 Hz, 1.0 Hz, H3), 7.73–7.69 (2H, m, H2', H6'), 7.57 (1H, dd, J = 8.7 Hz, 2.5 Hz, H5), 7.28 (1H, dd, J = 8.5 Hz, 1.0 Hz, H6), 7.08–7.03 (2H, m, H3', H5'), 4.27–4.16 (4H, m, CHAHB), 1.30 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.3, 159.5 (1C, d, J 242.6 Hz, C4'), 146.6 (1C, d, J = 8.5 Hz), 135.2 (1C, d, J = 1.5 Hz), 134.5, 133.9 (1C, d, J = 2.9 Hz, C1'), 128.80 (1C, d, J = 6.1 Hz), 122.9 (1C, d, J = 3.0 Hz), 121.7 (2C, d, J = 7.8 Hz, C2', C6'), 119.0 (1C, d, J = 2.0 Hz), 115.6 (2C, d, J = 22.4 Hz, C3', C5'), 65.9 ( 2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C17H18BrFNO4PS (462.27): C, 44.17; H, 3.92; N, 3.03. Found: C, 44.30; H, 3.99; N, 2.87.
O-(4-Bromo-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl) O,O-diethyl phosphorothioate (1h). Yield: 38%, oily liquid; IR: 1677 (amide I), 1606 (ν CCaromatic), 1537 (amide II), 1492, 1472 (ν CCaromatic) cm−1; 1H-NMR: δ 9.21 (1H, bs, NH), 8.19 (1H, dd, J = 2.8 Hz, 1.1 Hz, H3), 8.04 (1H, s, H2'), 7.97 (1H, d, J = 8.2 Hz, H6'), 7.60 (1H, dd, J = 8.7 Hz, 2.6 Hz, H5), 7.49 (1H, t, J = 8.0 Hz, H5'), 7.41 (1H, d, J = 7.8 Hz, H4'),7.31 (1H, dd, J = 8.7 Hz, 1.3 Hz, H6), 4.29–4.17 (4H, m, CHAHB), 1.31 (6H, t, J = 7.1 Hz, CH3); 13C-NMR: δ 161.55, 146.76 (1C, d, J = 8.2 Hz), 138.39, 135.51 (1C, d, J = 1.8 Hz), 134.64 (1C, d, J = 1.0 Hz), 131.39 (1C, q, J = 32.5 Hz, C3'), 129.57, 128.27 (1C, d, J = 6.2 Hz), 123.83 (1C, q, J = 272.5 Hz, CF3), 123.12, 122.89 (1C, d, J = 2.9 Hz), 121.12 (1C, q, J = 3.9 Hz, C2'), 119.09 (1C, d, J = 2.1 Hz), 116.78 (1C, q, J = 3.9 Hz, C4'), 66.05 (2C, d, J = 5.6 Hz, CHAHB), 15.76 (2C, d, J = 7.3 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd for C18H18BrF3NO4PS (512.28): C, 42.20; H, 3.54; N, 2.73. Found: C, 42.41; H, 3.59; N, 2.64.
O-(4-Bromo-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl) O,O-diethyl phosphorothioate (1i). Yield: 61%, white solid; m.p. 83–84 °C (acetone-hexane); IR: 1683 (amide I), 1604 (ν CCaromatic), 1540 (amide II), 1472 (ν CCaromatic) cm−1; 1H-NMR: δ 9.23 (1H, bs, NH), 8.17 (1H, dd, J = 2.5 Hz, 1.3 Hz, H3), 7.89 (2H, d, J = 8.5 Hz, H3', H5'), 7.62 (2H, d, J = 8.5 Hz, H2', H6'), 7.60 (1H, dd, J = 9.0 Hz, 2.5 Hz, H5), 7.23 (1H, dd, J = 9.0 Hz, 1.3 Hz, H6), 4.29–4.16 (4H, m, CHAHB), 1.31 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.6, 146.7 (1C, d, J = 8.5 Hz), 140.9 (1C, d, J = 1.1 Hz), 135.5 (1C, d, J = 1.6 Hz), 134.6, 128.4 (1C, d, J = 6.1 Hz), 126.3 (2C, q, J = 3.8 Hz, C3', C5'), 126.3 (1C, q, J = 32.8 Hz, C4'), 124.0 (1C, q, J = 270.1 Hz, CF3), 122.9 (1C, d, J = 2.9 Hz), 119.7, 119.1 (1C, d, J = 2.0 Hz), 66.0 (2C, d, J =5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.5. Anal. Calcd for C18H18BrF3NO4PS (512.28): C, 42.20; H, 3.54; N, 2.73. Found: C, 42.39; H, 3.38; N, 2.91.
O-{4-Chloro-2-[(3-chlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1j). Yield: 75%, white solid; m.p. 52–54 °C; IR: 1677, 1658 (amide I), 1597 (ν CCaromatic), 1540 (amide II), 1476, 1428 (ν CCaromatic) cm−1; 1H-NMR: δ 9.07 (1H, bs, NH), 8.00 (1H, dd, J = 2.8 Hz, 1.3 Hz, H3), 7.87 (1H, t, J = 2.0 Hz, H2'), 7.59 (1H, dd, J = 8.3 Hz, 1.3 Hz, H6'), 7.43 (1H, dd, J = 8.8 Hz, 2.8 Hz, H5), 7.35 (1H, dd, J = 8.5 Hz, 1.5 Hz, H6), 7.28 (1H, t, J = 8.0 Hz, H5'), 7.14–7.11 (1H, m, H4'), 4.28–4.16 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 0.9 Hz, CH3); 13C-NMR: δ 161.5, 146.1 (1C, d, J = 8.4 Hz), 139.0, 134.6, 132.4 (1C, d, J = 1.8 Hz), 131.6, 131.6 (1C, d, J = 1.9 Hz), 130.0, 128.2 (1C, d, J = 6.1 Hz), 124.6, 122.6 (1C, d, J = 3.0 Hz), 120.1, 118.0, 66.0 (2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.5 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd for C17H18Cl2NO4PS (434.27): C, 47.02; H, 4.18; N, 3.23. Found: C, 47.15; H, 4.08; N, 3.32.
O-{4-Chloro-2-[(4-chlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1k). Yield: 62%, white solid; m.p. 53–54 °C; IR: 1666 (amide I), 1594 (ν CCaromatic), 1536 (amide II), 1492 (ν CCaromatic) cm−1; 1H-NMR: δ 9.06 (1H, bs, NH), 7.99 (1H, d, J = 2.5 Hz, H3), 7.71 (2H, d, J = 8.5 Hz, H2', H6'), 7.43 (1H, dd, J = 8.8 Hz, 2.5 Hz, H5), 7.35–7.29 (3H, m, H6, H3', H5'), 4.28–4.15 (4H, m, CHAHB), 1.30 (6H, t, J = 7.0 Hz, CH3); 13C-NMR: δ 161.5, 146.1 (1C, d, J = 8.6 Hz), 136.4, 132.3, 131.5, 129.5, 129.0, 128.4 (1C, d, J = 6.3 Hz), 122.6 (1C, d, J = 2.6 Hz), 121.3, 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C17H18Cl2NO4PS (434.27): C, 47.02; H, 4.18; N, 3.23. Found: C, 47.12; H, 4.30; N, 3.17.
O-{4-Chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1l). Yield: 87%, white solid; m.p. 90–91 °C (acetone-hexane); IR: 1677 (amide I), 1586 (ν CCaromatic), 1524 (amide II), 1477 (ν CCaromatic) cm−1; 1H-NMR: δ 9.14 (1H, bs, NH), 8.01–7.99 (2H, m, H3, H2'), 7.59 (1H, dd, J = 9.0 Hz, 2.5 Hz, H6'), 7.44 (1H, dd, J = 8.8 Hz, 2.8 Hz, H5), 7.41 (1H, dd, J = 9.0 Hz, 0.8 Hz, H5'), 7.34 (1H, dd, J = 8.8 Hz, 1.0 Hz, H6), 4.29–4.17 (4H, m, CHAHB), 1.32 (6H, tt, J = 7.2 Hz, 0.9 Hz, CH3); 13C-NMR: δ 161.5, 146.1 (1C, d, J = 8.5 Hz), 137.3, 132.8, 132.6 (1C, d, J = 1.8 Hz), 131.7, 131.6 (1C, d, J = 2.0 Hz), 130.5, 127.9 (1C, d, J = 6.3 Hz), 127. 8, 122.6 (1C, d, J = 3.0 Hz), 121.7, 119.3, 66.1 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C17H17Cl3NO4PS (468.72): C, 43.56; H, 3.66; N, 2.99. Found: C, 43.44; H, 3.57; N, 3.16.
O-{2-[(3-Bromophenyl)carbamoyl]-4-chlorophenyl} O,O-diethyl phosphorothioate (1m). Yield: 76%, white solid; m.p. 57–58 °C (acetone-hexane); IR: 1677 (amide I), 1592 (ν CCaromatic), 1530 (amide II), 1476 (ν CCaromatic) cm−1; 1H-NMR: δ 9.06 (1H, bs, NH), 8.01–7.99 (2H, m, H3, H2'), 7.66 (1H, ddd, J = 8.0 Hz, 2.0 Hz, 1.0 Hz, H6'), 7.44 (1H, dd, J = 8.8 Hz, 2.8 Hz, H5), 7.35 (1H, dd, J = 8.8 Hz, 1.3 Hz, H6), 7.28 (1H, ddd, J = 8.0 Hz, 2.0 Hz, 1.0 Hz, H4'), 7.22 (1H, t, J = 8.0 Hz, H5'), 4.28–4.16 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.5, 146.1 (1C, d, J = 8.3 Hz), 139.1, 132.4 (1C, d, J = 1.5 Hz), 131.6, 131.6 (1C, d, J = 1.8 Hz), 130.3, 128.2 (1C, d, J = 6.1 Hz), 127.6, 122.9, 122.6 (2C, d, J = 3.0 Hz), 122.6, 118.5, 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd for C17H18BrClNO4PS (478.72): C, 42.65; H, 3.79; N, 2.93. Found: C, 42.79; H, 3.65; N, 2.82.
O-{2-[(4-Bromophenyl)carbamoyl]-4-chlorophenyl} O,O-diethyl phosphorothioate (1n). Yield: 81%, white solid; m.p. 60–62 °C; IR: 1677, 1660 (amide I), 1600, 1572 (ν CCaromatic), 1538 (amide II), 1489 (ν CCaromatic) cm−1; 1H-NMR: δ 9.06 (1H, bs, NH), 7.99 (1H, dd, J = 2.7 Hz, 1.2 Hz, H3), 7.68–7.63 (2H, m, H2', H6'), 7.49–7.45 (2H, m, H3', H5'), 7.43 (1H, dd, J = 8.8 Hz, 2.7 Hz, H5), 7.33 (1H, dd, J = 8.8 Hz, 1.3 Hz, H6), 4.27–4.15 (4H, m, CHAHB), 1.31 (6H, dt, J = 7.0 Hz, 0.8 Hz, CH3); 13C-NMR: δ 161.8, 146.1 (1C, d, J = 8.4 Hz), 137.0, 132.3 (1C, d, J = 1.8 Hz), 132.0, 131.6 (1C, d, J = 2.0 Hz), 128.4 (1C, d, J = 6.3 Hz), 122.6 (1C, d, J = 2.9 Hz), 121.6, 117.2, 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.5 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd for C17H18BrClNO4PS (478.72): C, 42.65; H, 3.79; N, 2.93. Found: C, 42.49; H, 4.01; N, 3.10.
O-{4-Chloro-2-[(3-fluorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1o). Yield: 65%, white solid; m.p. 46–48 °C; IR: 1659 (amide I), 1612 (ν CCaromatic), 1548 (amide II), 1473, 1446 (ν CCaromatic) cm−1; 1H-NMR: δ 9.10 (1H, bs, NH), 8.00 (1H, dd, J = 2.5 Hz, 1.0 Hz, H3), 7.70 (1H, dt, J = 11.0 Hz, 2.2 Hz, H6'), 7.43 (1H, dd, J = 8.8 Hz, 2.5 Hz, H5), 7.35 (1H, dd, J = 8.8 Hz, 1.0 Hz, H6), 7.36–7.27 (2H, m, H2', H5'), 6.85 (1H, ddt, J = 8.0 Hz, 2.5 Hz, J = 0.9 Hz, H4'), 4.28–4.16 (4H, m, CHAHB), 1.31 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 162.9 (1C, d, J = 243.5 Hz, C3'), 161.6, 146.1 (1C, d, J = 8.4 Hz), 139.3 (1C, d, J = 10.9 Hz), 132.3 (1C, d, J = 1.6 Hz), 131.6 (1C, d, J = 1.3 Hz), 131.5, 130.0 (1C, d, J = 9.3 Hz), 128.3 (1C, d, J = 6.1 Hz), 122.6 (1C, d, J = 2.9 Hz), 115.3 (1C, d, J = 3.0 Hz, C6'), 111.3 (1C, d, J = 21.3 Hz), 107.5 (1C, d, J = 26.4 Hz), 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd for C17H18ClFNO4PS (417.82): C, 48.87; H, 4.34; N, 3.35. Found: C, 48.75; H, 4.23; N, 3.52.
O-{4-Chloro-2-[(4-fluorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1p). Yield: 68%, liquid; IR: 1670 (amide I), 1615 (ν CCaromatic), 1541 (amide II), 1509, 1473 (ν CCaromatic) cm−1; 1H-NMR: δ 9.02 (1H, bs, NH), 7.99 (1H, dd, J = 2.8 Hz, 1.2 Hz, H3), 7.75–7.69 (2H, m, H2', H6'), 7.42 (1H, dd, J = 8.8 Hz, 2.8 Hz, H5), 7.34 (1H, dd, J = 8.8 Hz, 1.2 Hz, H6), 7.09–7.03 (2H, m, H3', H5'), 4.27–4.14 (4H, m, CHAHB), 1.30 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.4, 159.5 (1C, d, J = 242.6 Hz, C4'), 146.1 (1C, d, J = 8.5 Hz), 133.9 (1C, d, J = 2.8 Hz, C1'), 132.2 (1C, d, J = 1.5 Hz), 131.5, 128.5 (1C, d, J = 6.1 Hz), 122.6 (1C, d, J = 2.9 Hz), 121.7 (2C, d, J = 7.8 Hz, C2', C6'), 115.6 (2C, d, J = 22.3 Hz, C3', C5'), 65.9 (2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd. for C17H18ClFNO4PS (417.82): C, 48.87; H, 4.34; N, 3.35. Found: C, 48.98; H, 4.50; N, 3.19.
O-(4-Chloro-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl) O,O-diethyl phosphorothioate (1q). Yield: 67%, white solid; m.p. 49–51 °C; IR: 1678 (amide I), 1606 (ν CCaromatic), 1564 (amide II), 1450 (ν CCaromatic) cm−1; 1H-NMR: δ 9.22 (1H, bs, NH), 8.06–8.02 (2H, m, H3, H2'), 7.98 (1H, d, J = 8.0 Hz, H6'), 7.49 (1H, t, J = 8.0 Hz, H5'), 7.45 (1H, dd, J = 8.8 Hz, 2.8 Hz, H5), 7.40 (1H, d, J = 8.0 Hz, H4'), 7.37 (1H, dd, J = 8.8 Hz, 1.3 Hz, H6), 4.29–4.16 (4H, m, CHAHB), 1.31 (6H, t, J = 7.0 Hz, CH3); 13C-NMR: δ 161.7, 146.2 (1C, d, J = 8.4 Hz), 138.4, 132.5 (1C, d, J = 1.6 Hz), 131.7 (1C, d, J = 1.1 Hz), 131.6 (1C, d, J = 1.9 Hz), 131.4 (1C, q, J = 32.3 Hz, C3'), 129.6, 128.0 (1C, d, J = 6.1 Hz), 123.8 (1C, q, J = 270.9 Hz, CF3), 123.1 (1C, d, J = 0.9 Hz), 122.6 (1C, d, J = 2.9 Hz), 121.1 (1C, q, J = 3.8 Hz, C2'), 116.8 (1C, q, J = 3.9 Hz, C4'), 66.0 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.7. Anal. Calcd for C18H18ClF3NO4PS (467.83): C, 46.21; H, 3.88; N, 2.99. Found: C, 45.98; H, 4.04; N, 3.19.
O-(4-Chloro-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl) O,O-diethyl phosphorothioate (1r). Yield: 93%, white solid; m.p. 81–82 °C; IR: 1684 (amide I), 1603 (ν CCaromatic), 1540 (amide II), 1475 (ν CCaromatic) cm−1; 1H-NMR: δ 9.24 (1H, bs, NH), 8.02 (1H, dd, J = 2.8 Hz, 1.2 Hz, H3), 7.89 (2H, d, J = 8.5 Hz, H3', H5'), 7.62 (2H, d, J = 8.5 Hz, H2', H6'), 7.45 (1H, dd, J = 8.8 Hz, 2.8 Hz, H5), 7.35 (1H, dd, J = 8.8 Hz, 1.3 Hz, H6), 4.28–4.16 (4H, m, CHAHB), 1.31 (6H, td, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.8, 146.1 (1C, d, J = 8.7 Hz), 140.9, 132.6 (1C, d, J = 1.8 Hz), 131.7 (1C, d, J = 1.0 Hz), 131.6 (1C, d, J = 2.6 Hz), 128.1 (1C, d, J = 6.3 Hz), 126.3 (1C, q, J = 32.6 Hz, C4'), 126.2 (2C, q, J = 3.9 Hz, C3', C5'), 124.0 (1C, q, J = 270.0 Hz, CF3), 122.6 (1C, d, J = 3.0 Hz), 119.7, 66.0 (2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.6. Anal. Calcd for C18H18ClF3NO4PS (467.83): C, 46.21; H, 3.88; N, 2.99. Found: C, 46.32; H, 3.93; N, 3.20.
O-{5-Chloro-2-[(3-chlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1s). Yield: 79%, semisolid; IR: 1660 (amide I), 1593 (ν CCaromatic), 1532 (amide II), 1482 (ν CCaromatic) cm−1; 1H-NMR: δ 9.06 (1H, bs, NH), 7.98 (1H, d, J = 8.5 Hz, H3), 7.87 (1H, t, J = 2.0 Hz, H2'), 7.58 (1H, dd, J = 8.3 Hz, 1.8 Hz, H6'), 7.40 (1H, t, J = 1.5 Hz, H6), 7.32 (1H, td, J = 8.5 Hz, 1.0 Hz, H4), 7.26 (1H, t, J = 8.0 Hz, H5'), 7.12–7.08 (1H, m, H4'), 4.28–4.17 (4H, m, CHAHB), 1.32 (6H, t, J = 7.0 Hz, CH3); 13C-NMR: δ 161.9, 147.9 (1C, d, J = 8.5 Hz), 139.1, 138.0 (1C, d, J = 1.9 Hz), 134.6, 132.9, 129.9, 126.3 (1C, d, J = 1.1 Hz), 125.2 (1C, d, J = 6.0 Hz), 124.5, 121.5 (1C, d, J = 3.0 Hz), 120.1, 118.0, 66.1 (2C, d, J = 5.4 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.4. Anal. Calcd for C17H18Cl2NO4PS (434.27): C, 47.02; H, 4.18; N, 3.23. Found: C, 46.91; H, 4.29; N, 3.34.
O-{5-Chloro-2-[(4-chlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1t). Yield: 91%, white solid; m.p. 67–68 °C (acetone-hexane); IR: 1658 (amide I), 1604, 1546 (ν CCaromatic), 1546 (amide II), 1492 (ν CCaromatic) cm−1; 1H-NMR: δ 9.07 (1H, bs, NH), 7.99 (1H, d, J = 8.5 Hz, H3), 7.74–7.69 (2H, m, H2', H6'), 7.40 (1H, d, J = 1.8 Hz, H6), 7.34–7.29 (3H, m, H4, H3', H5'), 4.29–4.17 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.9, 147.9 (1C, d, J = 8.8 Hz), 137.9 (1C, d, J = 1.6 Hz), 136.6, 132.8, 129.4, 129.0, 126.3, 125.4 (1C, d, J = 6.1 Hz), 121.5 (1C, d, J = 2.6 Hz), 121.2, 66.1 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.3. Anal. Calcd for C17H18Cl2NO4PS (434.27): C, 47.02; H, 4.18; N, 3.23. Found: C, 47.20; H, 4.02; N, 3.10.
O-{5-Chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1u). Yield: 91%, white solid; m.p. 70–71 °C; IR: 1650 (amide I), 1599, 1587 (ν CCaromatic), 1533 (amide II), 1472 (ν CCaromatic) cm−1; 1H-NMR: δ 9.14 (1H, bs, NH), 8.02 (1H, d, J = 2.5 Hz, H2'), 8.00 (1H, dd, J = 8.5 Hz, 1.0 Hz, H3), 7.58 (1H, dd, J = 8.8 Hz, 2.3 Hz, H6'), 7.42–7.39 (2H, m, H6, H5'), 7.32 (1H, td, J = 8.5 Hz, 1.0 Hz, H4), 4.31–4.18 (4H, m, CHAHB), 1.34 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 161.9, 148.0 (1C, d, J = 8.8 Hz), 138.2 (1C, d, J = 1.5 Hz), 137.5, 132.9, 132.8, 130.5, 127.6, 126.3, 124.9 (1C, d, J = 6.0 Hz), 121.7, 121.5 (1C, d, J = 2.6 Hz), 119.3, 66.2 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.3 Hz, CH3); 31P-NMR: δ 62.3. Anal. Calcd for C17H17Cl3NO4PS (468.72): C, 43.56; H, 3.66; N, 2.99. Found: C, 43.69; H, 3.90; N, 3.18.
O-{2-[(3-Bromophenyl)carbamoyl]-5-chlorophenyl} O,O-diethyl phosphorothioate (1v). Yield: 49%, colour liquid; IR: 1681 (amide I), 1589 (ν CCaromatic), 1532 (amide II), 1479, 1420 (ν CCaromatic) cm−1; 1H-NMR: δ 9.07 (1H, bs, NH), 8.05–8.96 (2H, m, H3, H2΄), 7.65 (1H, d, J = 7.5 Hz, H6'), 7.41 (1H, s, H6), 7.34–7.18 (3H, m, H4, H4', H5'), 4.33–4.16 (4H, m, CHAHB), 1.33 (6H, t, J = 7.0 Hz, CH3); 13C-NMR: δ 161.9, 147.9 (1C, d, J = 8.5 Hz), 139.2, 138.0 (1C, d, J = 1.9 Hz), 132.9, 130.2, 127.4, 126.3, 125.2 (1C, d, J = 6.0 Hz), 122.9, 122.6, 121.5 (1C, d, J = 2.4 Hz), 118.5, 66.1 (2C, d, J = 5.4 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.4. Anal. Calcd for C17H18BrClNO4PS (478.72): C, 42.65; H, 3.79; N, 2.93. Found: C, 42.54; H, 4.00; N, 3.01.
O-{2-[(4-Bromophenyl)carbamoyl]-5-chlorophenyl} O,O-diethyl phosphorothioate (1w). Yield: 93%, white solid; m.p. 80–82 °C; IR: 1681 (amide I), 1649, 1600, 1589 (ν CCaromatic), 1529 (amide II), 1488 (ν CCaromatic) cm−1; 1H-NMR: δ 9.06 (1H, bs, NH), 7.99 (1H, d, J = 8.5 Hz, H3), 7.68–7.64 (2H, m, H2', H6'), 7.49–7.44 (2H, m, H3', H5'), 7.40 (1H, t, J = 1.8 Hz, H6), 7.31 (1H, td, J = 8.5 Hz, 1.0 Hz, H4), 4.29–4.17 (4H, m, CHAHB), 1.33 (6H, tt, J = 7.1 Hz, 0.8 Hz, CH3); 13C-NMR: δ 161.9, 147.9 (1C, d, J = 8.8 Hz), 138.0 (1C, d, J = 1.4 Hz), 137.1, 132.9, 131.9, 126.3 (1C, d, J = 1.4 Hz), 125.4 (1C, d, J = 6.1 Hz), 121.6, 121.5 (1C, d, J = 2.8 Hz), 117.1, 66.1 (2C, d, J = 5.4 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.3. Anal. Calcd for C17H18BrClNO4PS (478.72): C, 42.65; H, 3.79; N, 2.93. Found: C, 42.77; H, 3.84; N, 2.78.
O-{5-Chloro-2-[(3-fluorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1x). Yield: 48%, oily liquid; IR: 1662 (amide I), 1599 (ν CCaromatic), 1541 (amide II), 1491 (ν CCaromatic) cm−1; 1H-NMR: δ 9.11 (1H, bs, NH), 7.99 (1H, dd, J = 8.5 Hz, 1.0 Hz, H3), 7.71 (1H, dt, J = 11.0 Hz, 2.0 Hz, H6'), 7.42–7.26 (4H, m, H4, H6, H2', H5'), 6.84 (1H, td, J = 8.5 Hz, 1.8 Hz, H4'), 4.30–4.18 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 0.9 Hz, CH3); 13C-NMR: δ 162.9 (1C, d, J = 243.3 Hz, C3'), 162.0, 147.9 (1C, d, J = 8.6 Hz), 139.4 (1C, d, J = 10.9 Hz), 138.0 (1C, d, J = 1.9 Hz), 132.9, 130.0 (1C, d, J = 9.4 Hz), 126.3 (1C, d, J = 1.4 Hz), 125.3 (1C, d, J = 6.1 Hz), 121.5 (1C, d, J = 3.0 Hz), 115.3 (1C, d, J = 3.0 Hz, C6'), 111.2 (1C, d, J = 21.3 Hz), 107.5 (1C, d, J = 26.4 Hz), 66.1 (2C, d, J = 5.5 Hz, CHAHB), 15.8 (2C, d, J = 7.3 Hz, CH3); 31P-NMR: δ 62.4. Anal. Calcd for C17H18ClFNO4PS (417.82): C, C, 48.87; H, 4.34; N, 3.35. Found: C, 49.02; H, 4.50; N, 3.24.
O-{5-Chloro-2-[(4-fluorophenyl)carbamoyl]phenyl} O,O-diethyl phosphorothioate (1y). Yield: 83%, white solid; m.p. 41–42 °C; IR: 1655 (amide I), 1640, 1616 (ν CCaromatic), 1560 (amide II), 1507, 1484 (ν CCaromatic) cm−1; 1H-NMR: δ 9.02 (1H, bs, NH), 7.99 (1H, dd, J = 8.8 Hz, 1.0 Hz, H3), 7.75–7.69 (2H, m, H2', H6'), 7.40 (1H, t, J = 1.8 Hz, H6), 7.31 (1H, ddd, J = 8.8 Hz, 2.0 Hz, 1.0 Hz, H4), 7.08–7.02 (2H, m, H3', H5'), 4.29–4.17 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 0.9 Hz, CH3); 13C-NMR: δ 161.8, 159.4 (1C, d, J = 242.5 Hz, C4'), 147.9 (1C, d, J = 8.9 Hz), 137.8 (1C, d, J = 1.9 Hz), 134.0 (1C, d, J = 2.8 Hz, C1'), 132.8, 126.2 (1C, d, J = 1.4 Hz), 125.5 (1C, d, J = 6.1 Hz), 121.7 (2C, d, J = 7.8 Hz, C2', C6'), 121.5 (1C, d, J = 3.0 Hz), 115.6 (2C, d, J = 21.2 Hz, C3', C5'), 66.0 (2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.4. Anal. Calcd for C17H18ClFNO4PS (417.82): C, 48.87; H, 4.34; N, 3.35. Found: C, 48.66; H, 4.30; N, 3.18.
O-(5-Chloro-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl) O,O-diethyl phosphorothioate (1z). Yield: 78%, oily liquid; IR: 1681 (amide I), 1598 (ν CCaromatic), 1552 (amide II), 1493, 1446 (ν CCaromatic) cm−1; 1H-NMR: δ 9.22 (1H, bs, NH), 8.07 (1H, s, H2'), 8.01 (1H, dd, J = 8.8 Hz, 1.0 Hz, H3), 7.96 (1H, d, J = 8.0 Hz, H6'), 7.48 (1H, t, J = 7.8 Hz, H5'), 7.43 (1H, t, J = 1.5 Hz, H6), 7.39 (1H, d, J = 8.0 Hz, H4'), 7.32 (1H, ddd, J = 8.5 Hz, 1.8 Hz, 1.0 Hz, H4), 4.30–4.18 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, 1.0 Hz, CH3); 13C-NMR: δ 162.0, 148.0 (1C, d, J = 8.6 Hz), 138.5, 138.2 (1C, d, J = 2.0 Hz), 132.9, 131.3 (1C, q, J = 32.3 Hz, C3'), 129.5, 126.3 (1C, d, J = 1.4 Hz), 125.0 (1C, d, J = 6.1 Hz), 123.8 (1C, q, J = 270.9 Hz, CF3), 123.1 (1C, d, J = 0.9 Hz), 121.5 (1C, d, J = 3.1 Hz), 121.0 (1C, q, J = 3.8 Hz, C2'), 116.7 (1C, q, J = 4.0 Hz, C4'), 66.1 (2C, d, J = 5.5 Hz, CHAHB), 15.7 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.5. Anal. Calcd for C18H18ClF3NO4PS (467.83): C, 46.21; H, 3.88; N, 2.99. Found: C, 46.37; H, 3.75; N, 3.13.
O-(5-Chloro-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl) O,O-diethyl phosphorothioate (1zz). Yield: 78%, white solid; m.p. 61–62 °C (acetone-hexane); IR: 1668 (amide I), 1594 (ν CCaromatic), 1542 (amide II), 1474 (ν CCaromatic) cm−1; 1H-NMR: δ 9.25 (1H, bs, NH), 8.01 (1H, dd, J = 8.7 Hz, 0.9 Hz, H3), 7.89 (2H, d, J = 8.4 Hz, H3', H5'), 7.62 (2H, d, J = 8.4 Hz, H2', H6'), 7.42 (1H, t, J = 1.8 Hz, H6), 7.31 (1H, ddd, J = 8.6 Hz, 2.0 Hz, 1.0 Hz, H4), 4.33–4.15 (4H, m, CHAHB), 1.32 (6H, dt, J = 7.0 Hz, J = 0.9 Hz, CH3); 13C-NMR: δ 162.1, 148.0 (1C, d, J = 8.9 Hz), 141.0, 138.2 (1C, d, J = 2.0 Hz), 132.9 (1C, d, J = 1.0 Hz), 126.3 (1C, d, J = 1.5 Hz), 125.1 (1C, d, J = 6.1 Hz), 126.2 (2C, q, J = 3.8 Hz, C3', C5'), 126.2 (1C, q, J = 32.5 Hz, C4'), 124.1 (1C, q, J = 269.9 Hz, CF3), 121.5 (1C, d, J = 3.0 Hz), 119.7, 66.1 (2C, d, J = 5.6 Hz, CHAHB), 15.8 (2C, d, J = 7.4 Hz, CH3); 31P-NMR: δ 62.3. Anal. Calcd for C18H18ClF3NO4PS (467.83): C, 46.21; H, 3.88; N, 2.99. Found: C, 46.06; H, 3.71; N, 2.82.