Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds
Abstract
:1. Introduction
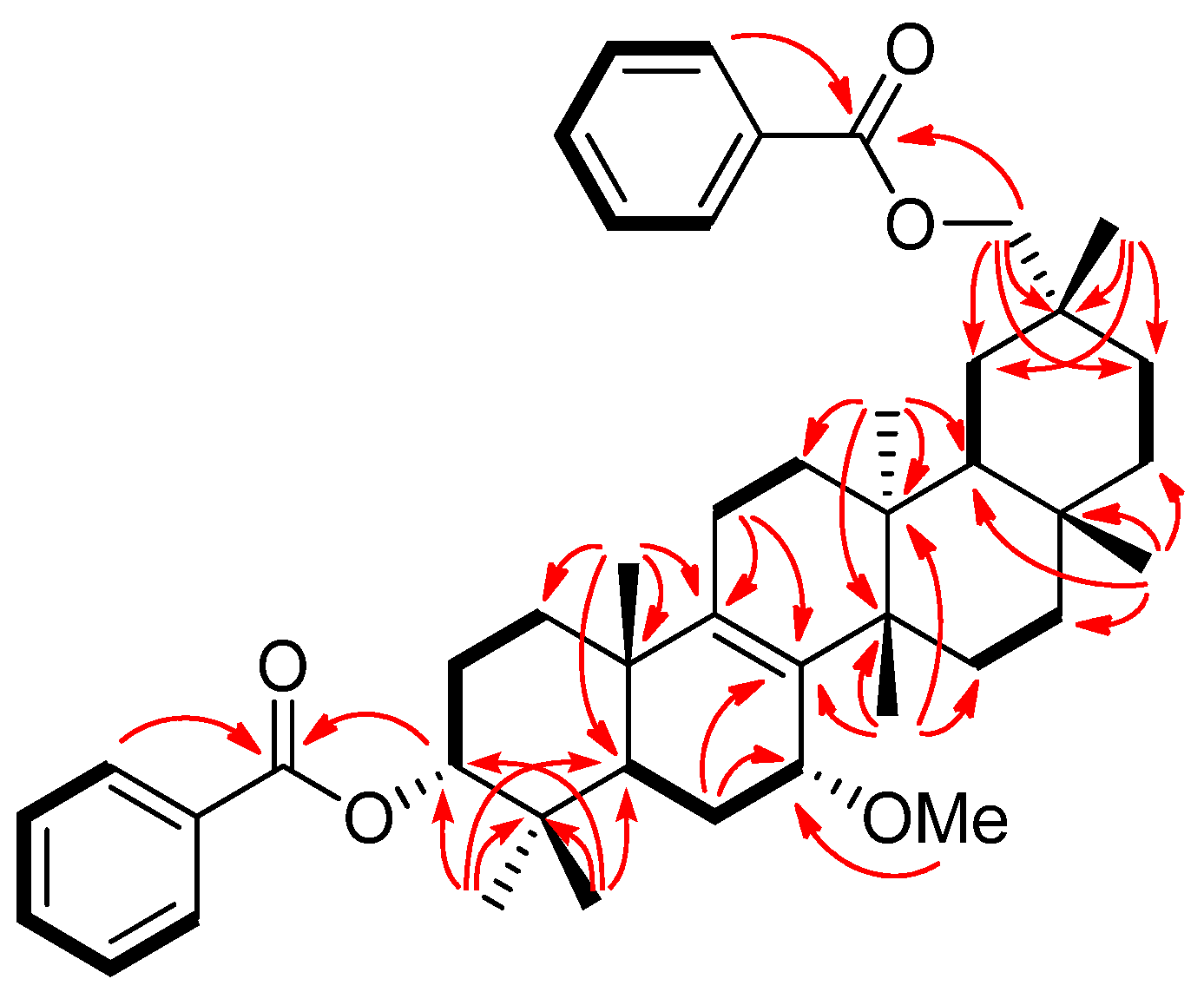
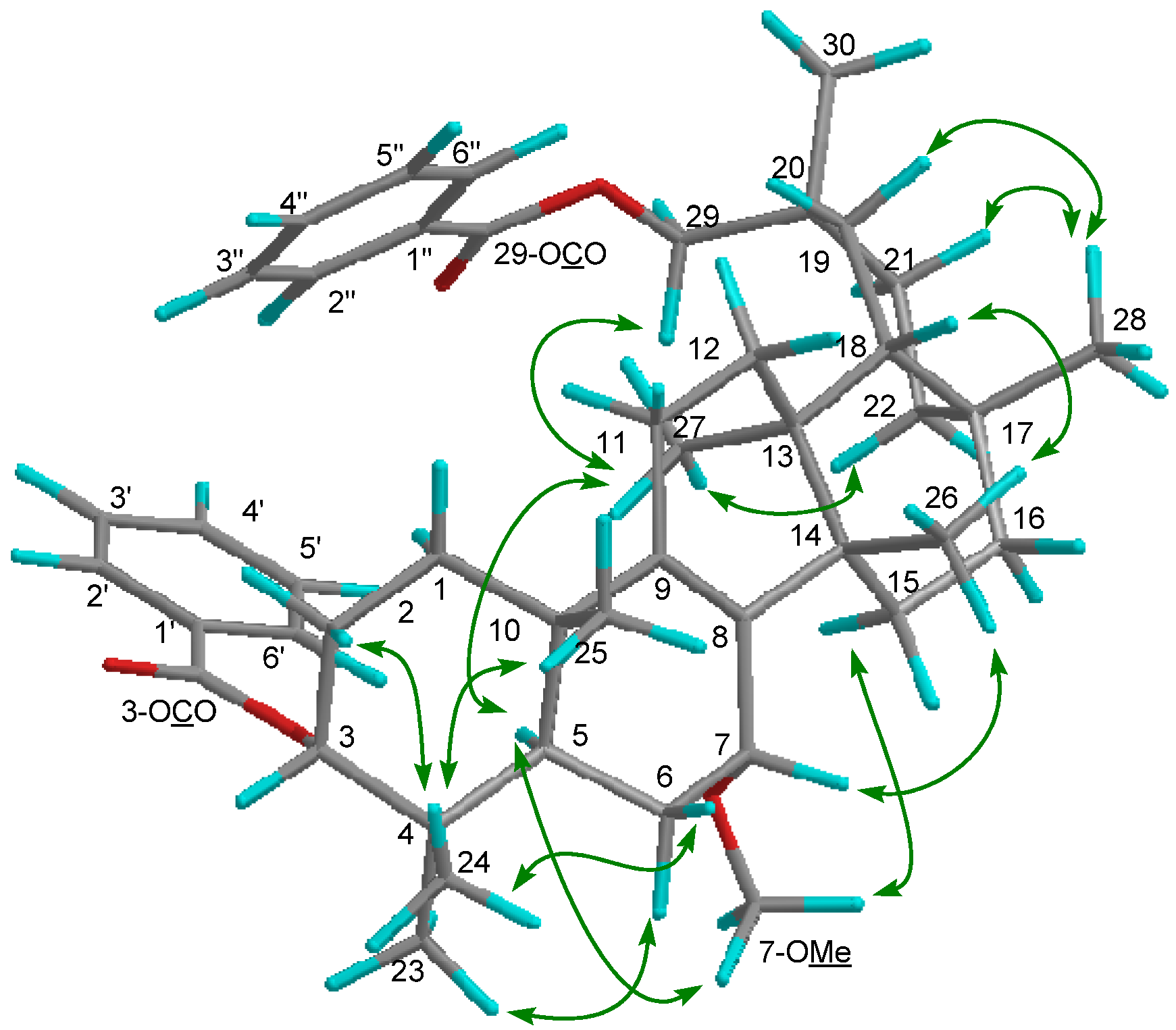
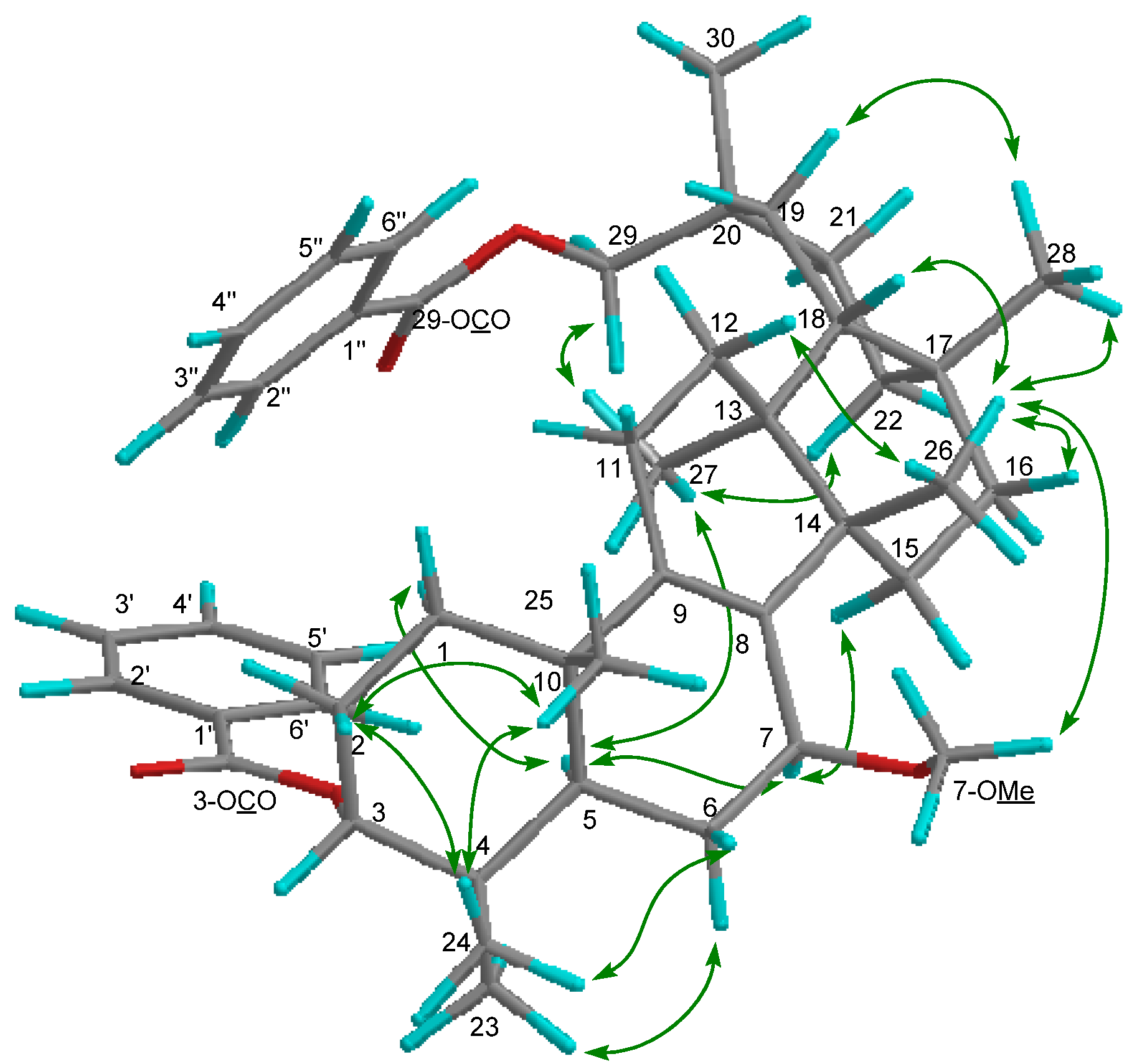
2. Results and Discussion
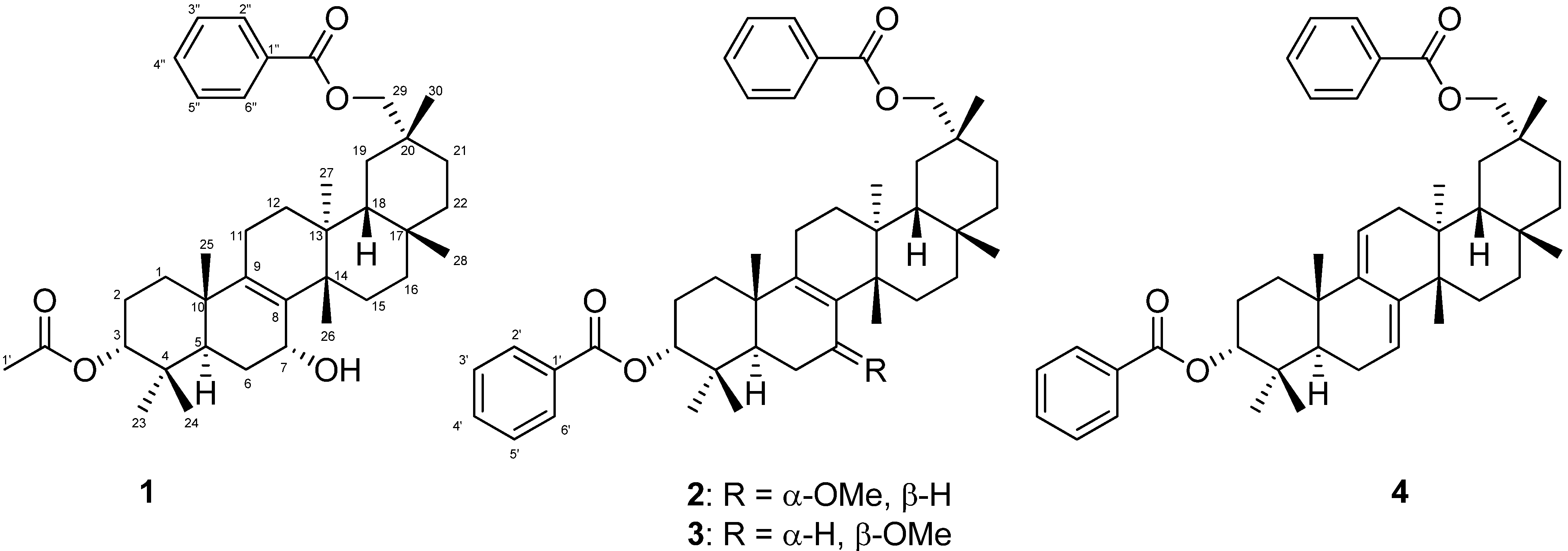
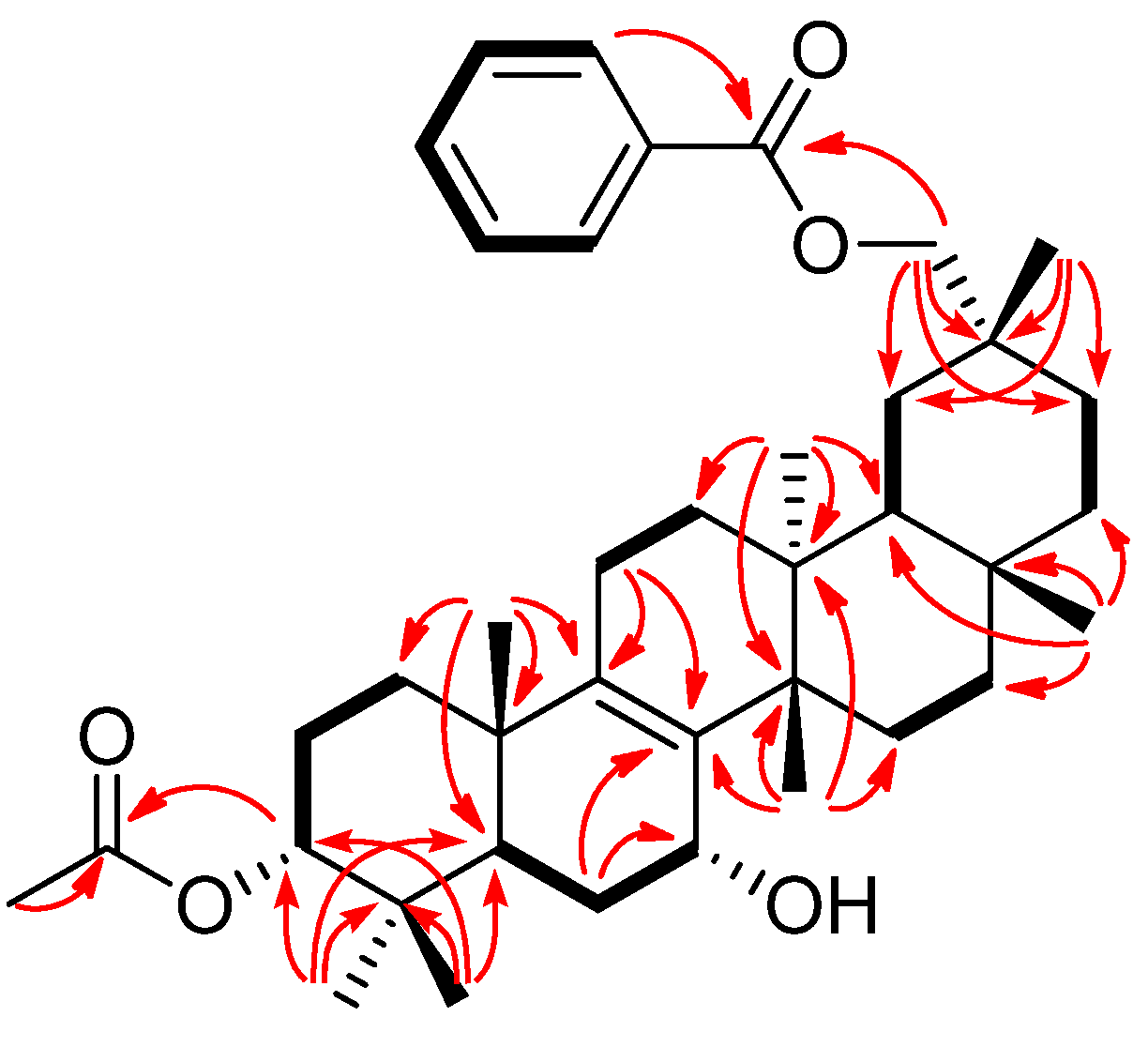
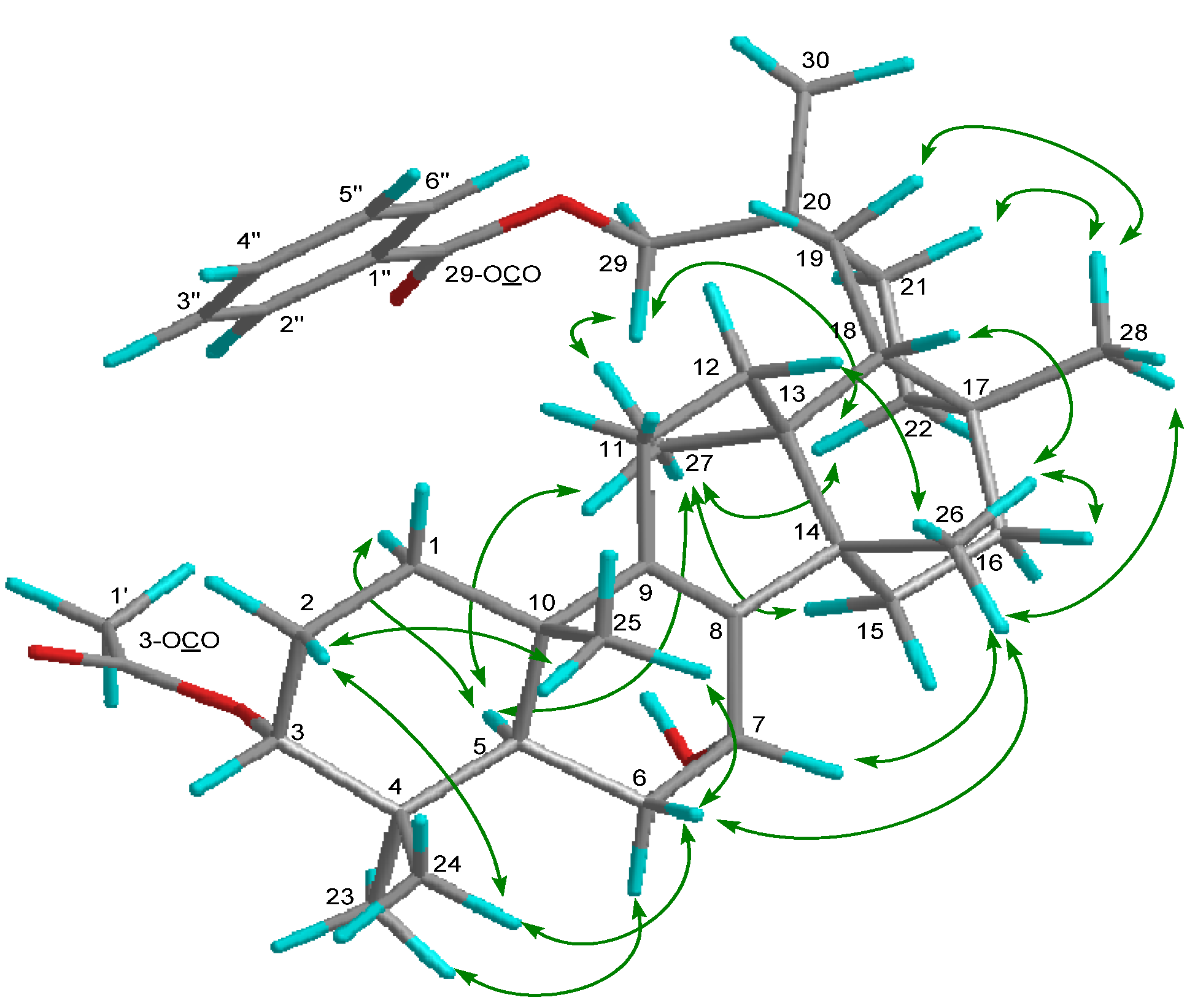
| 1 | 2 | 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |||||||||
| 1 | 29.6 | t | α | 1.33 | m | 29.8 | t | α | 1.48 | m | 30.6 | t | α | 1.40 | m |
| β | 1.47 | m | β | 1.53 | m | β | 1.52 | m | |||||||
| 2 | 23.3 | t | α | 1.66 | m | 23.6 | t | α | 1.80 | m | 23.3 | t | α | 1.80 | m |
| β | 1.88 | m | β | 1.98 | m | β | 1.97 | m | |||||||
| 3 | 77.4 | d | 4.69 | t (3.0) | 78.1 | d | 4.95 | t (2.9) | 78.2 | d | 4.91 | t (3.0) | |||
| 4 | 36.3 | s | 36.7 | s | 37.0 | s | |||||||||
| 5 | 39.6 | d | 1.91 | m | 39.9 | d | 2.19 | dd (12.6, 1.2) | 44.1 | d | 1.70 | m | |||
| 6 | 28.8 | t | α | 1.74 | m | 22.5 | t | α | 1.95 | m | 25.3 | t | α | 2.21 | m |
| β | 1.60 | m | β | 1.34 | m | β | 1.51 | m | |||||||
| 7 | 64.3 | d | 4.16 | brs | 73.8 | d | 3.54 | brs | 78.8 | d | 3.98 | brt (7.6) | |||
| 8 | 136.4 | s | 135.3 | s | 136.7 | s | |||||||||
| 9 | 140.1 | s | 139.7 | s | 140.3 | s | |||||||||
| 10 | 38.6 | s | 38.6 | s | 38.2 | s | |||||||||
| 11 | 20.9 | t | 1.93 | 2H, m | 20.9 | t | 1.97 | 2H, m | 20.8 | t | α | 2.03 | m | ||
| β | 1.91 | m | |||||||||||||
| 12 | 31.19 | t | α | 1.34 | m | 31.3 | t | α | 1.35 | m | 30.7 | t | α | 1.51 | m |
| β | 1.61 | m | β | 1.61 | m | β | 1.40 | m | |||||||
| 13 | 36.8 | s | 37.0 | s | 38.2 | s | |||||||||
| 14 | 41.9 | s | 41.8 | s | 40.9 | s | |||||||||
| 15 | 26.1 | t | α | 2.05 | m | 25.4 | t | α | 2.19 | m | 26.3 | t | α | 1.78 | m |
| β | 1.50 | m | β | 1.26 | m | β | 1.83 | m | |||||||
| 16 | 36.9 | t | α | 1.54 | m | 36.9 | t | α | 1.56 | m | 36.5 | t | α | 1.61 | m |
| β | 1.67 | m | β | 1.61 | m | β | 1.53 | m | |||||||
| 17 | 31.2 | s | 31.1 | s | 31.2 | s | |||||||||
| 18 | 44.4 | d | 1.61 | m | 44.0 | d | 1.60 | m | 42.8 | d | 1.66 | m | |||
| 19 | 28.4 | t | α | 1.90 | m | 28.8 | t | α | 1.86 | m | 29.8 | t | α | 1.40 | m |
| β | 1.56 | m | β | 1.49 | m | β | 1.50 | m | |||||||
| 20 | 31.7 | s | 31.9 | s | 32.2 | s | |||||||||
| 21 | 30.1 | t | α | 1.47 | m | 29.9 | t | α | 1.48 | m | 29.1 | t | 1.52 | 2H, m | |
| β | 1.59 | m | β | 1.53 | m | ||||||||||
| 22 | 35.0 | t | α | 1.87 | m | 35.6 | t | α | 1.84 | d (4.4) | 37.1 | t | α | 1.68 | m |
| β | 0.97 | m | β | 0.96 | m | β | 1.01 | m | |||||||
| 23 | 27.4 | q | 0.89 | s | 27.5 | q | 0.97 | s | 27.6 | q | 0.96 | s | |||
| 24 | 22.1 | q | 0.91 | s | 22.4 | q | 1.00 | s | 21.7 | q | 1.02 | s | |||
| 25 | 18.1 | q | 0.92 | s | 18.2 | q | 0.98 | s | 20.2 | q | 1.10 | s | |||
| 26 | 25.1 | q | 1.03 | s | 26.0 | q | 1.05 | s | 27.8 | q | 1.29 | s | |||
| 27 | 19.0 | q | 1.06 | s | 19.0 | q | 1.082 | s | 18.0 | q | 0.95 | s | |||
| 28 | 31.17 | q | 1.12 | s | 31.3 | q | 1.13 | s | 30.7 | q | 1.17 | s | |||
| 29 | 72.5 | t | 4.14 | 2H, brs | 72.9 | t | A | 4.08 | d (10.8) | 74.0 | t | A | 4.05 | d (10.6) | |
| B | 4.16 | d (10.8) | B | 4.11 | d (10.6) | ||||||||||
| 30 | 30.6 | q | 1.10 | s | 29.8 | q | 1.084 | s | 28.1 | q | 1.12 | s | |||
| 3-OCO | 170.9 | s | 166.3 | s | 165.9 | s | |||||||||
| 1' | 21.4 | q | 2.07 | s | 130.8b | s | 130.6 | s | |||||||
| 2', 6' | 129.6c | d | 8.05 b | dd (7.4, 1.4) | 129.4 | d | 7.99 | dd (7.4, 1.4) | |||||||
| 3', 5' | 128.4d | d | 7.45 c | tt (7.4, 1.4) | 128.5 | d | 7.45 | tt (7.4, 1.4) | |||||||
| 4' | 132.7e | d | 7.55 d | tt (7.4, 1.4) | 132.8 | d | 7.56 | tt (7.4, 1.4) | |||||||
| 29-OCO | 166.7 | s | 166.6 | s | 166.8 | s | |||||||||
| 1'' | 130.6 | s | 130.7b | s | 130.9 | s | |||||||||
| 2'', 6'' | 129.4 | d | 8.07 | dd (7.4, 1.2) | 129.4c | d | 8.04 b | dd (7.4, 1.4) | 129.5 | d | 8.04 | dd (7.3, 1.7) | |||
| 3'', 5'' | 128.4 | d | 7.47 | tt (7.4, 1.2) | 128.3d | d | 7.43 c | tt (7.4, 1.4) | 128.4 | d | 7.43 | tt (7.3, 1.7) | |||
| 4'' | 132.9 | d | 7.58 | tt (7.4, 1.2) | 132.6e | d | 7.54 d | tt (7.4, 1.4) | 132.7 | d | 7.55 | tt (7.3, 1.7) | |||
| 7-OMe | 54.9 | q | 3.24 | s | 55.0 | q | 3.35 | s | |||||||
| Compound | Conc. (μM) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 10 | 30 | 100 | 300 | |||
| 1 | melanin content | 88.5 ± 2.7 ** | 65.8 ± 1.7 ** | 35.7 ± 0.8 ** | 46.5 | |
| cell viability | 105.4 ± 4.5 | 88.0 ± 1.0 | 58.4 ± 7.7 ** | >100 | ||
| 2 | melanin content | 96.3 ± 2.2 | 105.5 ± 4.5 | 108.3 ± 1.0 | >100 | |
| cell viability | 103.4 ± 3.6 | 105.8 ± 5.0 | 104.9 ± 7.7 | >100 | ||
| 3 | melanin content | 96.9 ± 9.2 | 79.4 ± 4.5 ** | 60.1 ± 1.8 ** | >100 | |
| cell viability | 94.6 ± 0.4 | 86.3 ± 2.4 * | 67.2 ± 4.8** | >100 | ||
| 4 b | melanin content | 98.4 ± 3.2 | 102.2 ± 11.7 | 95.4 ± 8.4 | >100 | |
| cell viability | 110.8 ± 4.3 | 103.0 ± 8.2 | 101.1 ± 5.9 | >100 | ||
| Arbutin c | melanin content | 88.9 ± 2.3 ** | 72.3 ± 3.1 ** | 55.3 ± 1.0 ** | 33.8 ± 2.8 ** | 124.6 |
| cell viability | 100.0 ± 2.7 | 94.4 ± 1.2 | 89.9 ± 0.3 ** | 81.9 ± 3.2 ** | >300 | |
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Product Characterization Data
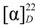 −85.8 (c = 0.2, CHCl3); UV (EtOH) λmax (logε) 205.0 (3.80), 220.0 (3.94), 232.5 (3.98), 270.5 (3.41), 280.0 (3.32), 321.0 (2.87) nm; IR (KBr) νmax 3437, 2938, 2876, 1718, 1272, 1244, 1110, 750, 729, 710 cm−1; 1H and 13C-NMR data see Table 1; EIMS m/z 586 [M–H2O]+ (26), 540 (33), 527 (40), 511 (100), 389 (12), 387 (9), 253 (15), 225 (16); HREIMS m/z 586.4019 (calcd for C39H54O4: 586.4023).
−85.8 (c = 0.2, CHCl3); UV (EtOH) λmax (logε) 205.0 (3.80), 220.0 (3.94), 232.5 (3.98), 270.5 (3.41), 280.0 (3.32), 321.0 (2.87) nm; IR (KBr) νmax 3437, 2938, 2876, 1718, 1272, 1244, 1110, 750, 729, 710 cm−1; 1H and 13C-NMR data see Table 1; EIMS m/z 586 [M–H2O]+ (26), 540 (33), 527 (40), 511 (100), 389 (12), 387 (9), 253 (15), 225 (16); HREIMS m/z 586.4019 (calcd for C39H54O4: 586.4023).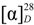 −20.7 (c = 0.7, CHCl3); UV (EtOH) λmax (logε) 238.5 (3.70), 271.5 (3.21), 278.5 (3.11) nm; IR (KBr) νmax:2948, 2883, 1717, 1456, 1367, 1314, 1274, 1113, 1069, 716 cm−1;1H and 13C-NMR data see Table 1; EIMS m/z 680 (6) [M]+, 648 [M–MeOH]+ (12), 526 (25), 511 (100), 389 (8), 355 (10), 324 (8); HREIMS m/z 680.4447 (calcd for C45H60O5: 680.4441).
−20.7 (c = 0.7, CHCl3); UV (EtOH) λmax (logε) 238.5 (3.70), 271.5 (3.21), 278.5 (3.11) nm; IR (KBr) νmax:2948, 2883, 1717, 1456, 1367, 1314, 1274, 1113, 1069, 716 cm−1;1H and 13C-NMR data see Table 1; EIMS m/z 680 (6) [M]+, 648 [M–MeOH]+ (12), 526 (25), 511 (100), 389 (8), 355 (10), 324 (8); HREIMS m/z 680.4447 (calcd for C45H60O5: 680.4441).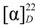 −43.7 (c = 0.2, CHCl3); UV (EtOH) λmax (logε) 206.5 (3.86), 220.0 (3.97), 235.0 (4.00), 271.0 (3.29), 280.0 (3.16) nm; IR (KBr) νmax:3437, 1717, 1272, 1110, 1028, 975, 711 cm−1; 1H and 13C-NMR data see Table 1; EIMS 680 (65) [M]+, 665 (38), 648 [M–MeOH]+ (14), 526 (29), 511 (100), 393 (18), 381 (18), 354 (28); HREIMS m/z 680.4446 [M]+ (calcd for C45H60O5: 680.4440).
−43.7 (c = 0.2, CHCl3); UV (EtOH) λmax (logε) 206.5 (3.86), 220.0 (3.97), 235.0 (4.00), 271.0 (3.29), 280.0 (3.16) nm; IR (KBr) νmax:3437, 1717, 1272, 1110, 1028, 975, 711 cm−1; 1H and 13C-NMR data see Table 1; EIMS 680 (65) [M]+, 665 (38), 648 [M–MeOH]+ (14), 526 (29), 511 (100), 393 (18), 381 (18), 354 (28); HREIMS m/z 680.4446 [M]+ (calcd for C45H60O5: 680.4440).3.5. Cell Cultures
3.6. Determination of B16 4A5 Cells Proliferation
3.7. Assay of Melanin Content
3.8. Cytotoxicity Assay against Cancer Cell Lines
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okada, M.; Mitsuhashi, H. Newly Revised Illustrated Medicinal Plants of World; Hokuryukan Pablishing Co., Ltd.: Tokyo, Japan, 2002; p. p. 514. [Google Scholar]
- Chevallier, A. The Encyclopedia of Medicinal Plants; Seibundo Shinkosha Pablishing Co., Ltd.: Tokyo, Japan, 2000; p. p. 194. [Google Scholar]
- Appendino, G.; Jakupovic, J.; Belloro, E.; Marchesini, A. Secondary metabolites from edible plants and spices. Part 4. Multiflorane triterpenoid esters from pumpkin. An unexpected extrafolic source of PABA. Phytochemistry 1999, 51, 1021–1026. [Google Scholar] [CrossRef]
- Appendino, G.; Jakupovic, J.; Belloro, E.; Marchesini, A. Triterpenoid p-aminobenzoates from the seeds of zucchini. Fitoterapia 2000, 71, 258–263. [Google Scholar] [CrossRef]
- Akihisa, T.; Ghosh, P.; Thakur, S.; Rosenstein, F.U.; Tamura, T.; Matsumoto, T. Widespread occurrence of cucurbita-5,24-dienol in Cucurbitaceae. Yukagaku 1986, 35, 1036–1040. [Google Scholar]
- Cattel, L.; Balliano, G.; Caputo, O. Sterols and triterpenes from Cucurbita maxima. Planta Med. 1979, 37, 264–267. [Google Scholar] [CrossRef]
- Fenner, G.P.; Patterson, G.W.; Koines, P.M. Sterol composition during the life cycle of the soybean and the squash. Lipids 1986, 21, 48–51. [Google Scholar] [CrossRef]
- Fenner, G.P.; Patterson, G.W.; Lusby, W.R. Developmental regulation of sterol biosynthesis in Cucurbita. maxima L. Lipids 1989, 24, 271–277. [Google Scholar] [CrossRef]
- Kikuchi, T.; Takebayashi, M.; Shinto, M.; Yamada, T.; Tanaka, R. Three new multiflorane-type triterpenes from pumpkin (Cucurbita maxima) seeds. Molecules 2013, 18, 5568–5579. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Toriumi, M.; Mukainaka, T.; Banno, N.; Kimura, Y.; Hasegawa, J.; Nishino, H. Inhibitory effects of cucurbitane glycosides and other triterpenoids from the fruit of Momordica grosvenori on Epstein-Barr virus early antigen induced by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 2002, 50, 6710–6715. [Google Scholar] [CrossRef]
- De Marino, S.; Festa, C.; Zollo, F.; Iorizzi, M. Phenolic glycosides from Cucumis melo var. inodorus seeds. Phytochem. Lett. 2009, 2, 130–133. [Google Scholar] [CrossRef]
- De Shan, M.; Hu, L.H.; Chen, Z.L. A new multiflorane triterpenoid ester from Momordica cochinchinensis Spreng. Nat. Prod. Lett. 2001, 15, 139–145. [Google Scholar] [CrossRef]
- Ma, Y.P.; Li, N.; Gao, J.; Fu, K.L.; Qin, Y.; Li, G.Y.; Wang, J.H. A new peroxy-multiflorane triterpene ester from the processed seeds of Trichosanthes kirilowii. Helv. Chim. Acta 2011, 94, 1881–1887. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ichiishi, E.; Mukainaka, T.; Toriumi, M.; Ukiya, M.; Yasukawa, K.; Nishino, H. Anti-tumor promoting effects of multiflorane-type triterpenoids and cytotoxic activity of karounidiol against human cancer cell lines. Cancer Lett. 2001, 173, 9–14. [Google Scholar] [CrossRef]
- Liu, C.H.; Yen, M.H.; Tsang, S.F.; Gan, K.H.; Hsu, H.Y.; Lin, C.N. Antioxidant triterpenoids from the stems of Momordica charantia. Food Chem. 2009, 118, 751–756. [Google Scholar]
- Tanaka, R.; Kikuchi, T.; Nakasuji, S.; Ue, Y.; Shuto, D.; Igarashi, K.; Okada, R.; Yamada, T. A novel 3α-p-nitrobenzoylmultiflora-7:9(11)-diene-29-benzoate and two new triterpenoids from the seeds of zucchini (Cucurbita pepo L.). Molecules 2013, 18, 7448–7459. [Google Scholar] [CrossRef]
- Lin, J.W.; Chiang, H.M.; Lin, Y.C.; Wen, K.C. Natural products with skin-whitening effects. J. Food Drug Anal. 2008, 16, 1–10. [Google Scholar]
- Lim, Y.J.; Lee, E.H.; Kang, T.H.; Ha, S.K.; Oh, M.S.; Kim, S.M.; Yoon, T.J.; Kang, C.; Park, J.H.; Kim, S.Y. Inhibitory effects of arbutin on melanin biosynthesis of α-melanocyte stimulating hormone-induced hyperpigmentation in cultured Brownish guinea pig skin tissues. Arch. Pharm. Res. 2009, 32, 367–373. [Google Scholar] [CrossRef]
- Yamada, T.; Muroga, Y.; Jinno, M.; Kajimoto, T.; Usami, Y.; Numata, A.; Tanaka, R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorg. Med. Chem. 2011, 19, 4106–4113. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kikuchi, T.; Ueda, S.; Kanazawa, J.; Naoe, H.; Yamada, T.; Tanaka, R. Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds. Molecules 2014, 19, 4802-4813. https://doi.org/10.3390/molecules19044802
Kikuchi T, Ueda S, Kanazawa J, Naoe H, Yamada T, Tanaka R. Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds. Molecules. 2014; 19(4):4802-4813. https://doi.org/10.3390/molecules19044802
Chicago/Turabian StyleKikuchi, Takashi, Shinsuke Ueda, Jokaku Kanazawa, Hiroki Naoe, Takeshi Yamada, and Reiko Tanaka. 2014. "Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds" Molecules 19, no. 4: 4802-4813. https://doi.org/10.3390/molecules19044802
APA StyleKikuchi, T., Ueda, S., Kanazawa, J., Naoe, H., Yamada, T., & Tanaka, R. (2014). Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds. Molecules, 19(4), 4802-4813. https://doi.org/10.3390/molecules19044802





