Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells
Abstract
:1. Introduction
2. Results and Discussion
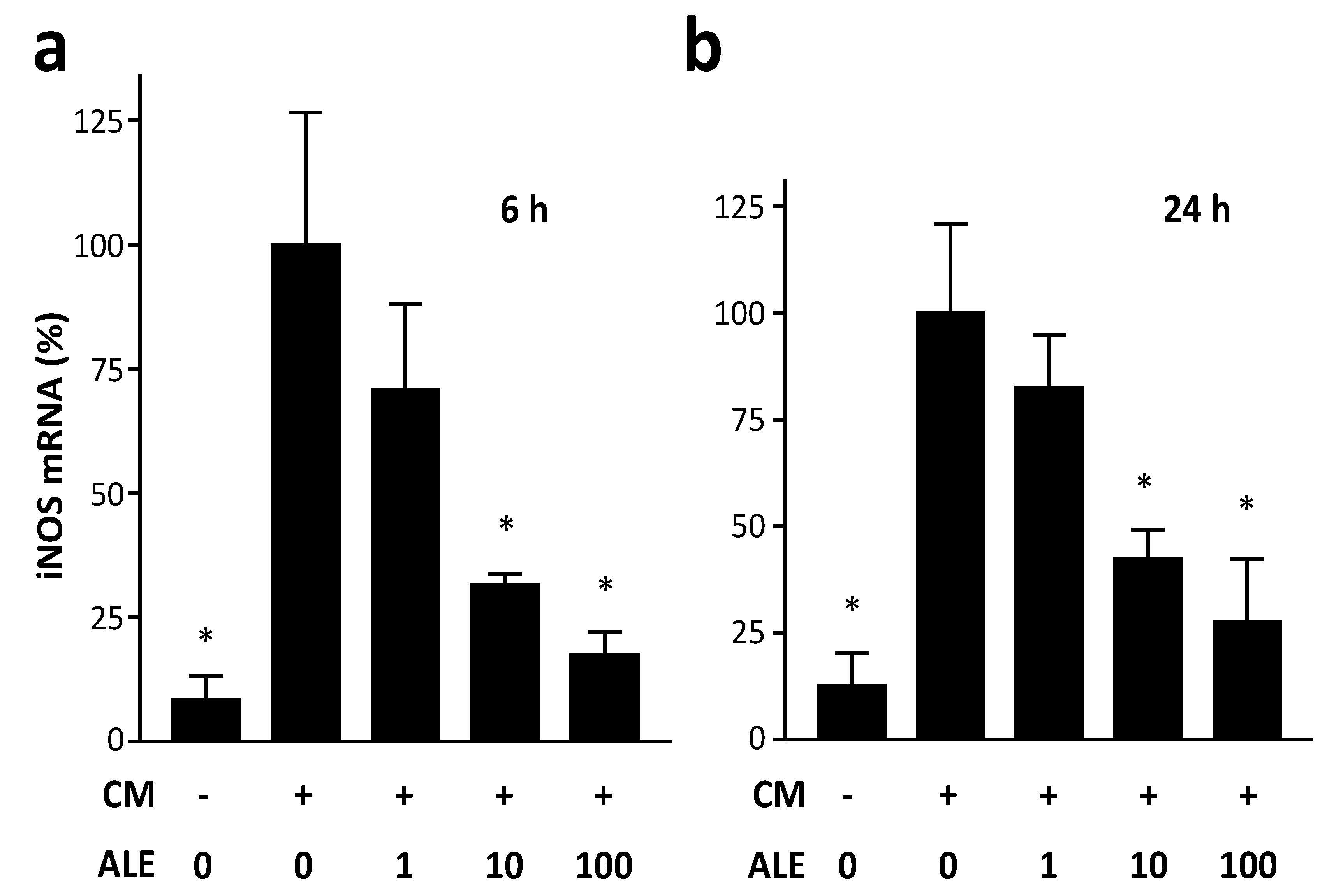
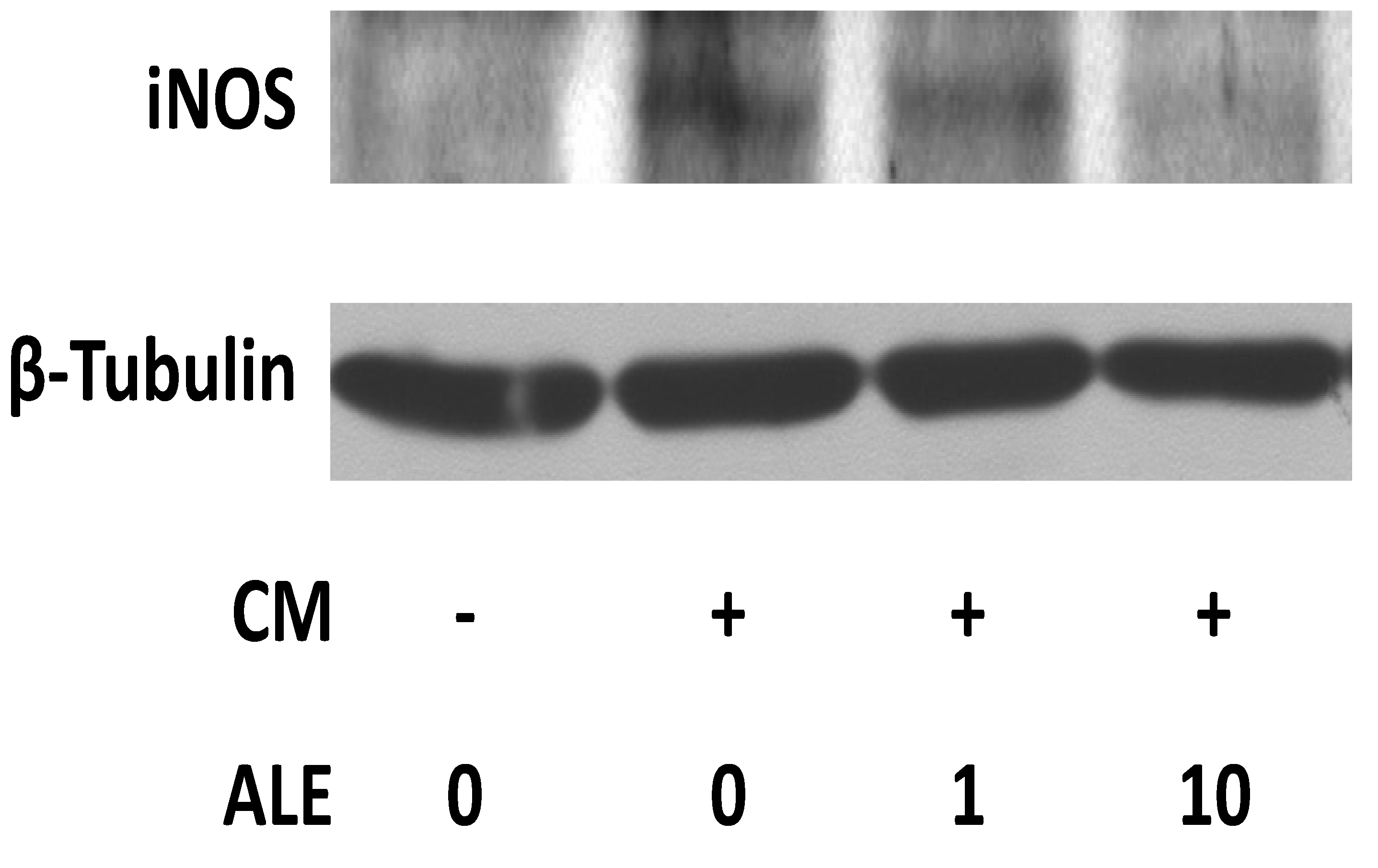
2.1. Artichoke Leaf Extracts Downregulate iNOS Expression
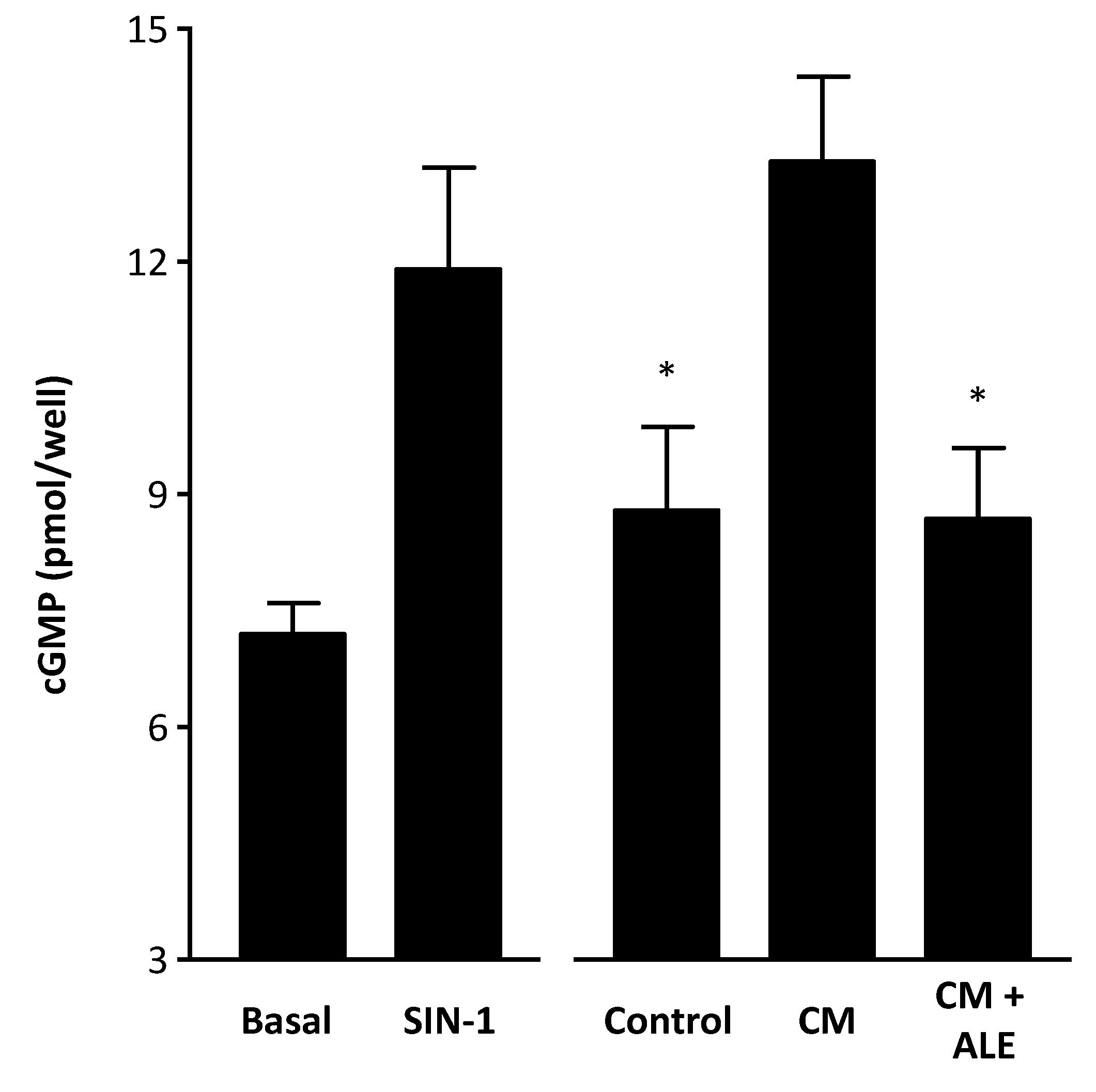
2.2. Artichoke Leaf Extracts Reduce Cytokine-Induced NO Production
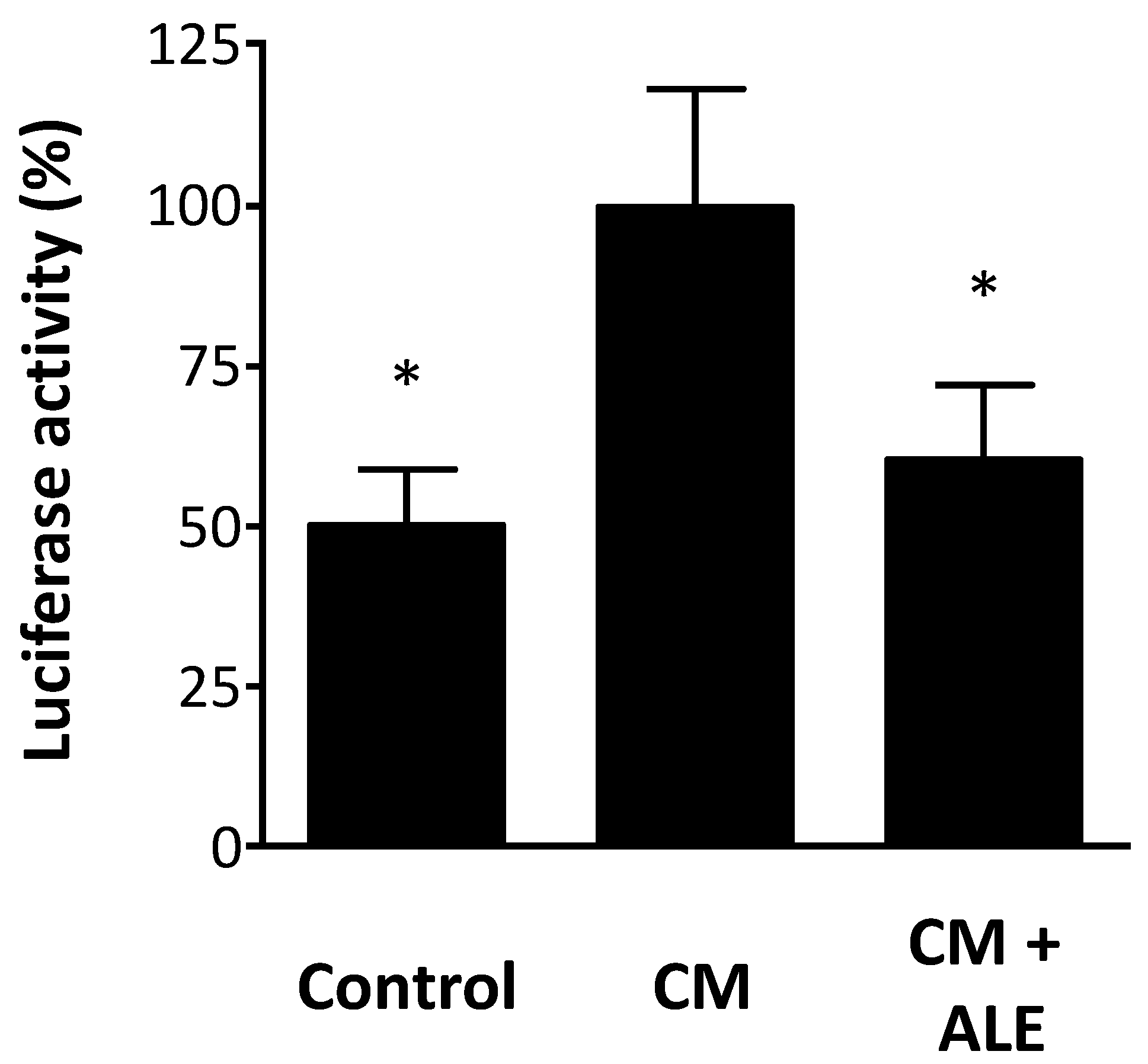
2.3. Artichoke Leaf Extracts Inhibit iNOS Promoter Activity
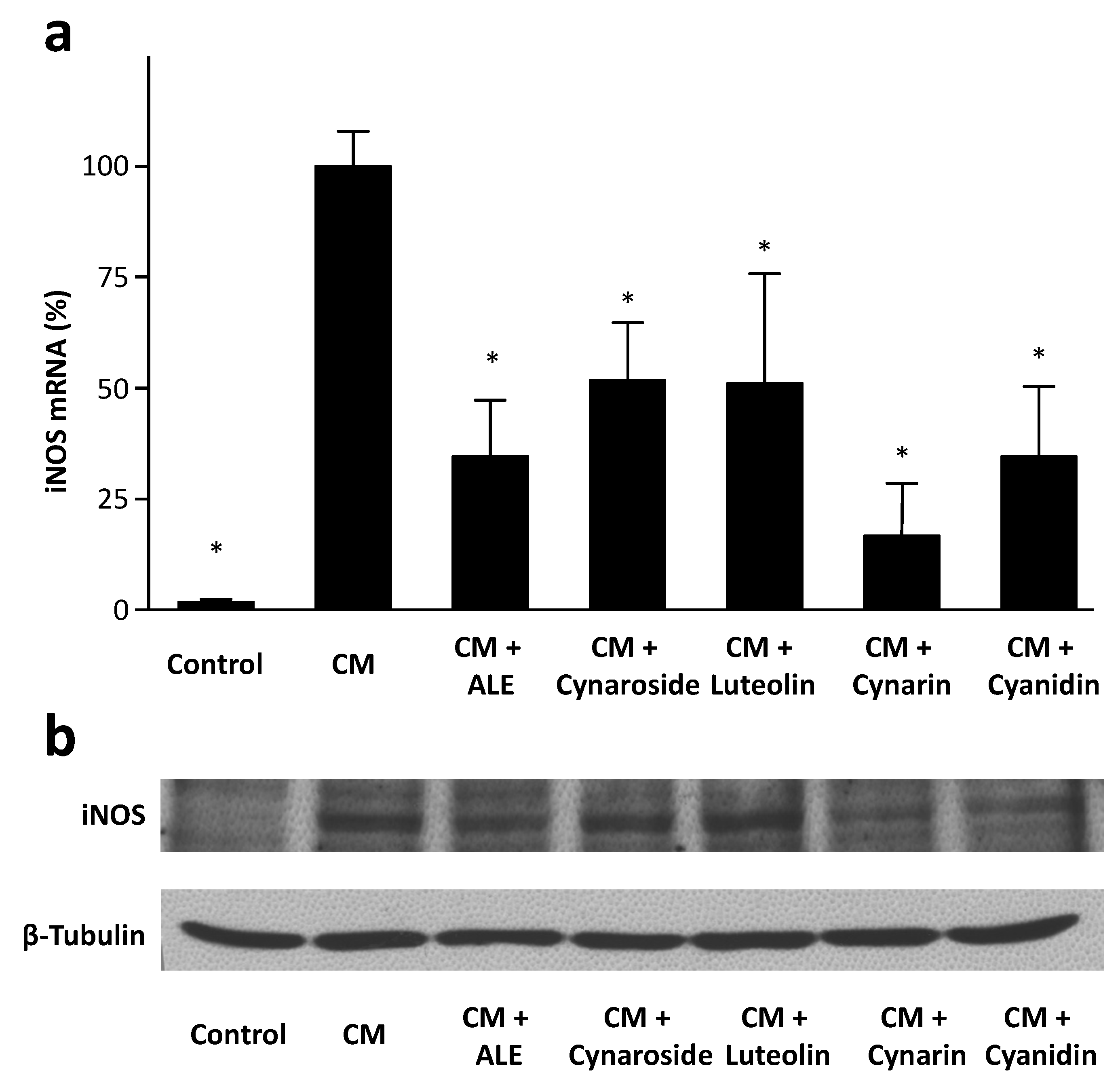
2.4. Artichoke Compounds Inhibit iNOS Expression
2.5. Potential Therapeutic Relevance
3. Experimental
3.1. Cell Culture
3.2. Real-Time RT-PCR for iNOS mRNA Analyses
3.3. Western Blot for iNOS Protein Analyses
3.4. Reporter Cell Assay for Determination of NO Production
3.5. Reporter Gene Assay for Determination of iNOS Promoter Activity
3.6. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Li, H.; Forstermann, U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009, 15, 3133–3145. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- Liu, V.W.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar]
- Gunnett, C.A.; Lund, D.D.; McDowell, A.K.; Faraci, F.M.; Heistad, D.D. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1617–1622. [Google Scholar] [CrossRef]
- Chen, S.J.; Li, S.Y.; Shih, C.C.; Liao, M.H.; Wu, C.C. NO contributes to abnormal vascular calcium regulation and reactivity induced by peritonitis-associated septic shock in rats. Shock 2010, 33, 473–478. [Google Scholar]
- Shelkovnikov, S.; Gonick, H.C. Peroxynitrite but not nitric oxide donors destroys epinephrine: HPLC measurement and rat aorta contractility. Life Sci. 2004, 75, 2765–2773. [Google Scholar] [CrossRef]
- Kessler, P.; Bauersachs, J.; Busse, R.; Schini-Kerth, V.B. Inhibition of inducible nitric oxide synthase restores endothelium-dependent relaxations in proinflammatory mediator-induced blood vessels. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1746–1755. [Google Scholar] [CrossRef]
- Virdis, A.; Colucci, R.; Fornai, M.; Blandizzi, C.; Duranti, E.; Pinto, S.; Bernardini, N.; Segnani, C.; Antonioli, L.; Taddei, S.; et al. Cyclooxygenase–2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: Role of inducible nitric-oxide synthase and oxidative stress. J. Pharmacol. Exp. Ther. 2005, 312, 945–953. [Google Scholar]
- Eguchi, D.; D'Uscio, L.V.; Wambi, C.; Weiler, D.; Kovesdi, I.; O'Brien, T.; Katusic, Z.S. Inhibitory effect of recombinant iNOS gene expression on vasomotor function of canine basilar artery. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2560–H2566. [Google Scholar]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Julou-Schaeffer, G.; Gray, G.A.; Fleming, I.; Schott, C.; Parratt, J.R.; Stoclet, J.C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am. J. Physiol. 1990, 259, H1038–H1043. [Google Scholar]
- Pittner, A.; Nalos, M.; Asfar, P.; Yang, Y.; Ince, C.; Georgieff, M.; Bruckner, U.B.; Radermacher, P.; Froba, G. Mechanisms of inducible nitric oxide synthase (iNOS) inhibition-related improvement of gut mucosal acidosis during hyperdynamic porcine endotoxemia. Intensiv. Care Med. 2003, 29, 312–316. [Google Scholar]
- Petros, A.; Lamb, G.; Leone, A.; Moncada, S.; Bennett, D.; Vallance, P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc. Res. 1994, 28, 34–39. [Google Scholar] [CrossRef]
- MacMicking, J.D.; Nathan, C.; Hom, G.; Chartrain, N.; Fletcher, D.S.; Trumbauer, M.; Stevens, K.; Xie, Q.W.; Sokol, K.; Hutchinson, N.; et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 1995, 81, 641–650. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, K.; Yue, M.; Lu, X.; Zheng, Q.; Zhang, H.; Zhai, Y.; Li, P.; Yu, L.; Cai, M.; et al. A non-synonymous SNP in the NOS2 associated with septic shock in patients with sepsis in Chinese populations. Hum. Genet. 2013, 132, 337–346. [Google Scholar] [CrossRef]
- Xia, Y.; Zweier, J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA 1997, 94, 6954–6958. [Google Scholar] [CrossRef]
- Marfella, R.; di Filippo, C.; Esposito, K.; Nappo, F.; Piegari, E.; Cuzzocrea, S.; Berrino, L.; Rossi, F.; Giugliano, D.; D’Amico, M. Absence of inducible nitric oxide synthase reduces myocardial damage during ischemia reperfusion in streptozotocin-induced hyperglycemic mice. Diabetes 2004, 53, 454–462. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Liaudet, L.; Rosenblatt-Velin, N.; Pacher, P. Role of peroxynitrite in the cardiovascular dysfunction of septic shock. Curr. Vasc. Pharmacol. 2013, 11, 196–207. [Google Scholar]
- Kuhlencordt, P.J.; Chen, J.; Han, F.; Astern, J.; Huang, P.L. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E–knockout mice. Circulation 2001, 103, 3099–3104. [Google Scholar] [CrossRef]
- Iadecola, C.; Zhang, F.; Xu, X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am. J. Physiol. 1995, 268, R286–R292. [Google Scholar]
- Iadecola, C.; Zhang, F.; Casey, R.; Nagayama, M.; Ross, M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997, 17, 9157–9164. [Google Scholar]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Forstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef]
- Rodriguez-Pascual, F.; Hausding, M.; Ihrig-Biedert, I.; Furneaux, H.; Levy, A.P.; Forstermann, U.; Kleinert, H. Complex contribution of the 3'-untranslated region to the expressional regulation of the human inducible nitric-oxide synthase gene. Involvement of the RNA-binding protein HuR. J. Biol. Chem. 2000, 275, 26040–26049. [Google Scholar] [CrossRef]
- Ishii, K.; Sheng, H.; Warner, T.D.; Forstermann, U.; Murad, F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am. J. Physiol. 1991, 261, H598–H603. [Google Scholar]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric. Oxide. 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Hausding, M.; Witteck, A.; Rodriguez-Pascual, F.; von Eichel-Streiber, C.; Forstermann, U.; Kleinert, H. Inhibition of small G proteins of the rho family by statins or clostridium difficile toxin B enhances cytokine-mediated induction of NO synthase II. Br. J. Pharmacol. 2000, 131, 553–561. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Saenz Rodriguez, T.; Garcia Gimenez, D.; de la Puerta Vazquez, R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine 2002, 9, 687–693. [Google Scholar] [CrossRef]
- Kucukgergin, C.; Aydin, A.F.; Ozdemirler-Erata, G.; Mehmetcik, G.; Kocak-Toker, N.; Uysal, M. Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol. Trace. Elem. Res. 2010, 135, 264–274. [Google Scholar] [CrossRef]
- Angelini, A.; di Pietro, R.; Centurione, L.; Castellani, M.L.; Conti, P.; Porreca, E.; Cuccurullo, F. Inhibition of P-glycoprotein-mediated transport by S-adenosylmethionine and cynarin in multidrug-resistant human uterine sarcoma MES-SA/Dx5 cells. J. Biol. Regul. Homeost. Agents 2012, 26, 495–504. [Google Scholar]
- Lopez-Molina, D.; Navarro-Martinez, M.D.; Rojas Melgarejo, F.; Hiner, A.N.; Chazarra, S.; Rodriguez-Lopez, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bauerlein, M.; Frohberg, C.; Landschutze, V.; Gibson, G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef]
- Llorach, R.; Espin, J.C.; Tomas-Barberan, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Zapolski-Downar, A.; Naruszewicz, M.; Siennicka, A.; Krasnodebska, B.; Koldziej, B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002, 71, 2897–2808. [Google Scholar] [CrossRef]
- Juzyszyn, Z.; Czerny, B.; Pawlik, A.; Drozdzik, M. The effect of artichoke (Cynara scolymus L.) extract on ROS generation in HUVEC cells. Phytother. Res. 2008, 22, 1159–1161. [Google Scholar] [CrossRef]
- Perez-Garcia, F.; Adzet, T.; Canigueral, S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radic. Res. 2000, 33, 661–665. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Dragsted, L.O.; Daneshvar, B.; Pulido, R.; Saura-Calixto, F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarkers of antioxidants in rats. J. Agric. Food Chem. 2003, 51, 5540–5545. [Google Scholar] [CrossRef]
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215. [Google Scholar] [CrossRef]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef]
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane. Database. Syst. Rev. 2013, 3, CD003335. [Google Scholar]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Wallis, C.; Simpson, H.C. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine 2008, 15, 668–675. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Faliva, M.A.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef]
- Grande, S.; Bogani, P.; de Saizieu, A.; Schueler, G.; Galli, C.; Visioli, F. Vasomodulating potential of mediterranean wild plant extracts. J. Agric. Food Chem. 2004, 52, 5021–5026. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Kamkhah, A.F. Artichoke leaf juice contains antihypertensive effect in patients with mild hypertension. J. Diet. Suppl. 2009, 6, 328–341. [Google Scholar] [CrossRef]
- Wittemer, S.M.; Ploch, M.; Windeck, T.; Muller, S.C.; Drewelow, B.; Derendorf, H.; Veit, M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine 2005, 12, 28–38. [Google Scholar] [CrossRef]
- Azzini, E.; Bugianesi, R.; Romano, F.; di Venere, D.; Miccadei, S.; Durazzo, A.; Foddai, M.S.; Catasta, G.; Linsalata, V.; Maiani, G. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: A pilot study. Br. J. Nutr. 2007, 97, 963–969. [Google Scholar]
- Dong, G.C.; Chuang, P.H.; Chang, K.C.; Jan, P.S.; Hwang, P.I.; Wu, H.B.; Yi, M.; Zhou, H.X.; Chen, H.M. Blocking effect of an immuno-suppressive agent, cynarin, on CD28 of T-cell receptor. Pharm. Res. 2009, 26, 375–381. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Interaction of natural dietary and herbal anionic compounds and flavonoids with human organic anion transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11). Evid. Based Complement. Alternat. Med. 2013, 2013, 612527. [Google Scholar]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Serra, D.; Paixao, J.; Nunes, C.; Dinis, T.C.; Almeida, L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS One 2013, 8, e73001. [Google Scholar]
- Wang, Q.; Xia, M.; Liu, C.; Guo, H.; Ye, Q.; Hu, Y.; Zhang, Y.; Hou, M.; Zhu, H.; Ma, J.; et al. Cyanidin-3-O-beta-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008, 83, 176–184. [Google Scholar] [CrossRef]
- Sorrenti, V.; di Giacomo, C.; Acquaviva, R.; Bognanno, M.; Grilli, E.; D’Orazio, N.; Galvano, F. Dimethylarginine dimethylaminohydrolase/nitric oxide synthase pathway in liver and kidney: Protective effect of cyanidin 3-O-beta-d-glucoside on ochratoxin-A toxicity. Toxins (Basel) 2012, 4, 353–363. [Google Scholar] [CrossRef]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.S.; Yoo, H.; Choung, M.G.; Sung, M.K. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 8427–8433. [Google Scholar] [CrossRef]
- Schmidt, N.; Pautz, A.; Art, J.; Rauschkolb, P.; Jung, M.; Erkel, G.; Goldring, M.B.; Kleinert, H. Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. Biochem. Pharmacol. 2010, 79, 722–732. [Google Scholar] [CrossRef]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Forstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Structure-activity relationship of staurosporine analogs in regulating expression of endothelial nitric-oxide synthase gene. Mol. Pharmacol. 2000, 57, 427–435. [Google Scholar]
- Sample Availability: Samples of the compounds (cynarin, cyanidin, luteolin and cynaroside) are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells. Molecules 2014, 19, 3654-3668. https://doi.org/10.3390/molecules19033654
Xia N, Pautz A, Wollscheid U, Reifenberg G, Förstermann U, Li H. Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells. Molecules. 2014; 19(3):3654-3668. https://doi.org/10.3390/molecules19033654
Chicago/Turabian StyleXia, Ning, Andrea Pautz, Ursula Wollscheid, Gisela Reifenberg, Ulrich Förstermann, and Huige Li. 2014. "Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells" Molecules 19, no. 3: 3654-3668. https://doi.org/10.3390/molecules19033654
APA StyleXia, N., Pautz, A., Wollscheid, U., Reifenberg, G., Förstermann, U., & Li, H. (2014). Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells. Molecules, 19(3), 3654-3668. https://doi.org/10.3390/molecules19033654




