1. Introduction
Indoleamine 2,3-dioxygenase (IDO) is one of the three heme-containing dioxygenases [
1]. This enzyme is involved in the kynurenine pathway which is the major pathway for the catabolism of the essential amino acid tryptophan (Trp), being responsible for catalysing the rate-limiting step of the Trp degradation to N-formylkynurenine [
2]. Elevated Trp catabolism has been associated with rheumathoid arthritis [
3] and cancer [
4]. It has been shown that after chemotherapy, inhibition of IDO could delay the recurrence of tumour antigens tolerance [
5], and also that its inhibition could be a new route to improve immunity to leishmania-infected humans [
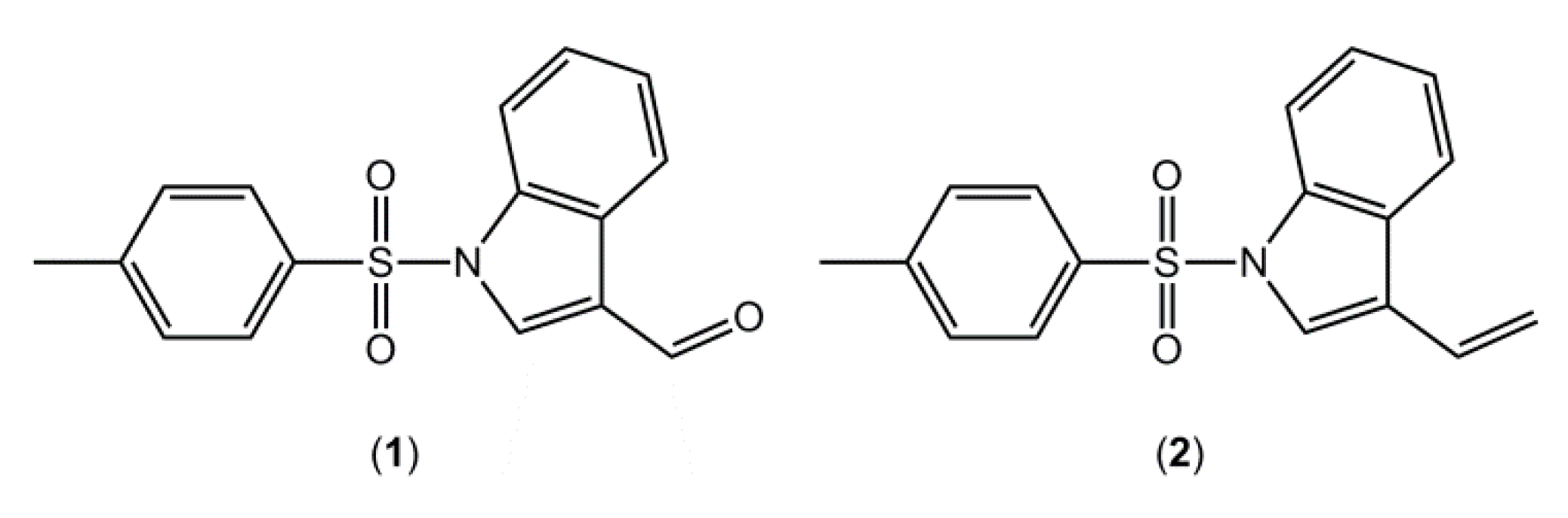
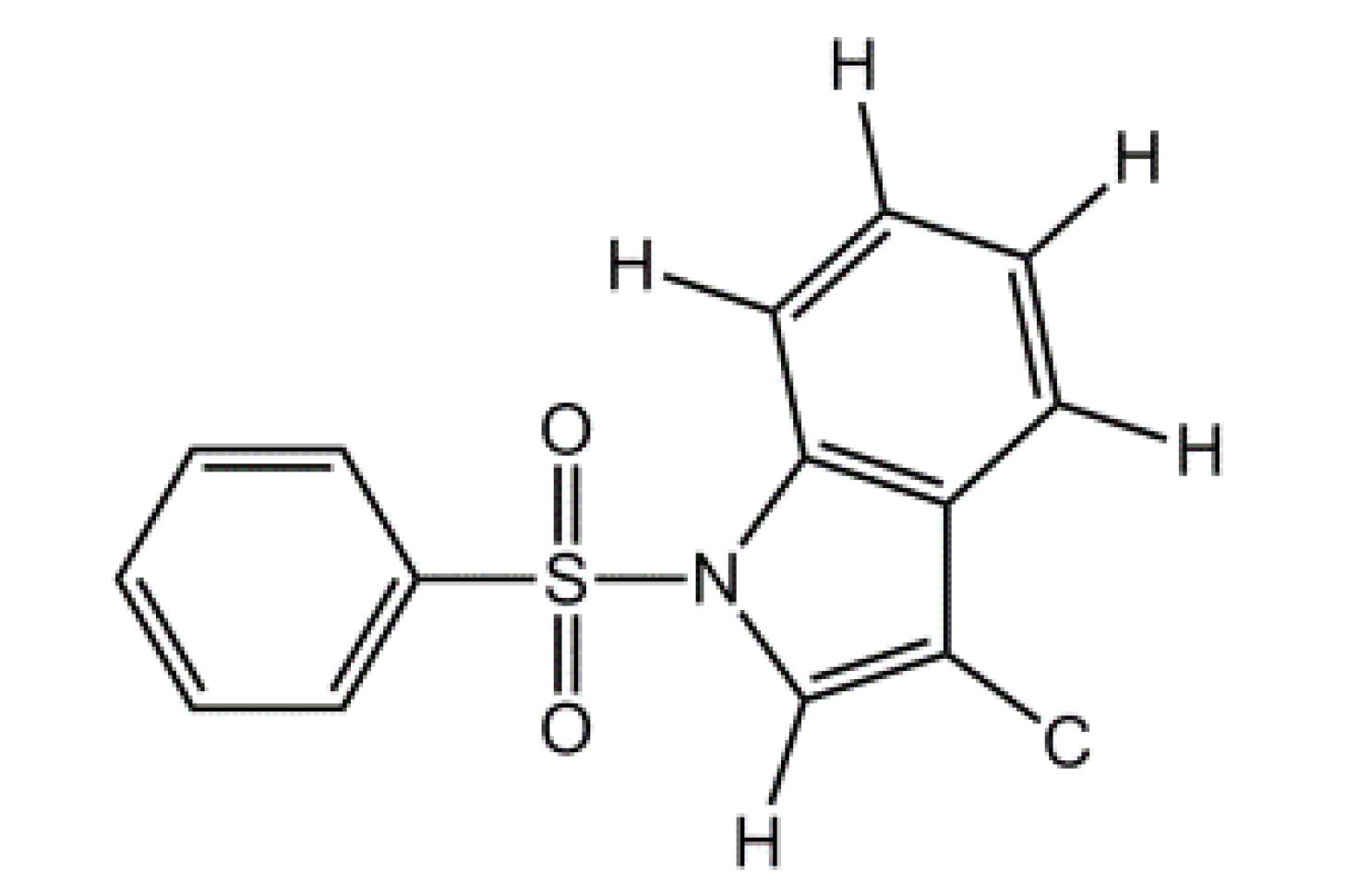
6]. As part of our research aimed at the synthesis of potential IDO inhibitors, using indole as a scaffold, 1-[(4-methylbenzene)sulfonyl]indole-3-carbaldehyde (
1,
Figure 1), was synthesized and its crystal structure determined.
Figure 1.
Chemical structures of (1) and (2).
Figure 1.
Chemical structures of (1) and (2).
In a previous contribution in this area [
7], we reported the crystal structure of a closely related compound, 3-ethenyl-1-[(4-methylbenzene)sulfonyl]indole (
2), where an ethenyl moiety was in the 3-postion rather than the carbaldehyde of compound
1 (
Figure 1). It is noted that in both
1 and
2 there are two independent molecules in the asymmetric unit,
i.e.,
Z' = 2, and that in each case these are conformers, each being related to each other via a rotation about the central S-N bond. As this situation seemed unusual [
8,
9], a search of the Cambridge Structure Database (CSD) [
10] was performed. For all-organic molecules only, there were 148,842 hits for structures crystallising with
Z' = 1 compared with 20807 with
Z' = 2,
i.e., indicating examples of structures
Z' = 2 are only 14% as likely to ocurr, compared with structures with
Z' = 1. With this background, a search was conducted for structures related to
1,
i.e., 1-(arylsulfonyl)indole derivatives, to ascertain how prevalent this phenomenon was for this class of compound. The results of this survey are also presented herein. Finally, DFT calculations on
1,
2 and related compounds were performed in order to understand the energetics of these systems, in particular the nature of the rotational barrier around the S-N bond.
2. Results and Discussion
There are two independent molecules in the asymmetric unit of
1 which are shown in
Figure 2. The dihedral angles between the fused ring system (r.m.s. deviations = 0.011 and 0.022 Å for the N1- and N2-containing rings, respectively) and the benzene ring are close,
i.e., 79.08(6)° and 72.83(5)°, respectively. The values found in similar structures are 82.98(12)° and 84.46(13)° for the two independent molecules of 3-ethenyl-1-(4-methylphenylsulfonyl)-1
H-indole (
2) [
7]; 80.37(8)° for (2-methyl-1-(phenylsulfonyl)-1
H-indol-3-yl)methanol [
11], 77.41(5)° for ethyl 2-bromo-3-(1-phenyl-sulfonyl-1
H-indol-3-yl)acrylate [
12] and 66.47(15)° for benzyl(3-bromo-1-(phenylsulfonyl)indol-2-ylmethyl)(
p-tolyl)amine [
13]. In
1, each carbaldehyde moiety is almost co-planar with the ring to which it is connected as seen in the values of the C8-C7-C9-O3 and C24-C23-C25-O6 torsion angles of −175.4(2)° and −174.1(2)°, respectively. Further discussion on the relationship between the two independent molecules in
1 is found below in the context of the DFT calculations performed on these species.
Figure 2.
Molecular structures for the two independent molecules of 1 showing the atom numbering scheme.
Figure 2.
Molecular structures for the two independent molecules of 1 showing the atom numbering scheme.
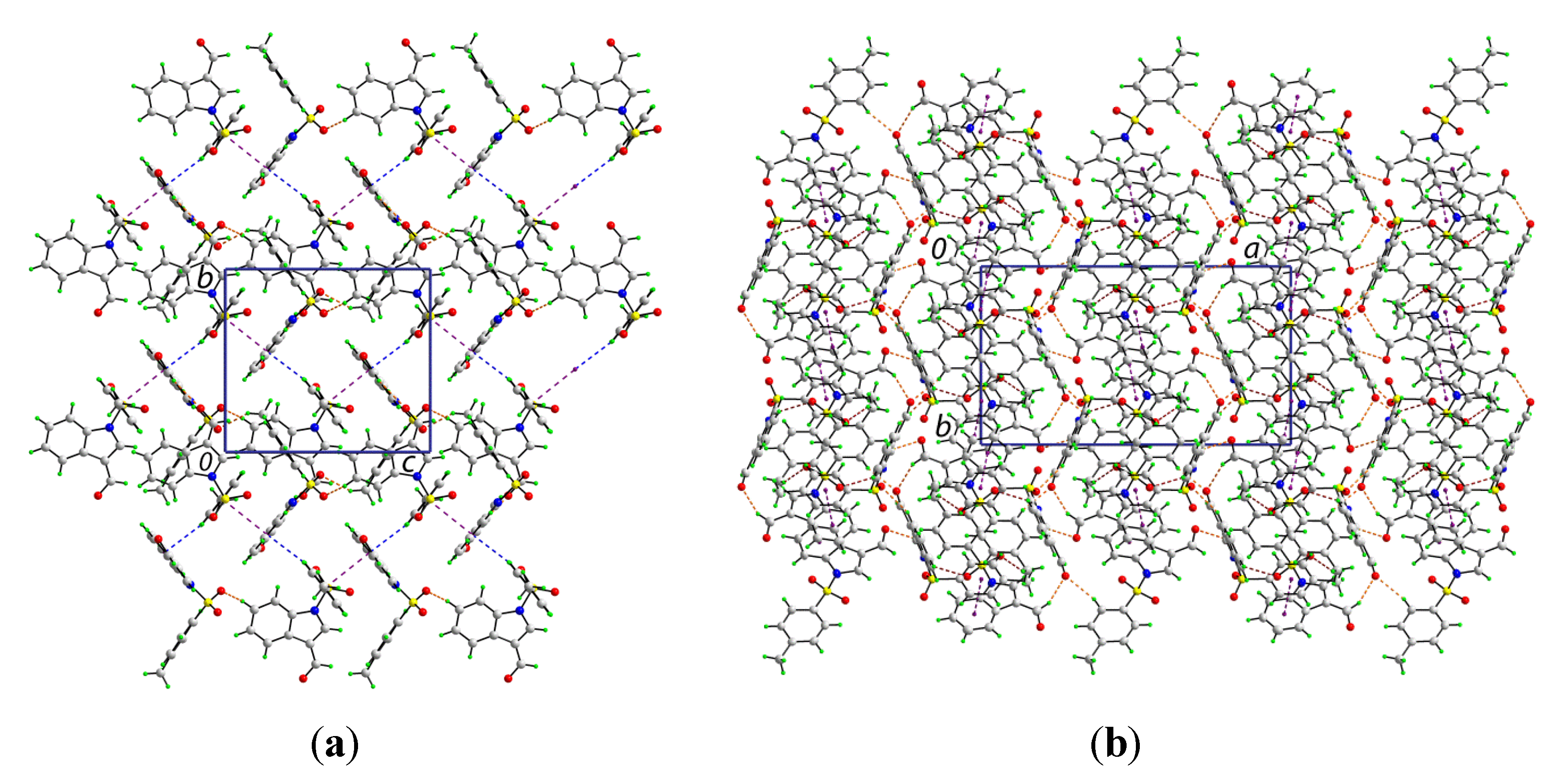
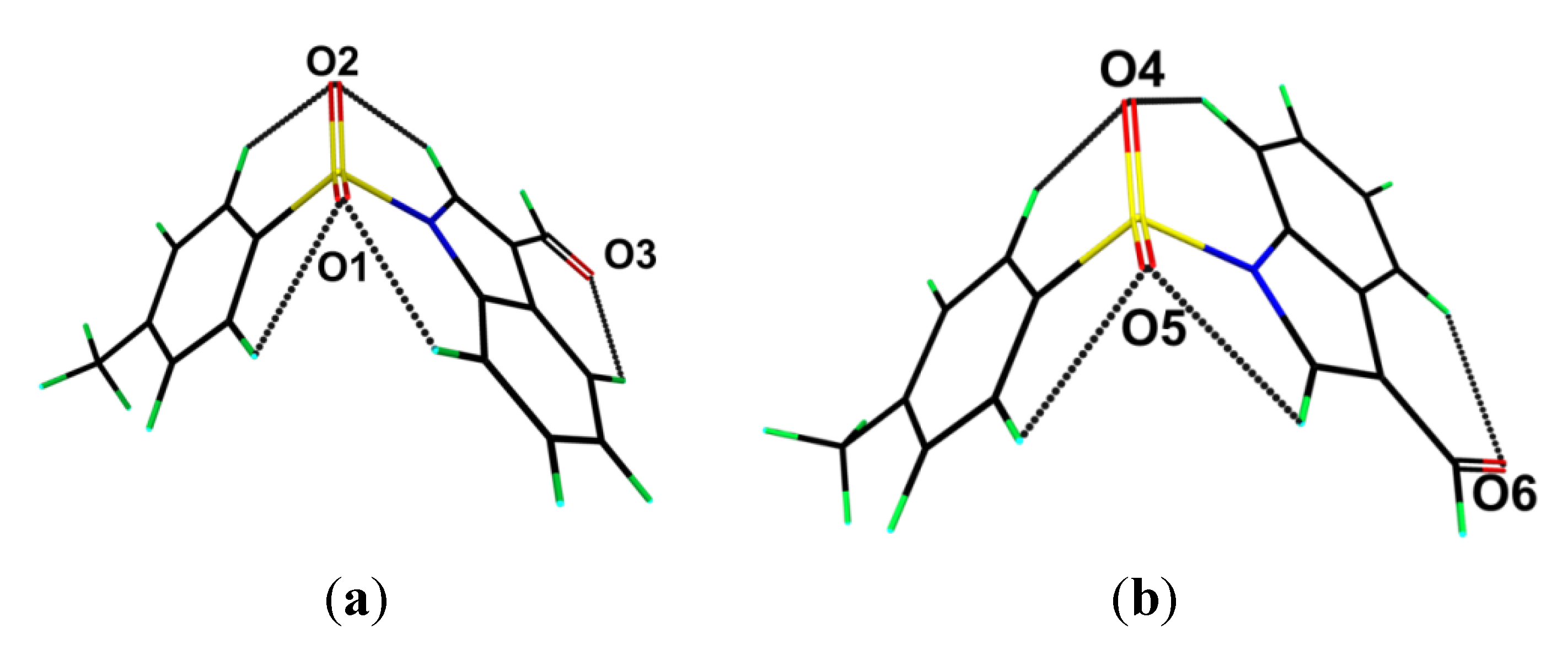
The crystal structure features a range of intermolecular interactions as summarized in
Table 1, with the most significant of these being π···π interactions occurring between the tolyl ring of the S1-containing molecule and the C
6 ring of the indole fused ring system of the S2-containing molecule. The two-molecule aggregates thus formed are connected into a supramolecular chain along the c-axis via C-H···O interactions involving each of the sulfoxide-O atoms of the S2-containing molecule whereby the O4 and O5 atoms accept H atoms bound to indole- and methyl- residues derived from the S1- and S2-containing molecules, respectively. Links between these chains are of the type C-H···π and involve a tolyl-H atom of the S1-containing molecule interacting with the C
6 ring of the indole fused ring system of the S2-containing molecule, resulting in the formation of supramolecular layers in the
bc-plane, as shown in
Figure 3a. Links between layers are exclusively of the type C-H···O and involve both aldehyde-O3 and O6 atoms as well as the sulfoxide-O2 atoms as acceptors,
Figure 3b.
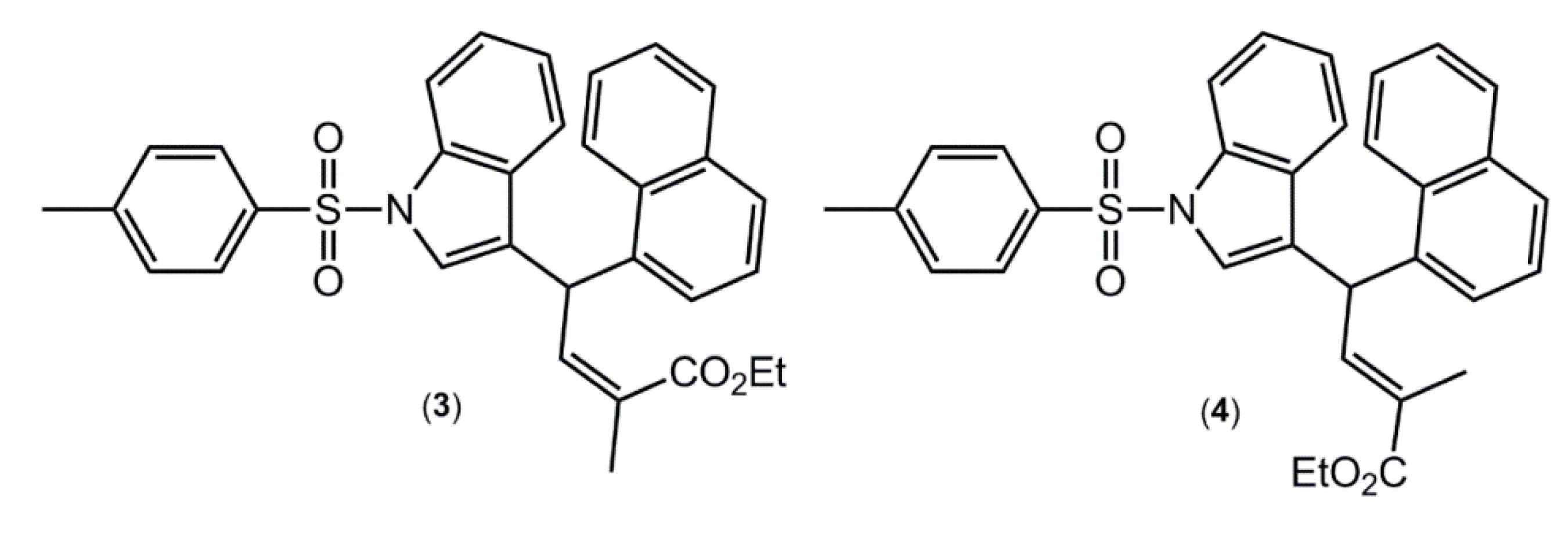
A search of the CSD [
10,
14] resulted in 34 related 1-(arylsulfonyl)indole structures of which five have two independent molecules in the asymmetric unit, that is
Z' = 2, a ratio consistent with the general findings mentioned in the Introduction (see the
Supplementary Material of this article for the complete list of all 34 hits). Arguably, the most interesting of these structures are the (
E)- and (
Z)- conformers of ethyl 2-methyl-4-(1-((4-methylphenyl)sulfonyl)-1
H-indol-3-yl)-4-(1-naphthyl)but-2-enoate [
15] (
Figure 4), where the (Z)- conformer crystallises with
Z' = 1 (compound
3), while the (
E) conformer crystallises with
Z' = 2 (compound
4). These molecules were also subjected to DFT calculations along with
1 and
2, as described below.
Table 1.
Geometric parameters describing intermolecular interactions (A–H···B; Å) operating in the crystal structure of 1.
Table 1.
Geometric parameters describing intermolecular interactions (A–H···B; Å) operating in the crystal structure of 1.
| A | H | B | A‒H | H···B | A···B | A‒H···B | Symmetry Operation |
|---|
| Cg(C10–C15) | - | Cg(C17–C22) | - | - | 3.5917(13) | 1.41(11) | 2 – x, 1 – y, ½ + z |
| C3 | H3 | O4 | 0.95 | 2.56 | 3.370(3) | 143 | x, y, – 1 + z |
| C32 | H32A | O5 | 0.98 | 2.55 | 3.401(3) | 148 | – x, 1 – y, – ½ + z |
| C31 | H31 | Cg(C1–C6) | 0.95 | 2.99 | 3.901(2) | 160 | –x, 1 – y, ½ + z |
| C5 | H5 | O2 | 0.95 | 2.58 | 3.458(3) | 155 | ½ – x, ½ + y, –½ + z |
| C8 | H8 | O6 | 0.95 | 2.40 | 3.129(3) | 134 | ½ + x, ½ – y, z |
| C25 | H25 | O3 | 0.95 | 2.60 | 3.452(3) | 150 | –x, 1 – y, ½ + z |
| C27 | H27 | O3 | 0.95 | 2.33 | 3.175(3) | 147 | ½ – x, –½ + y, ½ + z |
Figure 3.
Views of the supramolecular association in the crystal structure of 1: (a) supramolecular layer in the bc-plane, and (b) in projection down the c-axis showing the stacking of layers along the a-axis. The π···π, C-H···O (within layers), C-H···O (between layers), and C-H···π interactions (obscured in the (b) projection) are shown as purple, brown, orange and blue dashed lines, respectively.
Figure 3.
Views of the supramolecular association in the crystal structure of 1: (a) supramolecular layer in the bc-plane, and (b) in projection down the c-axis showing the stacking of layers along the a-axis. The π···π, C-H···O (within layers), C-H···O (between layers), and C-H···π interactions (obscured in the (b) projection) are shown as purple, brown, orange and blue dashed lines, respectively.
Figure 4.
Chemical structures of ethyl 2-methyl-4-(1-((4-methylphenyl)sulfonyl)-1H-indol-3-yl)-4-(1-naphthyl)but-2-enoate (3), the Z-conformer with Z' = 1, and 4, the E-conformer with Z' = 2.
Figure 4.
Chemical structures of ethyl 2-methyl-4-(1-((4-methylphenyl)sulfonyl)-1H-indol-3-yl)-4-(1-naphthyl)but-2-enoate (3), the Z-conformer with Z' = 1, and 4, the E-conformer with Z' = 2.
In all calculations the experimental structure,
i.e., as determined by X-ray crystallography, was the starting point for geometry optimisations. In cases where
Z' = 2 and the independent molecules are conformers, as is the cases of
1 and
4 [
15], calculations, as well as the Intrinsic Reaction Coordinate (IRC) calculations, were performed for both molecules. For
2 [
7], where the two independent molecules were almost identical, the 180° related-conformer was generated. This was done so that comparable IRC calculations for
2 could be performed as for
1 and
4. The two independent molecules of
1 are conformers, being twisted about the S-N and S-C bonds (
Figure 2). At this point it is important to stress that the appearance of conformers is a solid-state effect. A variable temperature
1H-NMR study was conducted (see
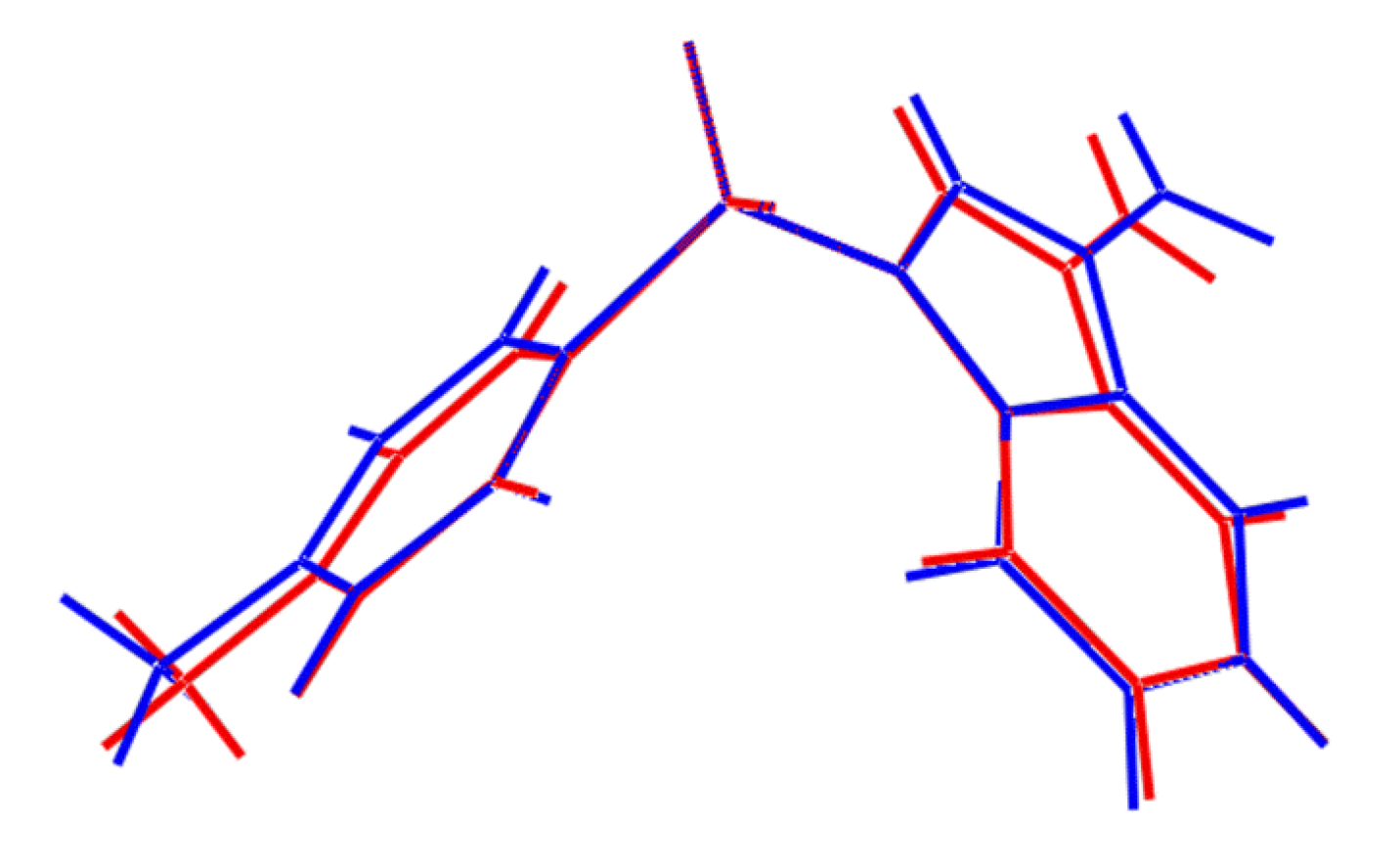
Supplementary Material) down to −50 °C in deuterated chloroform solution and no evidence was found for more than one conformation. As seen from the overlay diagram in
Figure 5, where the S1-containing molecule has been super-imposed upon the inverted form of the S2-containing molecule using QMol [
16], the molecular geometries closely resemble each other. The major difference in the molecules is quantified in the C10-S1-N1-C8 and C26-S2-N2-C24 torsion angles of 109.18(17)° and −116.34(18)°, respectively; a smaller difference in the O1-S1-C10-C11 and O4-S2-C26-C27 torsion angles of −2.1(2)° and 6.1(2)°, respectively, is noted.
Figure 5.
Superposition of the two independent molecules comprising the asymmetric unit of
1 [
16], drawn so that the SO
2 groups are superimposed. The inverted form of the S2-containing molecule (blue image) has been employed.
Figure 5.
Superposition of the two independent molecules comprising the asymmetric unit of
1 [
16], drawn so that the SO
2 groups are superimposed. The inverted form of the S2-containing molecule (blue image) has been employed.
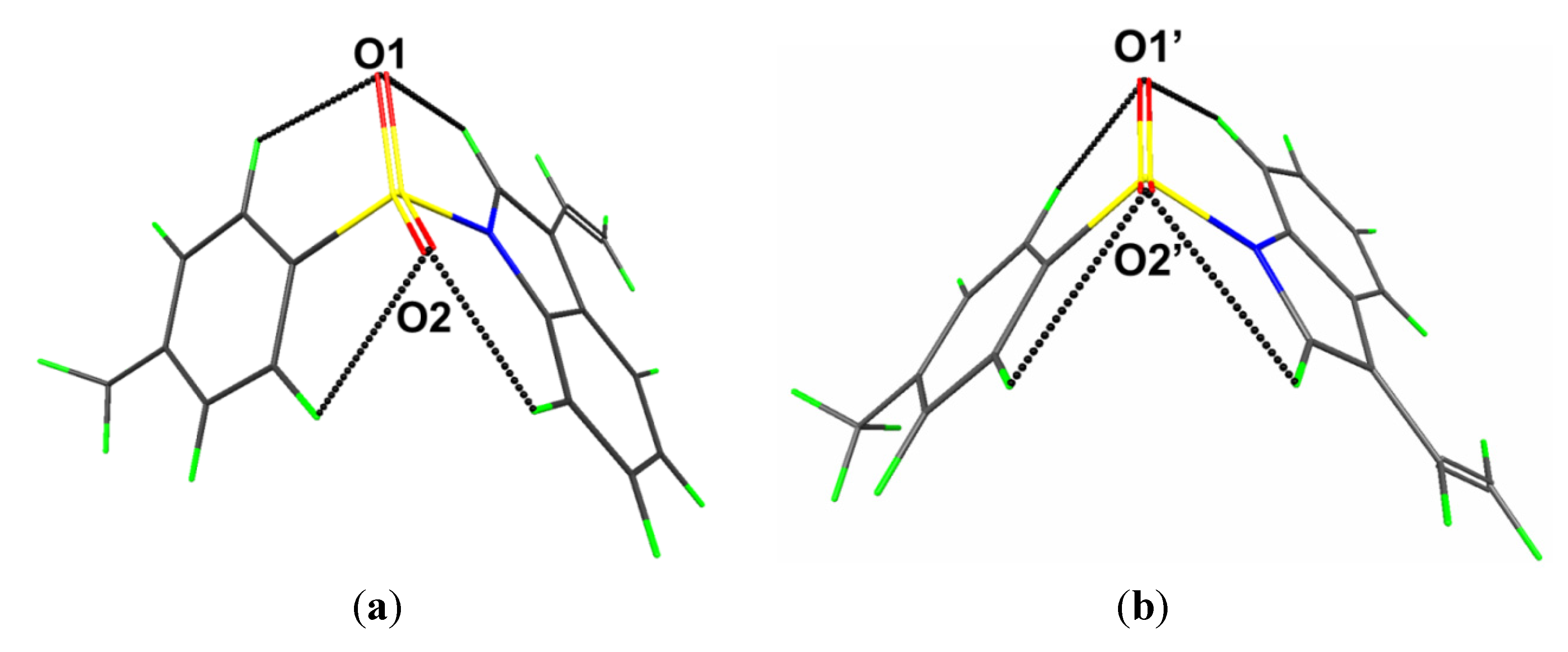
Geometry optimisation calculations conducted on the two independent molecules of each of
1 and
2 show that in each case both conformers optimise to the same energy-minimised molecule. This observation is readily ascribed to the fact that in both cases the structures are stabilized by comparable intramolecular C-H···O interactions as illustrated in
Figure 6 and
Figure 7 and quantified in
Table 2, where the geometric parameters and NBO analyses of these interactions are listed.
Figure 6.
Geometry optimised molecules for the (
a) S1- and (
b) S2-containing molecules of
1. Details of the intramolecular C-H···O interactions are tabulated in
Table 2.
Figure 6.
Geometry optimised molecules for the (
a) S1- and (
b) S2-containing molecules of
1. Details of the intramolecular C-H···O interactions are tabulated in
Table 2.
Figure 7.
Geometry optimised molecules for the (
a) S1- and (
b) S1'-containing molecules of (
2). Details of the intramolecular C-H···O interactions are tabulated in
Table 2.
Figure 7.
Geometry optimised molecules for the (
a) S1- and (
b) S1'-containing molecules of (
2). Details of the intramolecular C-H···O interactions are tabulated in
Table 2.
Table 2.
Geometric parameters describing intramolecular interactions (A–H···B; Å, °) for (1) and (2).
Table 2.
Geometric parameters describing intramolecular interactions (A–H···B; Å, °) for (1) and (2).
| A | H | B | A–H | H···B | A···B | A‒H···B | NBO energy/kcal·mol−1 |
|---|
| (1) S1-containing molecule |
| C2 | H2 | O1 | 1.10 | 2.35 | 3.05 | 120.2 | −2.09 |
| C11 | H11 | O1 | 1.10 | 2.69 | 3.08 | 99.7 | −0.29 |
| C8 | H8 | O2 | 1.09 | 2.71 | 3.05 | 97.2 | −0.26 |
| C15 | H15 | O2 | 1.10 | 2.56 | 3.00 | 102.7 | −0.60 |
| C5 | H5 | O3 | 1.10 | 2.65 | 3.25 | 113.9 | −0.76 |
| (1) S2-containing molecule |
| C18 | H18 | O4 | 1.10 | 2.36 | 3.05 | 120.0 | −2.01 |
| C27 | H27 | O4 | 1.10 | 2.70 | 3.08 | 99.5 | −0.27 |
| C24 | H24 | O5 | 1.09 | 2.70 | 3.04 | 97.4 | −0.27 |
| C31 | H31 | O5 | 1.10 | 2.56 | 3.00 | 102.8 | −0.62 |
| C21 | H21 | O6 | 1.10 | 2.65 | 3.25 | 113.9 | −0.74 |
| (2) S1-containing molecule |
| C9 | H9 | O2 | 1.10 | 2.32 | 3.03 | 120.8 | −2.32 |
| C16 | H16 | O2 | 1.10 | 2.70 | 3.08 | 99.5 | −0.27 |
| C8 | H8 | O1 | 1.09 | 2.71 | 3.05 | 97.5 | −0.25 |
| C20 | H20 | O1 | 1.10 | 2.56 | 3.00 | 102.9 | −064 |
| (2) S1'-containing molecule |
| C9 | H9 | O1' | 1.10 | 2.33 | 3.04 | 120.6 | −2.22 |
| C20 | H20 | O1' | 1.10 | 2.68 | 3.07 | 100.1 | −0.31 |
| C8 | H8 | O2' | 1.09 | 2.70 | 3.05 | 97.7 | −0.28 |
| C16 | H16 | O2' | 1.10 | 2.58 | 3.01 | 102.4 | −0.57 |
NBO data calculated using the Giambiagi-Mayer and Wiberg models show that the S-O bonds have significant ionic character in both the energy minimised and in the transition state structures (
Table 3), indicating that S
+‒O
− is the best Lewis structure description, rather than S=O. The consequence of this is that each sulfoxide-O atom has three lone pairs. Moreover, the NBO results indicate that the strength of the S-N bond is due to n
N→σ
*S-N and, and particularly, n
N→σ
*S-C hyperconjugative interactions which do not persist in the transition state (
Table 4). The S-N bond orders do not change significantly upon rotation as the n
O→σ
*S-N interactions do not achieve significant π-bond character from the n
N→σ
*S-C and n
N→σ
*S-O conjugation (
Table 3 and
Table 4), so that three-dimensional hyperconjugation results.
Table 3.
Bond orders calculated for the optimised and transition state structures 1 and 2 a.
Table 3.
Bond orders calculated for the optimised and transition state structures 1 and 2 a.
| Giambiagi-Mayer bond orders |
|---|
| Bond | 1–S1 | 1–S2 | (1)TS1 | (1)TS2 | 2–S1 | 2–S2 | (2)TS1 | (2)TS2 |
| S-O | 1.778 | 1.778 | 1.771 | 1.769 | 1.776 | 1.776 | 1.770 | 1.768 |
| S-N | 0.666 | 0.666 | 0.651 | 0.640 | 0.677 | 0.677 | 0.661 | 0.650 |
| S-C | 0.744 | 0.744 | 0.742 | 0.749 | 0.742 | 0.742 | 0.741 | 0.747 |
| Wiberg bond index |
| Bond | 1–S1 | 1–S2 | (1)TS1 | (1)TS2 | 2–S1 | 2–S2 | (2)TS1 | (2)TS2 |
| S-O | 1.484 | 1.484 | 1.484 | 1.482 | 1.483 | 1.483 | 1.482 | 1.481 |
| S-N | 0.580 | 0.580 | 0.568 | 0.560 | 0.590 | 0.590 | 0.578 | 0.569 |
| S-C | 0.694 | 0.694 | 0.693 | 0.701 | 0.691 | 0.691 | 0.691 | 0.698 |
Table 4.
Stabilization energies (kcal·mol−1) of hyperconjugative interactions in the sulfonylgroup a,b.
Table 4.
Stabilization energies (kcal·mol−1) of hyperconjugative interactions in the sulfonylgroup a,b.
| 1 |
|---|
| Interaction | 1–S1 | 1–S2 | (1)TS1 | (1)TS2 |
| nN→σ*S-C | 4.6 | 4.7 | 0.0 | 0.0 |
| nN→σ*S-N | 0.2 | 0.2 | 0.0 | 0.0 |
| nN→σ*S-O1 | 2.0 | 1.8 | 6.0 | 5.9 |
| nN→σ*S-O2 | 1.6 | 1.7 | 6.5 | 6.4 |
| nO1 →σ*S-C | 32.0 | 32.0 | 32.8 | 30.2 |
| nO1 →σ*S-N | 51.2 | 51.2 | 52.1 | 52.0 |
| nO1→σ*S-O1 | 0.2 | 0.2 | 0.2 | 0.3 |
| nO1 →σ*S-O2 | 30.3 | 30.3 | 30.3 | 30.3 |
| 2 |
| Interaction | 2–S1 | 2-S2 | (2)TS1 | (2)TS2 |
| nN→σ*S-C | 4.5 | 4.5 | 0.0 | 0.0 |
| nN→σ*S-N | 0.2 | 0.2 | 0.0 | 0.0 |
| nN→σ*S-O1 | 2.0 | 2.2 | 6.5 | 6.3 |
| nN→σ*S-O2 | 2.0 | 1.8 | 6.5 | 6.0 |
| nO1 →σ*S-C | 32.2 | 32.2 | 33.0 | 32.6 |
| nO1 →σ*S-N | 50.1 | 50.1 | 51.1 | 51.6 |
| nO1→σ*S-O1 | 0.2 | 0.2 | 0.2 | 0.2 |
| nO1 →σ*S-O2 | 30.3 | 30.3 | 30.3 | 30.3 |
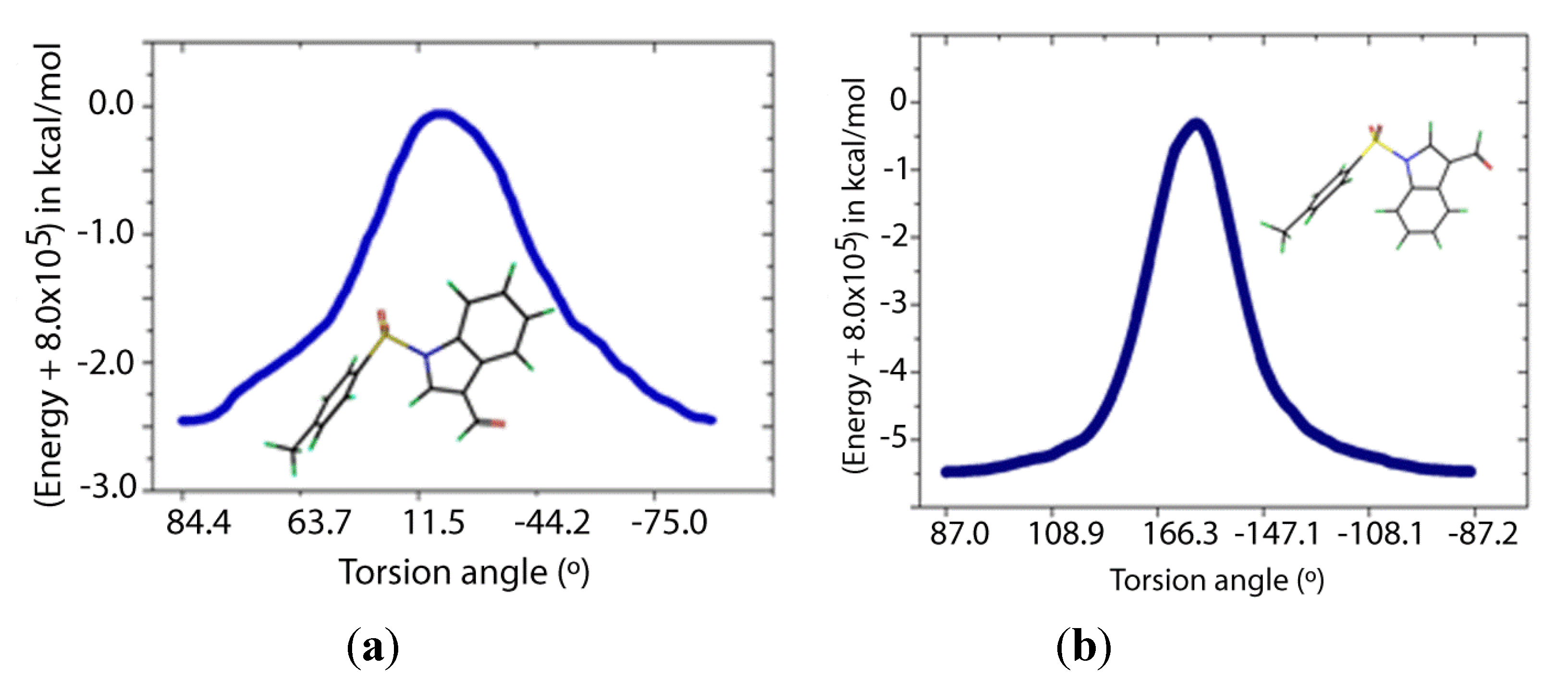
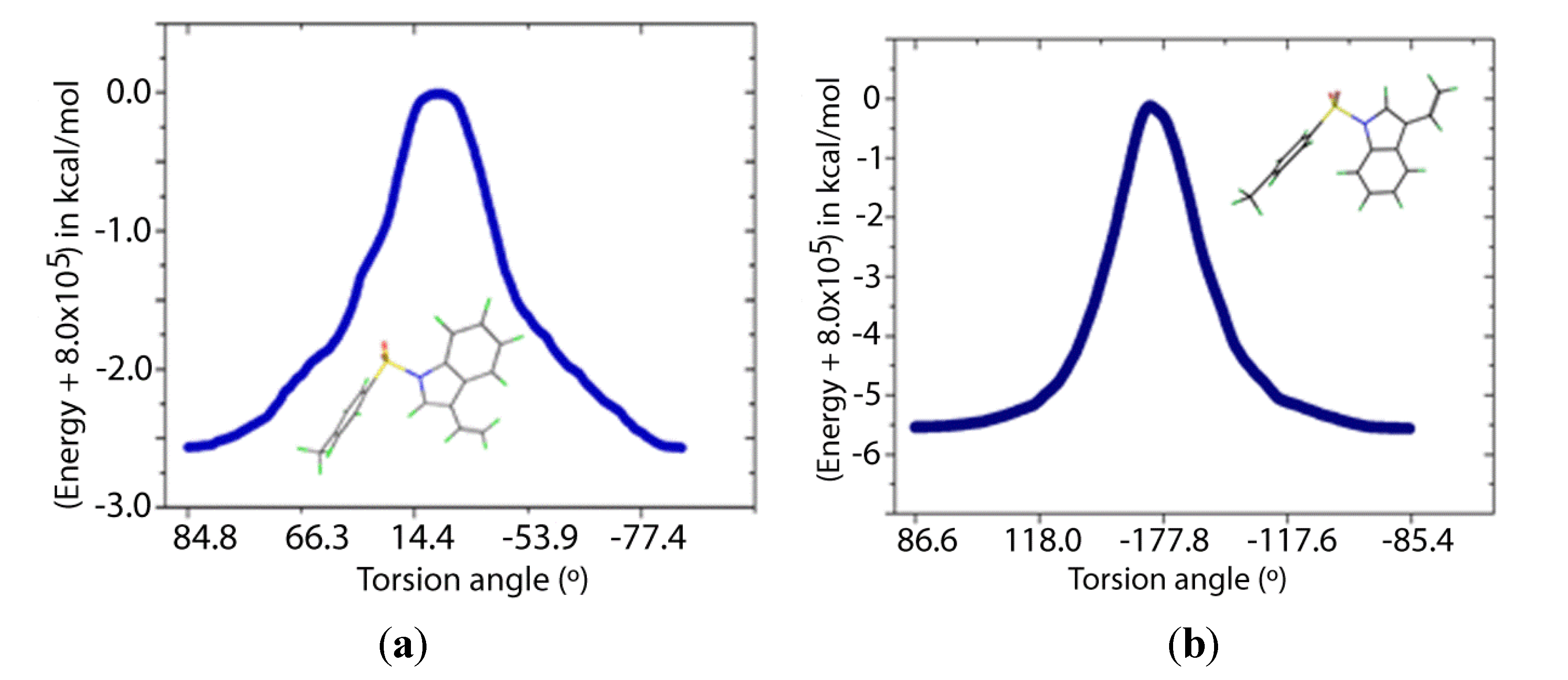
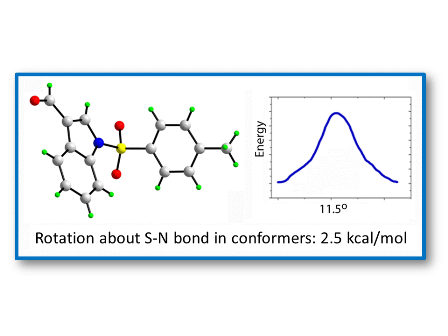
Two transition states for
1 and
2 were found (
TS1 and
TS2,
Figure 8 and
Figure 9), with C-N-S-C torsion angles near 0° and 180° and energy barriers
ca 2.5 and 5.5 kcal/mol, respectively (
Table 5). The lone pairs of
TS1 have lower occupancies (
Table 6) thus greater hyperconjugative effects than those of
TS2 according to the NBO analysis (
Table 4) and that higher delocalization explains why the
TS1 has a more stable structure.
Figure 8.
IRC of the rotational barrier about the S-N bond in 1: (a) 1TS1 and (b) 1TS2. Insets are the corresponding molecular structures Torsion angle (°) (Energy + 8.0 × 105) in kcal/mol.
Figure 8.
IRC of the rotational barrier about the S-N bond in 1: (a) 1TS1 and (b) 1TS2. Insets are the corresponding molecular structures Torsion angle (°) (Energy + 8.0 × 105) in kcal/mol.
Figure 9.
IRC of the rotational barrier about the S-N bond in 2: (a) 2TS1 and (b) 2TS2. Insets are the corresponding molecular structures.
Figure 9.
IRC of the rotational barrier about the S-N bond in 2: (a) 2TS1 and (b) 2TS2. Insets are the corresponding molecular structures.
According to
Table 7, the nitrogen and oxygen lone pairs in the transition states have higher repulsion than in the minimum energy structures. Therefore, the origin of the rotational barrier of these sulfonamides lies in the strong hyperconjugative effects of n
N→σ
*S-C in the S-N bond coupled with the high repulsions between lone pairs in the transition state structures. This energy difference between the transition states affords an effective Gibbs free energy of activation close to that of TS1, which ensures that rotation occurs mainly via TS1. The relatively low rotational barrier indicates that there is a facile interconversion between the conformers.
Table 5.
Relative energies (kcal·mol−1) and dihedral angles (°) of transition and ground states a.
Table 5.
Relative energies (kcal·mol−1) and dihedral angles (°) of transition and ground states a.
| Structure | ∆G# (298.15 K) | ∆E#ZPE | ∆E# | ∟C-S-N-C |
|---|
| 1 |
| 1 | 0.0 | 0.0 | 0.0 | 84.4 |
| (1)TS1 | 3.8 | 2.3 | 2.4 | 2.0 |
| (1)TS2 | 6.7 | 5.1 | 5.2 | 179.4 |
| ∆Geff# | 3.8 | | | |
| 2 |
| 2 | 0.0 | 0.0 | 0.0 | 84.8 |
| (2)TS1 | 3.8 | 2.5 | 2.4 | 1.7 |
| (2)TS2 | 7.0 | 5.3 | 5.4 | 179.5 |
| ∆Geff# | 3.8 | | | |
As mentioned above, an interesting case is found in the structures of ethyl 2-methyl-4-(1-((4-methylphenyl)sulfonyl)-1
H-indol-3-yl)-4-(1-naphthyl)but-2-enoate (
Figure 4), for which the (
E)-isomer
4 crystallizes with two independent molecules in the asymmetric unit, and the (
Z)-isomer
3 with only one [
15]; in the latter, the experimental structure was artificially manipulated to generate the other conformer. The conformers for each of
3 and
4 were also optimised. This showed that the conformers converged to the same energy in the gas-phase for
3 and
4, respectively. Interestingly, the (
Z)-isomer
3 has an energy 4.1 kcal·mol
−1 lower than that calculated for the (
E)-isomer
4. An evaluation of the molecular structures, in particular the intramolecular C-H···O interactions, provides a clear explanation for the energy difference. Thus, the carbonyl-O atom in (3) forms two significant C‑H···O interactions (–11.9 and –14.6 kcal·mol
−1) to provide considerable stability to the molecular structure. In
4, analogous intramolecular C-H···O interactions are also present but these provide considerably less stabilisation to the molecular structure (−1.8 and −8.7 kcal·mol
−1).
Table 6.
Occupancies (e) of lone pairs in the transition states according to NBO analyses a–c.
Table 6.
Occupancies (e) of lone pairs in the transition states according to NBO analyses a–c.
| Orbital | (1)TS1 | (1)TS2 | Δn | (2)TS1 | (2)TS2 | Δn |
|---|
| nN | 1.57042 | 1.57054 | −0.000120 | 1.99357 | 1.99359 | −0.00002 |
| nO1(1) | 1.99349 | 1.99354 | −0.00005 | 1.67815 | 1.67935 | −0.00120 |
| nO1(2) | 1.67855 | 1.67991 | −0.00136 | 1.6318 | 1.63041 | 0.00139 |
| nO1(3) | 1.62852 | 1.62776 | 0.00076 | 1.99356 | 1.99358 | −0.00002 |
| nO2(1) | 1.99350 | 1.99355 | −0.00005 | 1.67799 | 1.67935 | −0.00136 |
| nO2(2) | 1.67817 | 1.67999 | −0.00182 | 1.63202 | 1.63044 | 0.00158 |
| nO2(3) | 1.62936 | 1.62766 | 0.00170 | 1.57986 | 1.58039 | −0.00053 |
| nO3(1) | 1.99690 | 1.99679 | 0.00011 | | | |
| nO3(2) | 1.88105 | 1.88124 | −0.00019 | | | |
| Total | 16.04996 | 16.05098 | −0.00102 | 12.18695 | 12.18711 | −0.00016 |
Table 7.
Relative NBO Steric Exchange Energies between oxygen and nitrogen lone pairs.
Table 7.
Relative NBO Steric Exchange Energies between oxygen and nitrogen lone pairs.
| Structure | ∆Esteric/kcal·mol−1 | | Structure | ∆Esteric/kcal·mol−1 |
|---|
| 1 | 0.0 | | 2 | 0.0 |
| (1)TS1 | 0.9 | | (2)TS1 | 0.9 |
| (1)TS2 | 0.8 | | (2)TS2 | 0.9 |
3. Experimental
3.2. Synthesis and Characterization
Into a previously flamed two-necked round-bottomed flask under a nitrogen atmosphere was poured the 1H-indole-3-carbaldehyde (0.145 g, 1 mmol, 1 eq.), CH2Cl2 (5 mL), TsCl (0.22 g, 1.15 mmol, 1.15 eq.), Et3N (0.20 mL, 1.5 mmol, 1.5 eq.) and 4-dimethylaminopyridine (4-DMAP, 0.012 g, 10 mol%, 0.1 eq.). This was followed by vigorous stirring for 2 h at room temperature. The mixture was acidified with a 1 N HCl solution and extracted with EtOAc, washed with a saturated solution of NH4Cl and H2O, and dried under MgSO4. The remaining solvent was removed under reduced pressure. The solid was washed with MeOH three times (yield = 88%). Crystals for X-ray analysis were obtained by slow evaporation from its EtOAc solution held at 293 K; M.p: 420–423 K. NMR 1H (CDCl3, 300 MHz) δ (ppm): 10.09 (s, 1H), 8,25 (dd, J = 9.2 and 0.7 Hz, 1H), 7,94 (dd, J = 9.2 and 0.7 Hz, 1H), 7.86 (t, J = 1.8 Hz 1H), 7.83 (t, J = 1.8 Hz 1H), 7.38 (qtd. J= 15.3, 8.0 and 1.5 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 7.25 (s, 1H), 2.37 (s, 3H). NMR 13C (CDCl3, 75 MHz) δ (ppm): 185.27, 146.15, 136.17, 135.30, 134.48, 130.33 (2C), 127.25 (2C), 126.35, 126.32, 125.07, 122.63, 122.44, 113.29, 21.68.
3.3. X-ray Data Collection and Processing
Data for a colourless block (0.20 × 0.25 × 0.30 mm) were collected at 100(2)K on an Agilent Super Nova-Dual diffractometer (Agilent Technologies Inc., Santa Clara, CA, USA) using Cu Kα radiation (mirror monochromator) and an Atlas detector using the ω scan technique to θ
max = 76.5°. No. of unique data = 5053, No. of parameters = 381,
R (4882 data with
I ≥ 2σ(
I)) = 0.034,
wR(all data) = 0.094. The structure was solved by direct methods [
17] and refined by full-matrix least-squares on
F2, with anisotropic displacement parameters for non-hydrogen atoms. The H atoms were geometrically placed (C—H = 0.95–0.98 Å) and refined as riding with
Uiso(H) = 1.2–1.5
Ueq(C). The weighting scheme used was
w = 1/[σ
2(
Fo2) + 0.065
P2 + 0.201
P] where
P = (
Fo2 + 2
Fc2)/3) with SHELXL-97 [
18] on
F2. The programs WinGX [
19] and ORTEP3 for Windows [
19], PLATON [
20], MarvinSketch 5.1.10 [
21] and DIAMOND [
22] were used for geometric calculations and to prepare crystallographic material for publication and depositing. Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre as CCDC 935801. Copies of this information may be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: 44 1223 336 033; e-mail:
deposit@ccdc.cam.ac.uk or www:
http://www.ccdc.cam.ac.uk).
3.4. Theoretical Calculations
All calculations were carried out using the PC GAMESS package with the B3LYP hybrid function, the STO-3G** basis set and wxMacMolPlt software for structure visualization [
23,
24,
25,
26,
27,
28,
29,
30]. The optimization algorithm was based on the Quadratic Approximation (QA) and the threshold gradient value used was 10
−5 a.u. [
31]. Frequency analyses were carried out to verify the nature of the minimum state of all the stationary points obtained. The Intrinsic Reaction Coordinate (IRC) calculations were done using the Gonzalez-Schlegel second-order method [
32] with the former threshold gradient value and a step size between points of the reaction path of 0.2 a.u. The NBO donor-acceptor pairs were checked and second-order stabilization energies were calculated for the interaction studies [
33,
34,
35,
36,
37,
38]. The crystallographic structures were used as starting point for calculations.
3.5. CSD Survey Methodology
The Cambridge Structural Database (CSD: 5.32 + 4 updates) [
10] was searched using CONQUEST (Version 1.14) [
14] for the structural skeleton shown in
Figure 10; structures featuring disorder or errors were excluded.
Figure 10.
Generic 1-(arylsulfonyl)indole structure employed in the CSD search.
Figure 10.
Generic 1-(arylsulfonyl)indole structure employed in the CSD search.