In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Antioxidant and Prooxidant Activity and Phenolic Compound Composition from Different Pomace Grape Varieties
| Extraction Method | Grape Variety | Fraction | Sample | Epa (V) a | Epa (V) b | Phenolic Composition |
|---|---|---|---|---|---|---|
| Methanol/HCl | Cabernet Sauvignon | Ethyl acetate | Ground | 0.4 | 0.75 | vanillic acid, syringic acid, gallic acid, kaempferol, quercetin, protocatechuic |
| Carménère | Whole | 0.4 | 0.8 | vanillic acid, syringic acid, gallic acid, elagic acid, quercetin, 4-hydroxi-3,5-dimethoxibenzaldehyde | ||
| Syrah | Ground | 0.35 | 0.7 | gallic acid, p-coumaric, elagic acid, quercetin, kaempferol | ||
| Whole | 0.4 | 0.8 | gallic acid, protocatechuic, vanillic acid, syringic acid, quercetin, kaempferol | |||
| Ethanol (System 1) | Cabernet Sauvignon | Hexane | Whole | - | 0.6 | - |
| Ground | 0.35 | - | gallic acid, vanillic acid, syringic acid, (−) epicatechin, quercetin, kaempferol | |||
| Chloroform | Whole | - | 0.7 | gallic acid, protocatechuic acid, vanillic acid, syringic acid, elagic acid, quercetin, kaempferol | ||
| Ethyl acetate | Ground | - | 0.6 | gallic acid, (−) epicatechin, quercetin | ||
| Carmènére | Chloroform | Ground | 0.3 | - | vanillic acid, syringic acid, quercetin, kaempferol | |
| Whole | 0.41 | - | vanillic acid, syringic acid, quercetin, kaempferol | |||
| Ethyl acetate | 0.4 | - | gallic acid, protocatechuic acid, quercetin, (−) epicatechin | |||
| Syrah | Chloroform | Ground | 0.38 | - | gallic acid, vanillic acid, syringic acid, quercetin, kaempferol | |
| Whole | 0.34 | - | vanillic acid, syringic acid, quercetin, kaempferol | |||
| Ethyl acetate | Ground | 0.3 | - | gallic acid, vanillic acid, syringic acid, quercetin, (−) Epicatechin | ||
| Whole | 0.35 | 0.8 | gallic, protocatechuic acid, vanillic acid, p-coumárico, Elagic acid | |||
| Ethanol (System 2) | Cabernet Sauvignon | Chloroform | Ground | - | 0.58 | syringic acid, quercetin,kaempferol, 4-hydroxiphenyl acetic acid |
| Whole | - | 0.57 | 4-hydroxiphenil acetic acid, syringic acid, quercetin, kaempferol | |||
| Ethyl acetate | Ground | 0.31 | - | gallic acid, quercetin, (−) Epicatechin | ||
| Carménère | Chloroform | Ground | 0.29 | - | gallic acid, Catequin, quercetin, (−) Epicatechin | |
| Whole | - | 0.55 | gallic acid, Catequin, quercetin, (−) Epicatechin | |||
| Ground | 0.34 | - | - | |||
| Syrah | Hexane | Whole | - | 0.6 | quercetin | |
| Chloroform | Ground | 0.32 | - | 4-hydroxiphenil acetic acid, syringic acid, quercetin, kaempferol | ||
| Ethyl acetate | Ground | 0.33 | - | gallic acid, vanillic acid, quercetin, (−) Epicatechin |
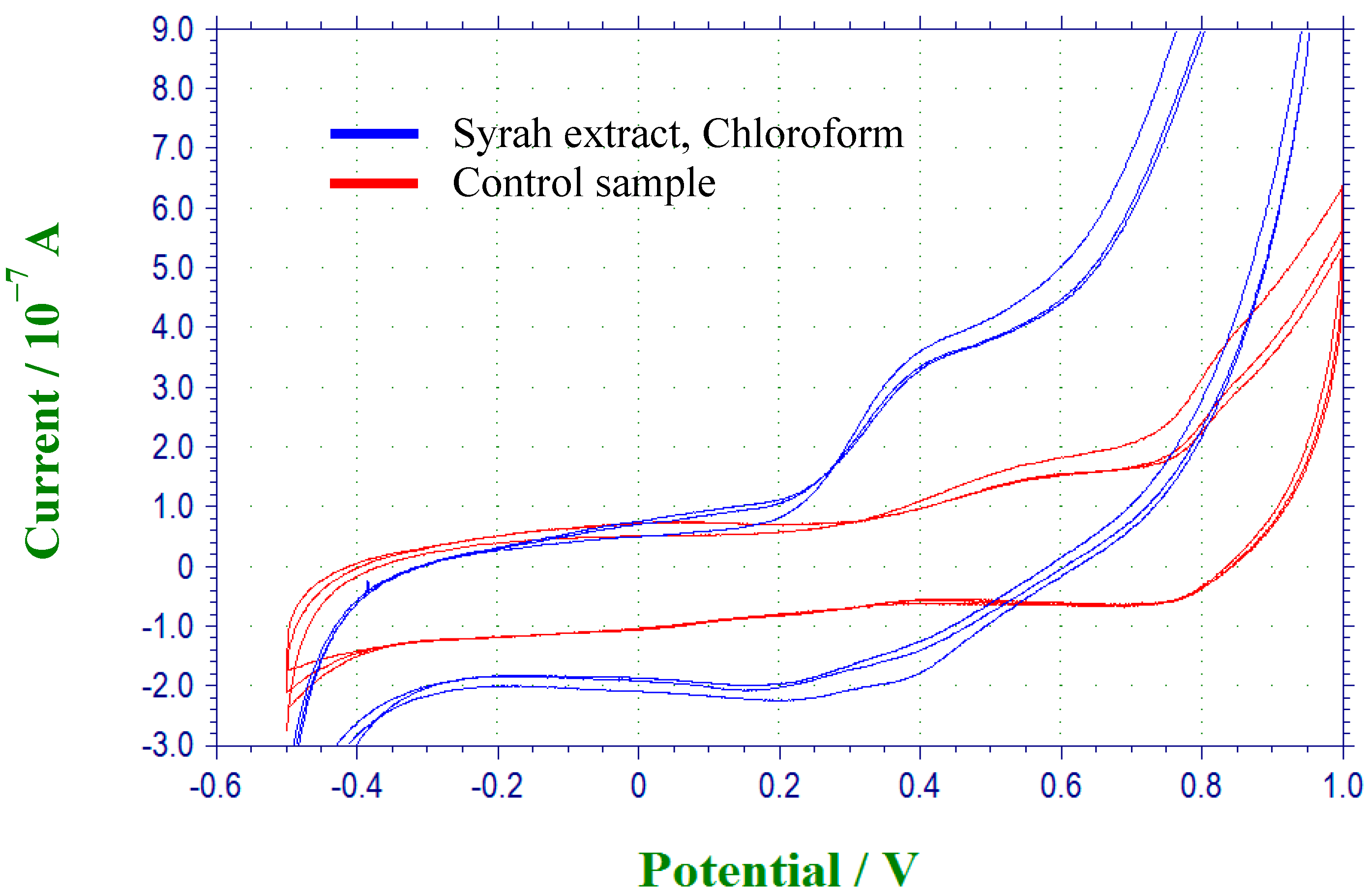
2.2. Evaluation of in Vivo Prooxidant Ability of the Extracts
| Extraction Solvent | Grape Variety | Fraction | Sample | Antioxidant Epa (V) a | Prooxidant Epa (V) b | ED50 ± SD (μg/mL) |
|---|---|---|---|---|---|---|
| Methanol/HCl | Carménère Syrah | Ethyl acetate | Whole | 0.4 0.4 | 0.8 0.8 | 52.04 ± 3.32 50.06 ± 3.34 |
| Ethanol 70%(System 1) | Cabernet Sauvignon Syrah | Chloroform | Ground Whole | 0.35 0.38 | - - | 49.34 ± 6.24 50.76 ± 2.58 |
| Syrah | Ethyl acetate | Whole | 0.35 | 0.8 | 44.59 ± 7.75 | |
| Ethanol 70%(System 2) | Cabernet Sauvignon | Ethyl acetate | Ground | 0.31 | - | 41.97 ± 5.23 |
| Carménère | Chloroform | Whole | - | 0.55 | 22.81 ± 3.28 | |
| Syrah | Ethyl acetate | Ground | 0.33 | - | 72.64 ± 7.27 |
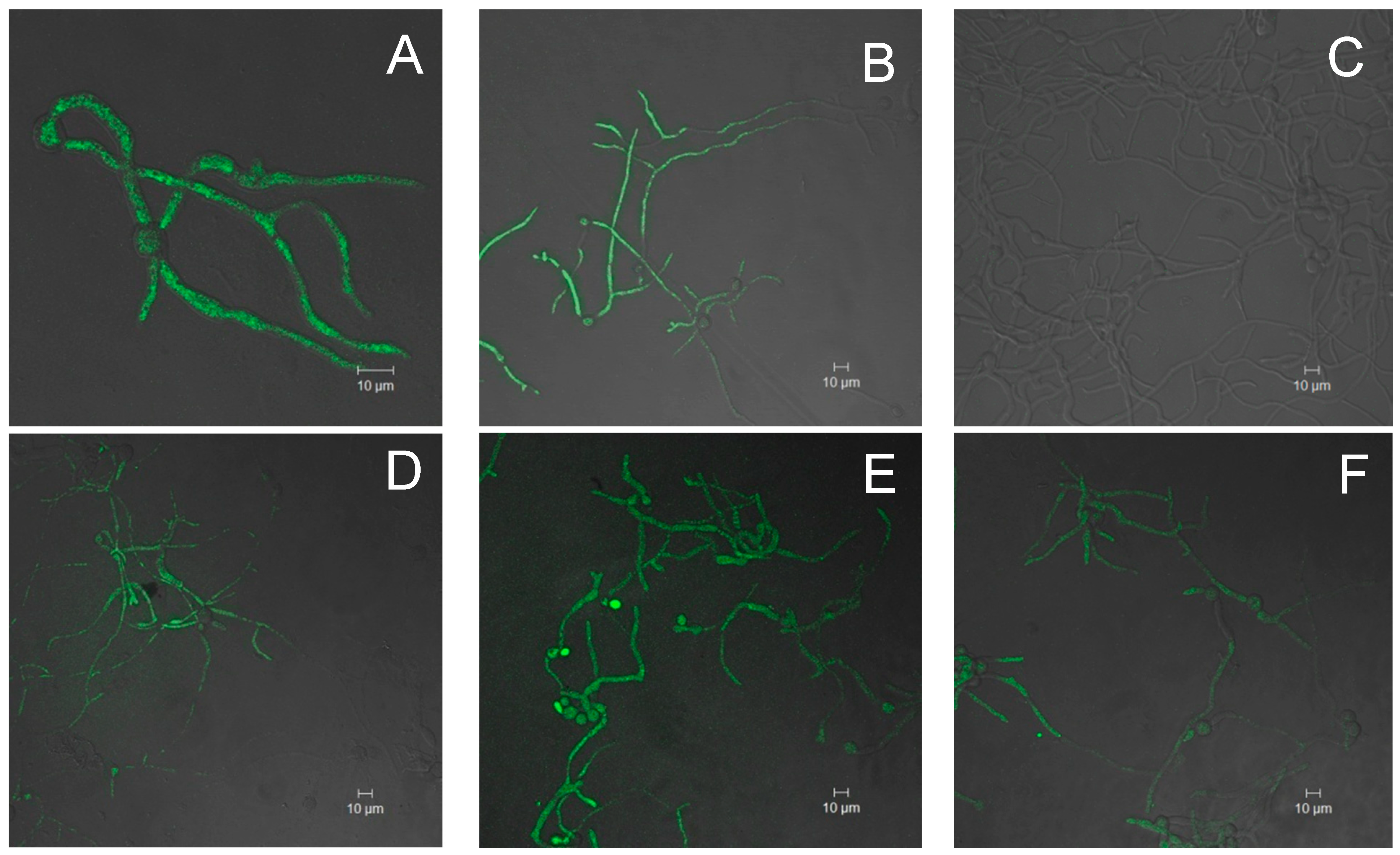
3. Experimental Section
3.1. Grape Pomaces
3.2. General Extraction Process
3.3. Determination of Total Phenol Content
3.4. Analysis of Phenolic Compounds in Different Extractions
3.5. Determination in Vitro of Antioxidant or Prooxidant Activity of Grape Pomace Extracts by Cyclic Voltammetry
3.6. Determination of in Vivo Prooxidant Activity of Grape Pomace Extracts
3.6.1. Fungal Isolate and Culture Conditions
3.6.2. Determination in Vivo Prooxidant Activity Using B. cinerea as Model Organism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ruggieri, L.; Cadena, E.; Martinez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrel, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of organic wastesin the Spanish wine industry: Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef]
- De Campos, L.M.; Leimann, F.; Pedrosa, R.; Ferreira, S. Free radical scavenging of grape pomace extracts from Cabernet Sauvingnon (Vitis vinifera). Bioresour. Technol 2008, 99, 8413–8420. [Google Scholar]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Struntze, K.; Poulsen, L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [PubMed]
- Cole, G.M.; Lim, G.P.; Yang, F.; Teter, B.; Begum, A.; Ma, Q.; Harris-White, M.E.; Frautschy, S.A. Prevention of Alzheimer’s disease: Omega-3 fatty and phenolic anti-oxidant interventions. Neurobiol. Aging 2006, 26 (Suppl. S1), 133–136. [Google Scholar]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary flavonoids and cancer risk in the Zutphen Elderly Study. Nutr. Cancer 1994, 22, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crop Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Jarvis, WR. Epidemiology in the Biology of Botrytis; Coley-Smith, J.R., Verhoeff, K., Jarvis, W.R., Eds.; Academic Press: London, UK, 1980; pp. 219–250. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mnoharlal, R.; Puri, N.; Prasad, R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 2010, 30, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Lupetti, A.; Danesi, R.; Del, C.M.M.; Kelly, S. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 2002, 8, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Latifa, B.; Bouchra, L.; Sanaa, A.; Najat, C.; Fouzia, C.; Adnane, R. Comparative study of the antifungal activity of some essential oils and their major phenolic components against Aspergillus niger using three different methods. Afr. J. Biotechnol. 2012, 11, 14083–14087. [Google Scholar]
- Cotoras, M.; Mendoza, L.; Muñoz, A.; Yañez, K.; Castro, P.; Aguirre, M. Fungitoxicity against Botrytis cinerea of a flavonoid isolated from Pseudognaphalium robustum. Molecules 2011, 16, 3885–3895. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Foods Health Dis. 2011, 7, 232–244. [Google Scholar]
- Andersen, M.L.; Lauridsen, R.K.; Skibsted, L.H. Optimizing the use of phenolic compounds in foods. In Phytochemical Functional Foods; Johnson, I., Williamson, G., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2003; pp. 315–346. [Google Scholar]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Dominguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Schwartz, K.; Bertelsen, G.; Nissen, L.R.; Gardner, P.T.; Heinonen, M.I.; Hopia, A.; Huynh-Ba, T.; Lambelet, P.; McPhail, D.; Skibsted, L.H.; et al. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 212, 319–328. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Samra, M.A.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundaram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Middleton, J.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation. Heart Dis. Cancer Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Lambert, J.; Elias, R. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gunckel, S.; Santander, P.; Cordano, G.; Ferreira, J.; Munoz, S.; Nunez-Vergara, L.; Squella, J.A. Antioxidant activity of gallates: An electrochemical study in aqueous media. Chem. Biol. Interact. 1998, 114, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Castro, P.; Vivanco, H.; Melo, R.; Mendoza, L. Farnesol induces apoptosis-like phenotype in the phytopathogenic fungus Botrytis cinerea. Mycologia 2013, 105, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Jetten, A.M. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2009, 287, 123–135. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Prenzler, P.; Autolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Mendoza, L.; Cotoras, M.; Vivanco, M.; Matsuhiro, B.; Torres, S.; Aguirre, M. Evaluation of antifungal properties against the phytopathogeic fungus Botrytis cinerea of anthocyanin rich-extracts obtained from grape pomaces. J. Chil. Chem. Soc. 2013, 58, 1725–1727. [Google Scholar] [CrossRef]
- Munoz, G.; Hinrichsen, P.; Brygoo, Y.; Giraud, T. Genetic characterization of Botrytis cinerea populations in Chile. Mycol. Res. 2002, 106, 594–601. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules 2014, 19, 21154-21167. https://doi.org/10.3390/molecules191221154
Cotoras M, Vivanco H, Melo R, Aguirre M, Silva E, Mendoza L. In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules. 2014; 19(12):21154-21167. https://doi.org/10.3390/molecules191221154
Chicago/Turabian StyleCotoras, Milena, Herman Vivanco, Ricardo Melo, María Aguirre, Evelyn Silva, and Leonora Mendoza. 2014. "In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace" Molecules 19, no. 12: 21154-21167. https://doi.org/10.3390/molecules191221154
APA StyleCotoras, M., Vivanco, H., Melo, R., Aguirre, M., Silva, E., & Mendoza, L. (2014). In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules, 19(12), 21154-21167. https://doi.org/10.3390/molecules191221154