Relevance of the Anti-Inflammatory Properties of Curcumin in Neurodegenerative Diseases and Depression
Abstract
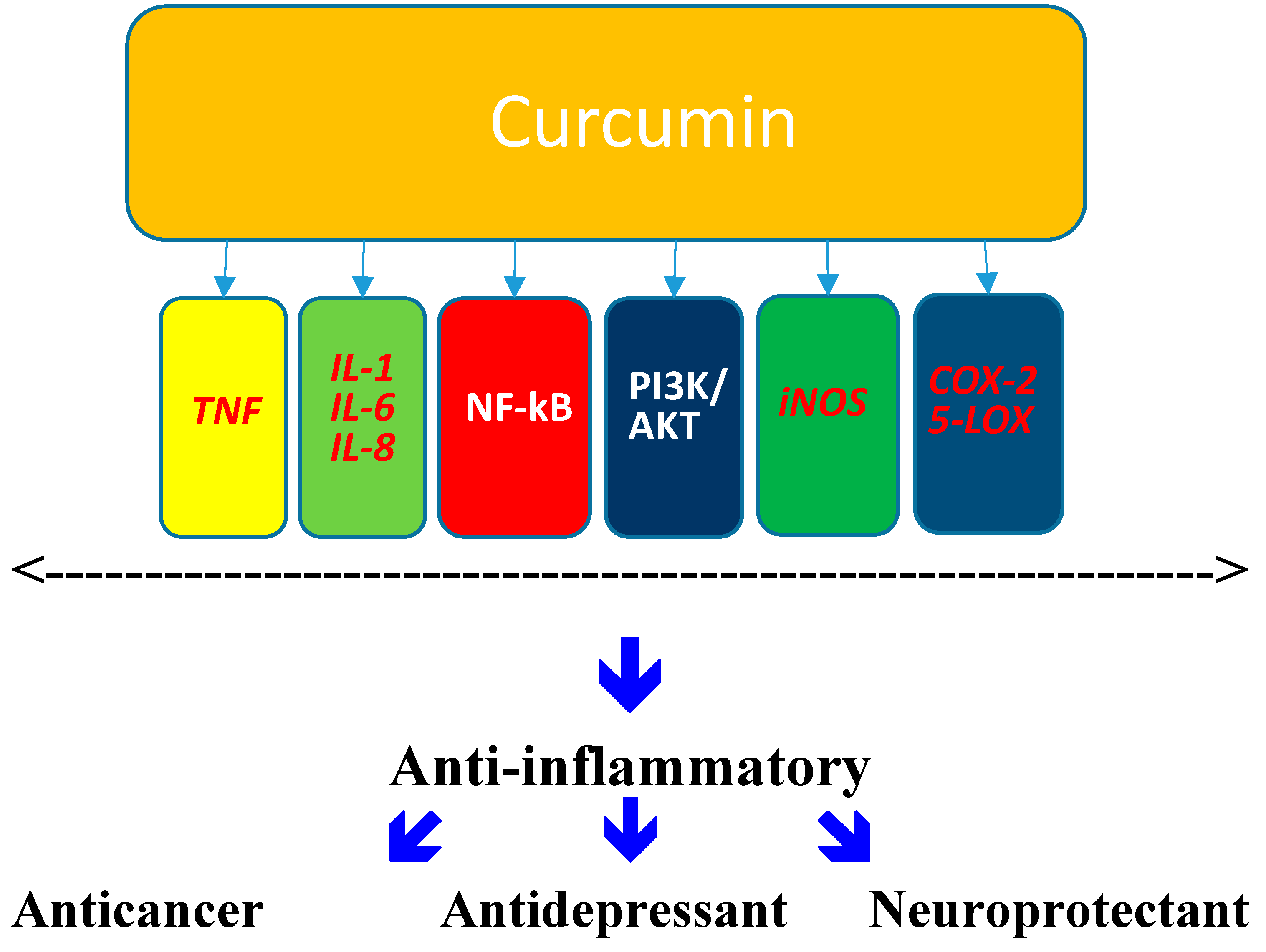
:1. Curcumin
2. Depression
2.1. General Considerations
2.2. Depression-Inflammation
3. Cytokines
4. Parkinson’s Disease
4.1. General Considerations
4.2. PD and Inflammation
5. Role of Stress
6. Curcumin: Antidepressant
7. Curcumin: Neuroprotectant
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manolova, Y.; Deneva, V.; Antonov, L. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta A 2014, 132, 815–820. [Google Scholar] [CrossRef]
- Sharma, O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef] [PubMed]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y.; Suzuki, Y.; Watanabe, N.; Oshima, Y.; Hikino, H. Antihepatotoxic principles of Curcuma longa Rhizomes1. Planta Med. 1983, 49, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Punithavathi, D.; Arumugam, V. Curcumin prevents adriamycin nephrotoxicity in rats. Br. J. Pharmacol. 2000, 129, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, P. Hepatoprotective effect of curcumin onlindane-induced oxidative stress in male Wistar rats. Toxicol. Int. 2011, 18, 124–129. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Tripanichkul, W.; Jaroensuppaperch, E.O. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur. Rev. Med. Pharmacol. Sci. 2013, 10, 1360–1368. [Google Scholar]
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H.; Li, X.J. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol. Biochem. Behav. 2005, 82, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Wang, F.M.; Pan, Y.; Qiang, L.Q.; Cheng, G.; Zhang, W.Y.; Kong, L.D. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Kuhad, A.; Tiwari, V.; Chopra, K. Curcumin ameliorates reserpine-induced pain depression dyad: Behavioural, neurochemical and molecular evidences. Psychoneuroendocrinology 2011, 36, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.A.; Tizabi, Y. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Pandav, R.; Dodge, H.H.; Johnston, J.M.; Belle, S.H.; DeKosky, S.T.; Ganguli, M. Incidence of Alzheimer’s disease in a rural community in India: The Indo-US study. Neurology 2001, 57, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Vas, C.J.; Pinto, C.; Panikker, D.; Noronha, S.; Deshpande, N.; Kulkarni, L.; Sachdeva, S. Prevalence of dementia in an urban Indian population. Int. Psychogeriatr. 2001, 13, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Muthane, U.; Yasha, T.C.; Shankar, S.K. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann. Neurol. 1998, 43, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Alladi, P.A.; Mahadevan, A.; Yasha, T.C.; Raju, T.R.; Shankar, S.K.; Muthane, U. Absence of age-related changes in nigral dopaminergic neurons of Asian Indians: Relevance to lower incidence of Parkinson’s disease. Neuroscience 2009, 159, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, A.S.; Carroll, R.T.; Bishayee, A.; Novotny, N.A.; Geldenhuys, W.J.; Van der Schyf, C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drugs 2012, 21, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, H.; Harter, M. Prevalence of mental disorders based on general population surveys. Soc. Psychiatry Psychiatr. Epidemiol. 2007, 42, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Thase, M.E.; Dube, S. Research issues in the study of difficult-to-treat depression. Biol. Psychiatry 2003, 53, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.j.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–191. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Malberg, J.; Nakagawa, S.; D’Sa, C. Neuronal plasticity and survival in mood disorders. Biol. Psychiatry 2000, 48, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Manji, H.K.; Duman, R.S. Impairments of neuroplasticity and cellular resilience in severe mood disorders: Implications for the development of novel therapeutics. Psychopharmacol. Bull. 2001, 35, 5–49. [Google Scholar] [PubMed]
- Lee, A.L.; Ogle, W.O.; Sapolsky, R.M. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord. 2002, 4, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.J.; Glabus, M.F.; Goodwin, G.M.; Ebmeier, K.P. Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br. J. Psychiatry 2002, 180, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Banasr, M.; Chowdhury, G.M.; Terwilliger, R.; Newton, S.S.; Duman, R.S.; Behar, K.L. Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 2010, 15, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Banasr, M.; Dwyer, J.M.; Duman, R.S. Cell atrophy and loss in depression: Reversal by antidepressant treatment. Curr. Opin. Cell Biol. 2011, 23, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Guan, Z.; Yang, X.; Fang, J. Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res. 2003, 991, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, L.R.; Marcinowska, A.; Obuchowicz, E. Antiapoptotic and neurotrophic effects of antidepressants: A review of clinical and experimental studies. Brain Res. Bull. 2009, 79, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, S.P.; Lucki, A.; Klein, E.; Ben-Shachar, D. Dexamethasone enhances the norepinephrine-induced ERK/MAPK intracellular pathway possibly via dysregulation of the α2-adrenergic receptor: Implications for antidepressant drug mechanism of action. Eur. J. Cell Biol. 2010, 89, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Dhabar, F.S.; Su, Y.; Blackburn, E.H. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress—Preliminary findings. PLoS One 2011, 6, e17837. [Google Scholar] [CrossRef] [PubMed]
- Yasui-Furukori, N.; Tsuchimine, S.; Nakagami, T.; Fujii, A.; Sato, Y.; Tomita, T.; Yoshizawa, K.; Inoue, Y.; Kaneko, S. Association between plasma paroxetine concentration and changes in plasma brain-derived neurotrophic factor levels in patients with major depressive disorder. Hum. Psychopharmacol. 2011, 26, 194–200. [Google Scholar] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Q.; Fang, L.; Cheng, K.; Zhang, R.; Zheng, P.; Zhan, Q.; Qi, Z.; Zhong, S.; Xie, P. Reduced neurogenesis and pre-synaptic dysfunction in the olfactory bulb of a rat model of depression. Neuroscience 2011, 192, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [PubMed]
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 1999, 57, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Jander, S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog. Neurobiol. 1999, 58, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Poulter, M.O.; Merali, Z.; Anisman, H. The pathogenesis of clinical depression: Stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 2005, 135, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Khairova, R.A.; Machado-Vieira, R.; Du, J.; Manji, H.K. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2009, 12, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Inflammation, depression and dementia: Are they connected? Neurochem. Res. 2007, 32, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E.; Myint, A. The psychoneuroimmunology of depression. Hum. Psychopharmacol. 2009, 24, 165–175. [Google Scholar] [PubMed]
- O’Sullivan, J.B.; Ryan, K.M.; Curtin, N.M.; Harkin, A.; Connor, T.J. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: Implications for depression and neurodegeneration. Int. J. Neuropsychopharmacol. 2009, 12, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Wirleitner, B.; Neurauter, G.; Schrocksnadel, K.; Frick, B.; Fuchs, D. Interferon-γ-induced conversion of tryptophan: Immunologic and neuropsychiatric aspects. Curr. Med. Chem. 2003, 10, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Role of pro- and anti-inflammatory cytokines during inflammation: Experimental and clinical findings. J. Biol. Regul. Homeost. Agents 1997, 11, 91–103. [Google Scholar] [PubMed]
- Dinarello, C.A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int. Rev. Immunol. 1998, 16, 457–499. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Irwin, M.R. Depression and immunity: Inflammation and depressive symptoms in multiple sclerosis. Immunol. Allergy Clin. North Am. 2009, 29, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Inada, K.; Takakuwa, T.; Yamada, Y.; Inoue, Y.; Shimamura, T.; Taniguchi, S.; Sato, S.; Wakabayashi, G.; Endo, S. Anti-inflammatory cytokine levels in patients with septic shock. Res. Commun. Mol. Pathol. Pharmacol. 1997, 98, 34–42. [Google Scholar] [PubMed]
- Munoz, C.; Carlet, J.; Fitting, C.; Misset, B.; Bleriot, J.P.; Cavaillon, J.M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Investig. 1991, 88, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- Barton, BE. IL-6: Insights into novel biological activities. Clin. Immunol. Immunopathol. 1997, 85, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.; Wu, Q.; Lu, Y.; Nie, J.; Xie, X.; Shi, J. Resveratrol Protects Cortical Neurons against Microglia-mediated Neuroinflammation. Phytother. Res. 2013, 27, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E.; Shortall, J.; Jackson, J.V. Interleukins 6 and 11 protect mice from mortality in a staphylococcal enterotoxin-induced toxic shock model. Infect. Immun. 1996, 64, 714–718. [Google Scholar] [PubMed]
- Hurst, S.M.; Wilkinson, T.S.; McLoughlin, R.M.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.M.; Topley, N.; Jones, S.A. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Takahashi, N.; Cauwels, A.; Brouckaert, P.; Bluethmann, H.; Fiers, W. Response of interleukin-6-deficient mice to tumor necrosis factor-induced metabolic changes and lethality. Eur. J. Immunol. 1994, 24, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Lanctot, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural. Transm. Suppl. 2000, 60, 277–290. [Google Scholar] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 2008, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Duman, R.S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.C.; Ross, F.M.; Heenan, L.E.; Davies, R.E.; Rothwell, N.J.; Allan, S.M. Neurodegenerative actions of interleukin-1 in the rat brain are mediated through increases in seizure activity. J. Neurosci. Res. 2006, 83, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Viviani, B.; Bartesaghi, S.; Corsini, E.; Galli, C.L.; Marinovich, M. Cytokines role in neurodegenerative events. Toxicol. Lett. 2004, 149, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.Y.; Crews, F.T. TNF α potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NF kappa B inhibition. Brain Res. 2005, 1034, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1. [Google Scholar] [CrossRef]
- Mravec, B. Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: A review of recent developments. Physiol. Res. 2006, 55, 353–364. [Google Scholar] [PubMed]
- Dostert, P.; Strolin Benedetti, M.; Dordain, G. Dopamine-derived alkaloids in alcoholism and in Parkinson’s and Huntington’s diseases. J. Neural Transm. 1988, 74, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T. Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci. Res. 1997, 29, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Matsubara, K.; Hashizume, Y. A neutral N-methyltransferase activity in the striatum determines the level of an endogenous MPP+-like neurotoxin, 1,2-dimethyl-6,7-dihydroxyisoquinolinium ion, in the substantia nigra of human brains. Neurosci. Lett. 1997, 235, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Antkiewicz-Michaluk, L. Endogenous risk factors in Parkinson’s disease: Dopamine and tetrahydroisoquinolines. Pol. J. Pharmacol. 2002, 54, 567–572. [Google Scholar] [PubMed]
- Maruyama, W.; Dostert, P.; Matsubara, K.; Naoi, M. N-methyl(R)salsolinol produces hydroxyl radicals: Involvement to neurotoxicity. Free Radic. Biol. Med. 1995, 19, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.; Siebecker, F.; Vieregge, P.; Jaskowski, P.; Kompf, D. Salsolinol, catecholamine metabolites, and visual hallucinations in L-dopa treated patients with Parkinson’s disease. J. Neural Transm. 1996, 103, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Stone, J.M.; Heron, C.; Cooper, J.M.; Schapira, A.H. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: Relevance to Parkinson’s disease. J. Neurochem. 1994, 63, 1987–1990. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Hong, J.S.; Zhang, W.; Liu, B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J. Neurosci. 2002, 22, 782–790. [Google Scholar] [PubMed]
- Freestone, P.S.; Chung, K.K.; Guatteo, E.; Mercuri, N.B.; Nicholson, L.F.; Lipski, J. Acute action of rotenone on nigral dopaminergic neurons—involvement of reactive oxygen species and disruption of Ca2+ homeostasis. Eur. J. Neurosci. 2009, 30, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Sherer, T.B.; Zhang, N.; Taylor, G.; Na, H.M.; Greenamyre, J.T.; Casida, J.E. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem. Res. Toxicol. 2004, 17, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Hong, J.S.; Zhang, W.; Liu, B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: Relevance to the etiology of Parkinson’s disease. J. Neurosci. 2003, 23, 1228–1236. [Google Scholar] [PubMed]
- Qualls, Z.; Brown, D.; Ramlochansingh, C.; Hurley, L.L.; Tizabi, Y. Protective effects of curcumin against rotenone and salsolinol-induced toxicity: Implications for Parkinson’s disease. Neurotox. Res. 2014, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Hong, J.S. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kannarkat, G.T.; Boss, J.M.; Tansey, M.G. The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinson’s Dis. 2013, 4, 493–514. [Google Scholar]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 and neuroinflammation: Partners in crime in Parkinson’s disease? J. Neuroinflam. 2014, 21, 11–52. [Google Scholar]
- Kent, S.; Bluthe, R.M.; Kelley, K.W.; Dantzer, R. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 1992, 13, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Lue, L.F.; Adler, C.H.; Shill, H.A.; Caviness, J.N.; Sabbagh, M.N.; Akiyama, H.; Serrano, G.E.; Sue, L.I.; Beach, T.G. Changes in properties of serine 129 phosphorylated α-synuclein with progression of Lewy-type histopathology in human brains. Exp. Neurol. 2013, 240, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bosmans, E.; Meltzer, H.Y.; Scharpe, S.; Suy, E. Interleukin-1β: A putative mediator of HPA axis hyperactivity in major depression? Am. J. Psychiatry 1993, 150, 1189–1193. [Google Scholar] [PubMed]
- Yirmiya, R.; Barak, O.; Avitsur, R.; Gallily, R.; Weidenfeld, J. Intracerebral administration of Mycoplasma fermentans produces sickness behavior: Role of prostaglandins. Brain Res. 1997, 749, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Miller, A.H. Cytokines and psychopathology: Lessons from interferon-α. Biol. Psychiatry 2004, 56, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Pollak, Y.; Yirmiya, R. Cytokine-induced changes in mood and behaviour: Implications for “depression due to a general medical condition”, immunotherapy and antidepressive treatment. Int. J. Neuropsychopharmacol. 2002, 5, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Song, C.; Lin, A.; de Jongh, R.; van Gastel, A.; Kenis, G.; Bosmans, E.; de Meester, I.; Benoy, I.; Neels, H.; et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 1998, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Lichter, I.; Beilin, B.; Ofek, K.; Bessler, H.; Gruberger, M.; Shavit, Y.; Seror, D.; Grinevich, G.; Posner, E.; Reichenberg, A.; et al. Cytokines and cholinergic signals co-modulate surgical stress-induced changes in mood and memory. Brain Behav. Immun. 2008, 22, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Hamer, M.; Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Kitamura, H.; Kimura, K.; Saito, M. Brain interleukin-1 is involved in blood interleukin-6 response to immobilization stress in rats. Jpn. J. Vet. Res. 2001, 49, 19–25. [Google Scholar] [PubMed]
- Nguyen, K.T.; Deak, T.; Owens, S.M.; Kohno, T.; Fleshner, M.; Watkins, L.R.; Maier, S.F. Exposure to acute stress induces brain interleukin-1β protein in the rat. J. Neurosci. 1998, 18, 2239–2246. [Google Scholar] [PubMed]
- Kaster, M.P.; Gadotti, V.M.; Calixto, J.B.; Santos, A.R.; Rodrigues, A.L. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology 2012, 62, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Hepgul, N.; Pariante, C.M. Inflammation and Depression. Curr. Top. Behav. Neurosci. 2013, 14, 135–151. [Google Scholar] [PubMed]
- Wuwongse, S.; Chang, R.C.; Law, A.C. The putative neurodegenerative links between depression and Alzheimer’s disease. Prog. Neurobiol. 2010, 91, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, A.M.; Herman, J.P.; Seroogy, K.B. Stress, depression and Parkinson’s disease. Exp. Neurol. 2012, 233, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Anthwal, A.; Thakur, B.K.; Rawat, M.S.; Rawat, D.S.; Tyagi, A.K.; Aggarwal, B.B. Synthesis, characterization and in vitro anticancer activity of C-5 curcumin analogues with potential to inhibit TNF-α-induced NF-κB activation. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Li, X. Curcumin, an active constituent of the ancient medicinal herb Curcuma longa L.: Some uses and the establishment and biological basis of medical efficacy. CNS Neurol. Disord. Drug Targets 2013, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Wu, H.L.; Li, Y.B.; Li, Y.H.; Guo, J.B. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H. The effects of curcumin on depressive-like behaviors in mice. Eur. J. Pharmacol. 2005, 518, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; Panagaki, T.; Koelink, P.J.; Morgan, M.E.; Hendriksen, H.; Garssen, J.; Kraneveld, A.D.; Olivier, B.; Oosting, R.S. Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression. Neuropharmacology 2014, 79, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res. 2014, 28, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Sahebkar, A. Investigation of the Efficacy of Adjunctive Therapy with Bioavailability-Boosted Curcuminoids in Major Depressive Disorder. Phytother. Res. 2014. [Google Scholar] [CrossRef]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.R.; Campbell, I.L.; Gyengesi, E.; Münch, G. Chronic neuroinflammation in Alzheimer’s disease: New perspectives on animal models and promising candidate drugs. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Rajbhandari, L.; Shrestha, S.; Mithal, A.; Hosmane, S.; Venkatesan, A. Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp. Neurol. 2014, 253, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H.; Li, J.; Zhang, Y.; Han, B.; Zeng, Z.; Qiao, N.; Cui, X.; Lou, J.; Li, J. Amelioration of β-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associated with suppression of CRMP-2 hyperphosphorylation. Neurosci. Lett. 2013, 557, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Settembre, R. Safety and efficacy of an add-on therapy with curcumin phytosome and piperine and/or lipoic acid in subjects with a diagnosis of peripheral neuropathy treated with dexibuprofen. J. Pain Res. 2013, 6, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromolecular Med. 2014, 16, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, N.; Dwivedi, S.; Tota, S.K.; Kamat, P.K.; Hanif, K.; Nath, C.; Shukla, R. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur. J. Pharmacol. 2013, 715, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Singula, N.; Chawan, D.K. N-methyl N-nitrosourea induced functional and structural alterations in mice brain-role of curcumin. Neurotox. Res. 2012, 22, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamada, M. Review: Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Su, X.; Liu, A.; Zhang, L.; Yu, A.; Xi, Y.; Zhai, G. Advances in clinical study of curcumin. Curr. Pharm. Des. 2013, 19, 1966–1973. [Google Scholar] [PubMed]
- Brondino, N.; Re, S.; Boldrini, A.; Cuccomarino, A.; Lanati, N.; Barale, F.; Politi, P. Curcumin as a therapeutic agent in dementia: A mini systematic review of human studies. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Mythri, R.; Bharath, M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Seidl, S.E.; Potashkin, J.A. The promise of neuroprotective agents in Parkinson’s disease. Front. Neurol. 2011, 2. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 6, 807–818. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, neurodegeneration, and depression. Neurotox. Res. 2013, 23, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Akinfiresoye, L.; Tizabi, Y. Antidepressant effects of AMPA and ketamine combination: Role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology 2013, 230, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Haque, A.; Banik, N.L.; Nagarkatti, P.; Nagarkatti, M.; Ray, S.K. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res. Bull. 2014, 109, 22–31. [Google Scholar] [CrossRef]
- Banji, O.J.; Banji, D.; Ch, K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem. Toxicol. 2014, 74, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: Treatment implications. Curr. Pharm. Des. 2014, 20, 3812–3847. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Veleri, S.; Frautschy, S. Molecular Chaperone Dysfunction in Neurodegenerative Diseases and Effects of Curcumin. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Meesarapee, B.; Thampithak, A.; Jaisin, Y.; Sanvarinda, P.; Suksamrarn, A.; Tuchinda, P.; Morales, N.P.; Sanvarinda, Y. Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother. Res. 2014, 28, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Chen, G.; Zhang, W.; Hu, X.; Liu, Y.; Zhou, Q.; Zhu, L.X.; Zhao, Y.F. Curcumin dually inhibits both mammalian target of rapamycin and nuclear factor-κB pathways through a crossed phosphatidylinositol 3-kinase/Akt/IκB kinase complex signaling axis in adenoid cystic carcinoma. Mol. Pharmacol. 2011, 79, 106–118. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tizabi, Y.; Hurley, L.L.; Qualls, Z.; Akinfiresoye, L. Relevance of the Anti-Inflammatory Properties of Curcumin in Neurodegenerative Diseases and Depression. Molecules 2014, 19, 20864-20879. https://doi.org/10.3390/molecules191220864
Tizabi Y, Hurley LL, Qualls Z, Akinfiresoye L. Relevance of the Anti-Inflammatory Properties of Curcumin in Neurodegenerative Diseases and Depression. Molecules. 2014; 19(12):20864-20879. https://doi.org/10.3390/molecules191220864
Chicago/Turabian StyleTizabi, Yousef, Laura L. Hurley, Zakiya Qualls, and Luli Akinfiresoye. 2014. "Relevance of the Anti-Inflammatory Properties of Curcumin in Neurodegenerative Diseases and Depression" Molecules 19, no. 12: 20864-20879. https://doi.org/10.3390/molecules191220864
APA StyleTizabi, Y., Hurley, L. L., Qualls, Z., & Akinfiresoye, L. (2014). Relevance of the Anti-Inflammatory Properties of Curcumin in Neurodegenerative Diseases and Depression. Molecules, 19(12), 20864-20879. https://doi.org/10.3390/molecules191220864



