Biflavans, Flavonoids, and a Dihydrochalcone from the Stem Wood of Muntingia calabura and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses
Abstract
:1. Introduction
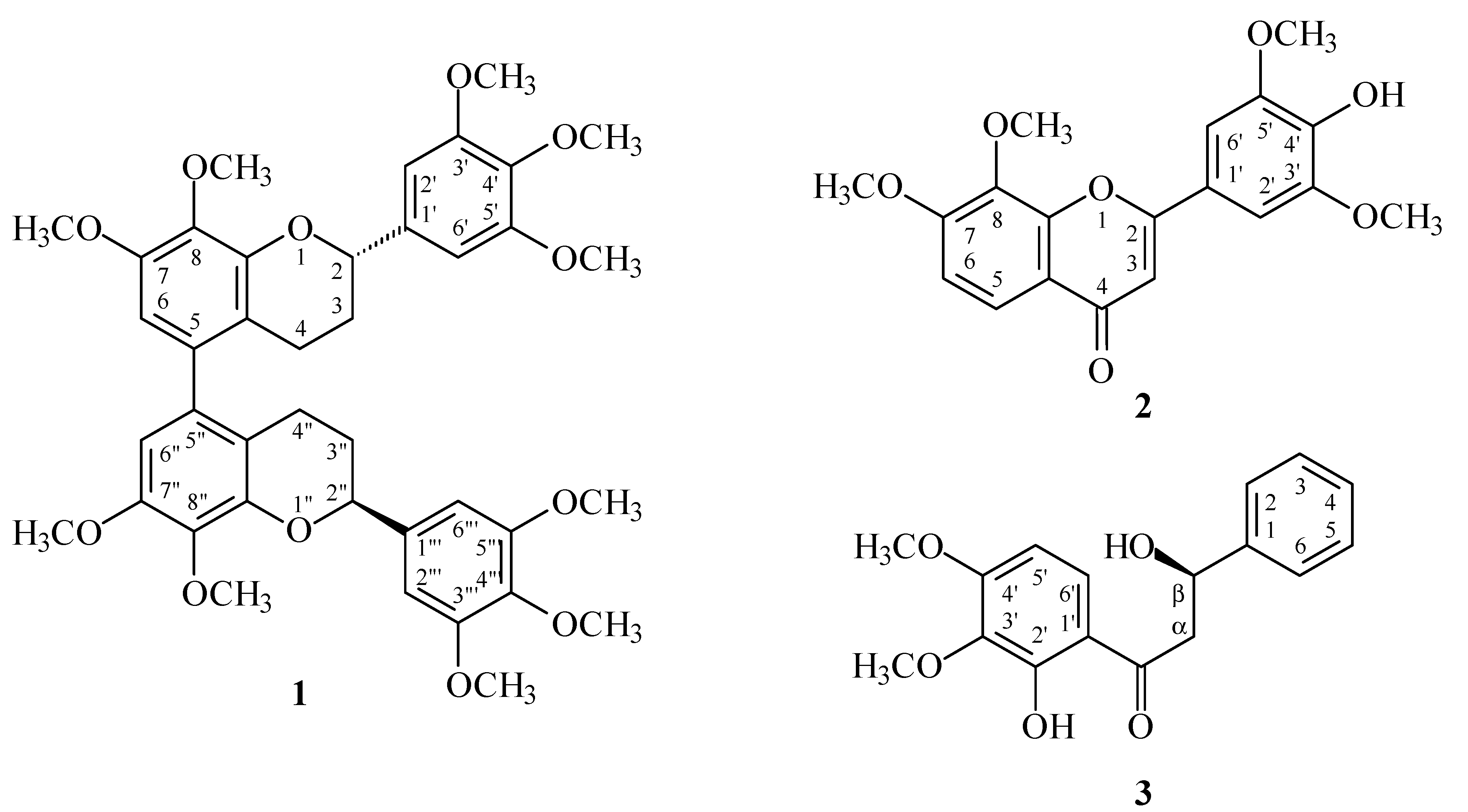
2. Results and Discussion
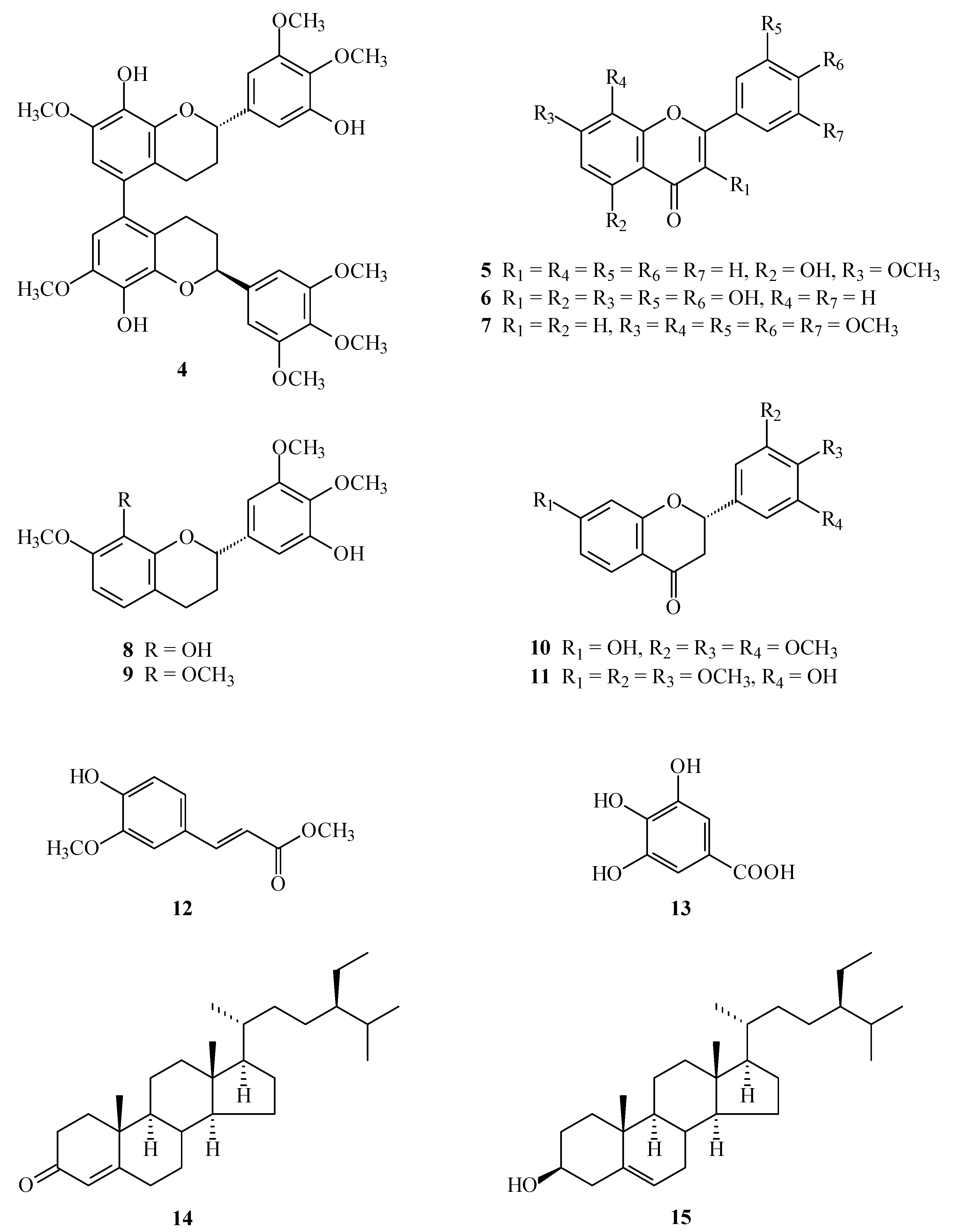
2.1. Results
| Position | δH | |||
|---|---|---|---|---|
| 1a | 4 b | |||
| M | P | M | P | |
| 2 | 5.02 dd (9.6, 3.0) | 5.03 dd (9.6, 3.0) | 4.90 d (9.4) | 4.90 d (9.4) |
| 3ax | 1.90–2.08 m | 1.90–2.08 m | 1.70–2.13 m | 1.70–2.13 m |
| 3eq | 2.09–2.18 m | 2.09–2.18 m | 1.70–2.13 m | 1.70–2.13 m |
| 4ax | 2.66 ddd (16.4, 11.0, 6.0) | 2.54 ddd (16.4, 11.0, 6.0) | 2.25–2.72 m | 2.25–2.72 m |
| 4eq | 2.23 ddd (16.4, 4.4, 4.4) | 2.50 ddd (16.4, 4.4, 4.4) | 2.25–2.72 m | 2.25–2.72 m |
| 6 | 6.42 s | 6.37 s | 6.38 s | 6.30 s |
| 2' | 6.64 s | 6.66 s | 6.59 d (2.1) | 6.59 d (2.1) |
| 6' | 6.64 s | 6.66 s | 6.61 s | 6.61 s |
| 2'' | 5.02 dd (9.6, 3.0) | 5.03 dd (9.6, 3.0) | 4.97 d (9.9) | 4.97 d (9.9) |
| 3''ax | 1.90–2.08 m | 1.90–2.08 m | 1.70–2.13 m | 1.70–2.13 m |
| 3''eq | 2.09–2.18 m | 2.09–2.18 m | 1.70–2.13 m | 1.70–2.13 m |
| 4''ax | 2.66 ddd (16.4, 11.0, 6.0) | 2.54 ddd (16.4, 11.0, 6.0) | 2.25–2.72 m | 2.25–2.72 m |
| 4''eq | 2.23 ddd (16.4, 4.4, 4.4) | 2.50 ddd (16.4, 4.4, 4.4) | 2.25–2.72 m | 2.25–2.72 m |
| 6'' | 6.42 s | 6.37 s | 6.38 s | 6.32 s |
| 2''' | 6.64 s | 6.66 s | 6.79 s | 6.82 s |
| 6''' | 6.64 s | 6.66 s | 6.79 s | 6.82 s |
| OMe-7 | 3.86 s | 3.87 s | 3.75 s | 3.74 s |
| OH-8 | 8.13 s | 8.13 s | ||
| OMe-8 | 3.90 s | 3.89 s | ||
| OMe-3' | 3.87 s | 3.88 s | 3.77 s | 3.79 s |
| OMe-4' | 3.85 s | 3.86 s | 3.66 s | 3.68 s |
| OH-5' | 9.17 s | 9.20 s | ||
| OMe-5' | 3.87 s | 3.88 s | ||
| OMe-7'' | 3.86 s | 3.87 s | 3.75 s | 3.74 s |
| OH-8'' | 8.13 s | 8.13 s | ||
| OMe-8'' | 3.90 s | 3.89 s | ||
| OMe-3''' | 3.87 s | 3.88 s | 3.77 s | 3.79 s |
| OMe-4''' | 3.85 s | 3.86 s | 3.66 s | 3.68 s |
| OMe-5''' | 3.87 s | 3.88 s | 3.81 s | 3.79 s |
| Position | δC | |||
|---|---|---|---|---|
| 1 a | 4 b | |||
| M | P | M | P | |
| 2 | 78.5 | 78.6 | 76.8 | 76.9 |
| 3 | 30.4 | 30.1 | 29.7 | 29.5 |
| 4 | 23.6 | 23.0 | 23.3 | 22.7 |
| 5 | 130.8 | 130.4 | 130.0 | 130.5 |
| 6 | 109.6 | 109.2 | 106.2 | 105.7 |
| 7 | 146.5 | 146.5 | 145.9 | 145.9 |
| 8 | 141.0 | 141.0 | 133.7 | 133.7 |
| 9 | 147.6 | 147.4 | 143.7 | 143.6 |
| 10 | 113.8 | 113.2 | 113.7 | 114.2 |
| 1' | 136.9 | 136.9 | 137.2 | 137.2 |
| 2' | 103.2 | 103.3 | 101.6 | 101.7 |
| 3' | 153.4 | 153.4 | 153.0 | 153.0 |
| 4' | 136.8 | 136.8 | 135.7 | 135.8 |
| 5' | 153.4 | 153.4 | 150.3 | 150.4 |
| 6' | 103.2 | 103.3 | 107.2 | 107.2 |
| 2'' | 78.5 | 78.6 | 77.0 | 77.1 |
| 3'' | 30.4 | 30.1 | 29.7 | 29.5 |
| 4'' | 23.6 | 23.0 | 23.5 | 22.9 |
| 5'' | 130.8 | 130.4 | 130.1 | 130.6 |
| 6'' | 109.6 | 109.2 | 106.4 | 105.9 |
| 7'' | 146.5 | 146.5 | 145.9 | 145.9 |
| 8'' | 141.0 | 141.0 | 133.7 | 133.7 |
| 9'' | 147.6 | 147.4 | 143.6 | 143.6 |
| 10'' | 113.8 | 113.2 | 113.7 | 114.2 |
| 1''' | 136.9 | 136.9 | 137.4 | 137.4 |
| 2''' | 103.2 | 103.3 | 103.7 | 103.8 |
| 3''' | 153.4 | 153.4 | 152.8 | 152.8 |
| 4''' | 136.8 | 136.8 | 136.9 | 137.0 |
| 5''' | 153.4 | 153.4 | 152.8 | 152.8 |
| 6''' | 103.2 | 103.3 | 103.7 | 103.8 |
| OMe-7 | 56.3 | 56.3 | 56.1 | 56.1 |
| OMe-8 | 60.8 | 60.8 | ||
| OMe-3' | 56.1 | 56.1 | 55.7 | 55.7 |
| OMe-4' | 60.9 | 60.9 | 59.9 | 59.9 |
| OMe-5' | 56.1 | 56.1 | ||
| OMe-7'' | 56.3 | 56.3 | 56.1 | 56.1 |
| OMe-8'' | 60.8 | 60.8 | ||
| OMe-3''' | 56.1 | 56.1 | 55.7 | 55.9 |
| OMe-4''' | 60.9 | 60.9 | 59.9 | 60.0 |
| OMe-5''' | 56.1 | 56.1 | 55.9 | |
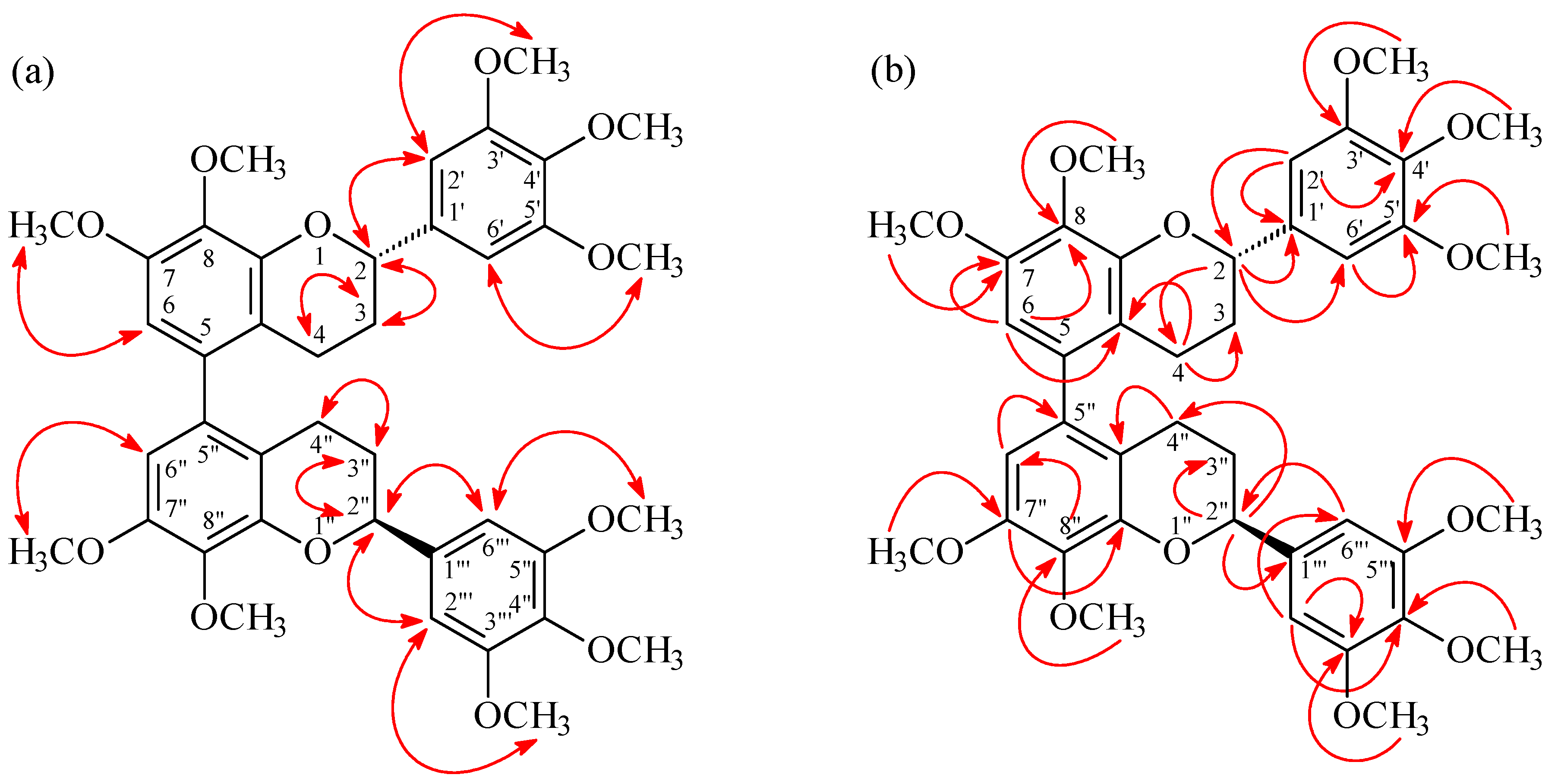
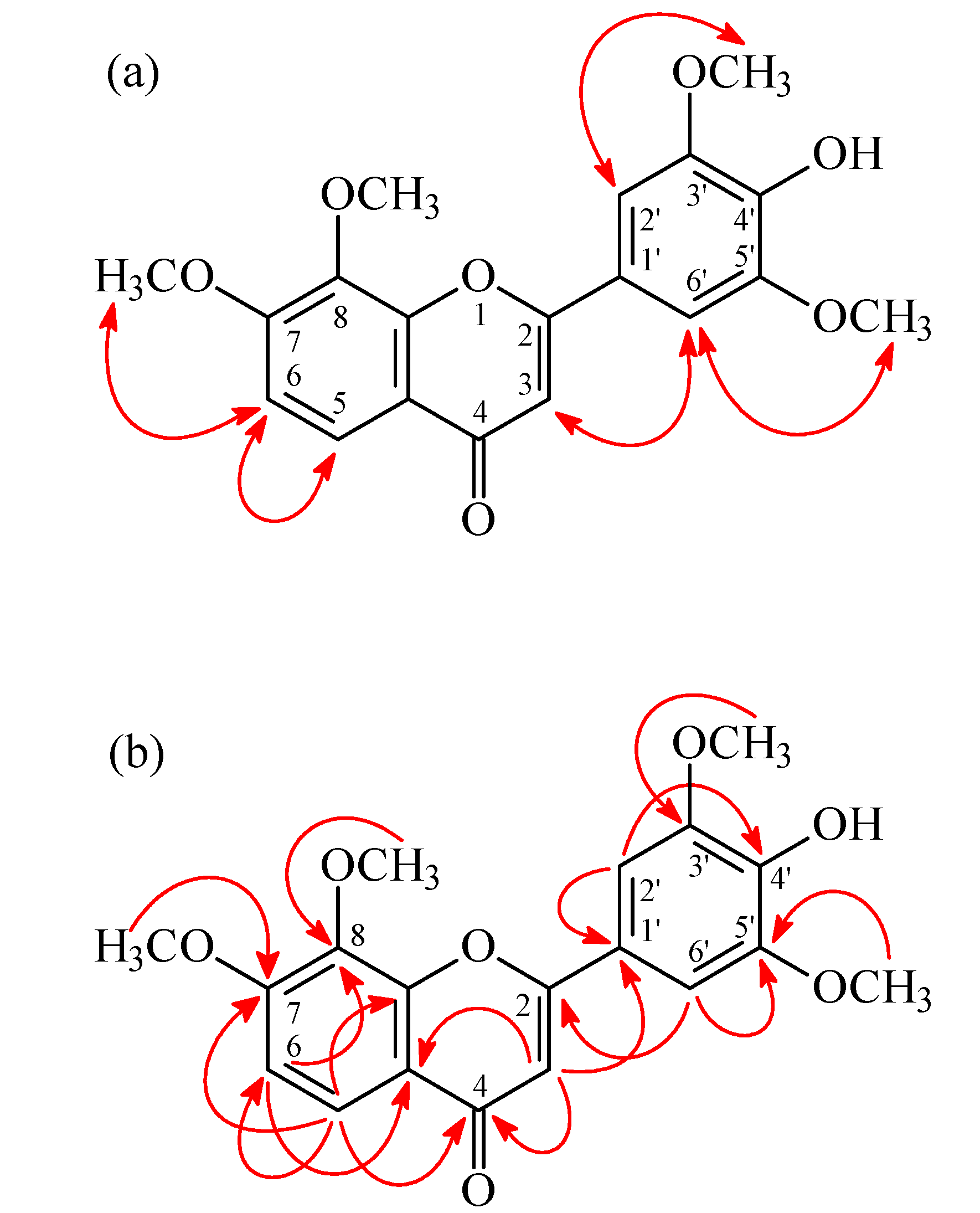
| Position | 3 a | 3a a,b | ||
|---|---|---|---|---|
| δH J (Hz) | NOESY | HMBC | δH J (Hz) | |
| 2 | 7.44 br d (7.6) | 3, α, β | 4, 6, β | 7.44 br d (7.6) |
| 3 | 7.39 br t (7.6) | 2, 4 | 1, 5 | 7.39 br t (7.6) |
| 4 | 7.31 br t (7.6) | 3, 5 | 2, 3, 5, 6 | 7.31 br t (7.6) |
| 5 | 7.39 br t (7.6) | 4, 6 | 1, 3 | 7.39 br t (7.6) |
| 6 | 7.44 br d (7.6) | 5, α, β | 2, 4, β | 7.44 br d (7.6) |
| α | 3.29 dd (17.2, 3.2) | 6, β | 1, 1', C=O | 3.29 dd (17.4, 3.2) |
| 3.36 dd (17.2, 8.8) | β, 6' | 1, 1', β | 3.36 dd (17.4, 8.8) | |
| β | 5.33 dd (8.8, 3.2) | 2, 6, α | 1, 2, 6, α, C=O | 5.34 dd (8.8, 3.2) |
| 5' | 6.46 d (9.0) | 6', OMe-4' | 1', 3', 4' | 6.50 d (9.0) |
| 6' | 7.47 d (9.0) | α, 5' | 2', 4', C=O | 7.40 d (9.0) |
| OH-β | 3.64 br s | 3.57 br s | ||
| OH-2' | 12.62 s | 1', 2', 3' | 12.67 s | |
| OH-4' | 6.41 br s | |||
| OMe-3' | 3.87 s | 3' | 4.00 s | |
| OMe-4' | 3.91 s | 5' | 4' | |
| Compounds | IC50 (μM) a |
|---|---|
| (M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-Decamethoxy-5,5''-biflavan (1) | >100 |
| 4'-Hydroxy-7,8,3',5'-tetramethoxyflavone (2) | 58.4 ± 6.2 |
| (R)-2',β-Dihydroxy-3',4'-dimethoxydihydrochalcone (3) | >100 |
| (M),(2S),(2''S)-,(P),(2S),(2''S)-8,5',8''-Trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy-5,5''-biflavan (4) | 54.2 ± 5.3 |
| 5-Hydroxy-7-methoxyflavone (5) | 1.77 ± 0.70 |
| Quercetin (6) | 3.82 ± 0.46 |
| 7,8,3',4',5'-Pentamethoxyflavone (7) | >100 |
| (2 S)-8,5'-Dihydroxy-7,3',4'-trimethoxyflavan (8) | 56.6 ± 6.2 |
| (2 S)-5'-Hydroxy-7,8,3',4'-tetramethoxyflavan (9) | >100 |
| (2 S)-7-Hydroxyflavanone (10) | 4.92 ± 1.71 |
| (2 S)-5'-Hydroxy-7,3',4'-trimethoxyflavanone (11) | >100 |
| (E)-Ferulic acid (12) | 24.18 ± 1.54 |
| Gallic acid (13) | >100 |
| β-Sitostenone (14) | >100 |
| β-Sitosterol (15) | >100 |
| Ibuprofen b | 27.5 ± 3.2 |
2.2. Discussion
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Biological Assay
3.4.1. Preparation of Human Neutrophils
3.4.2. Measurement of Superoxide Anion Generation
3.4.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, T.S.; Lo, H.C. Tiliaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1993; Volume 3, pp. 722–733. [Google Scholar]
- Chen, J.J.; Lee, H.H.; Shih, C.D.; Liao, C.H.; Chen, I.S.; Chou, T.H. New dihydrochalcones and anti-platelet aggregation constituents from the leaves of Muntingia calabura. Planta Med. 2007, 73, 572–577. [Google Scholar]
- Chen, J.J.; Lin, R.W.; Duh, C.Y.; Huang, H.Y.; Chen, I.S. Flavones and cytotoxic constituents from the stem bark of Muntingia calabura. J. Chin. Chem. Soc. 2004, 51, 665–670. [Google Scholar]
- Chen, J.J.; Lee, H.H.; Duh, C.Y.; Chen, I.S. Cytotoxic chalcones and flavonoids from the leaves of Muntingia calabura. Planta Med. 2005, 71, 970–973. [Google Scholar]
- Kaneda, N.; Pezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D.; Farnsworth, N.R.; Santisuk, T.; Tuchinda, P.; Udchachon, J.; Reutrakul, V. New cytotoxic flavonoids from Muntingia calabura roots. J. Nat. Prod. 1991, 54, 196–206. [Google Scholar]
- Nshimo, C.M.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Cytotoxic constituents of Muntingia calabura leaves and stems collected in Thailand. Int. J. Pharm. 1993, 31, 77–81. [Google Scholar]
- Chen, J.J.; Cho, J.Y.; Hwang, T.L.; Chen, I.S. Benzoic acid derivatives, acetophenones, and anti-inflammatory contituents from Melicope semecarpifolia. J. Nat. Prod. 2008, 71, 71–75. [Google Scholar]
- Chen, J.J.; Luo, Y.T.; Hwang, T.L.; Sung, P.J.; Wang, T.C.; Chen, I.S. A new indole alkaloid and anti-inflammatory constituents from Strychnos cathayensis. Chem. Biodivers. 2008, 5, 1345–1352. [Google Scholar]
- Chen, J.J.; Ting, C.W.; Hwang, T.L.; Chen, I.S. Benzophenone derivatives from the fruits of Garcinia multiflora and their anti-inflammatory activity. J. Nat. Prod. 2009, 72, 253–258. [Google Scholar]
- Chen, J.J.; Lin, Y.H.; Day, S.H.; Hwang, T.L.; Chen, I.S. New benzenoids and anti-inflammatory constituents from Zanthoxylum nitidum. Food Chem. 2011, 125, 282–287. [Google Scholar]
- Chen, J.J.; Tsai, Y.C.; Hwang, T.L.; Wang, T.C. Thymol, benzofuranoid, and phenylpropanoid derivatives: Anti-inflammatory constituents from Eupatorium annabinum. J. Nat. Prod. 2011, 74, 1021–1027. [Google Scholar]
- Bellini, A.; Venturella, P. The synthesis of hydroxychalcone and hydroxyflavone derivatives. VI. Flavones derived from syringic aldehyde. Ann. Chim. 1958, 48, 716–722. [Google Scholar]
- Cheon, C.H.; Yamamoto, H. N-Triflylthiophosphoramide catalyzed enantioselective mukaiyama aldol reaction of aldehydes with silyl enol ethers of ketones. Org. Lett. 2010, 12, 2476–2479. [Google Scholar]
- Thusoo, A.; Raina, N.; Minhaj, N.; Ahmed, S.R.; Zaman, A. Crystalline constituents from Daphne oleoides. Indian J. Chem. B 1981, 20, 937–938. [Google Scholar]
- Kim, S.M.; Kang, K.; Jho, E.H.; Jung, Y.J.; Nho, C.W.; Um, B.H.; Pam, C.H. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. 2011, 25, 1011–1017. [Google Scholar]
- Tanrisever, N.; Fronczek, F.R.; Fischer, N.H.; Williamson, G.B. Ceratiolin and other flavonoids from Ceratiola ericoides. Phytochemistry 1987, 26, 175–179. [Google Scholar]
- Machida, K.; Kikuchi, M. Norisoprenoids from Viburnum dilatatum. Phytochemistry 1992, 41, 1333–1336. [Google Scholar]
- Wang, Y.S.; Huang, R.; Yang, J.H. Chemical constituents of Litsea szemaois. Chem. Nat. Compd. 2011, 47, 122–123. [Google Scholar]
- Chen, J.J.; Huang, S.S.; Liao, C.H.; Wei, D.C.; Sung, P.J.; Wang, T.C.; Cheng, M.J. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010, 120, 379–384. [Google Scholar]
- Chen, J.J.; Wang, T.C.; Yang, C.K.; Liao, H.R.; Sung, P.J.; Chen, I.S.; Cheng, M.J.; Peng, C.F.; Chen, J.F. New pterosin sesquiterpenes and antitubercular constituents from Pteris ensiformis. Chem. Biodivers. 2013, 10, 1903–1908. [Google Scholar]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. 1968, 97, 77–89. [Google Scholar]
- Jauregui, H.O.; Hayner, N.T.; Driscoll, J.L.; Williams-Holland, R.; Lipsky, M.H.; Galletti, P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro 1981, 17, 1100–1110. [Google Scholar]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar]
- Sample Availability: Samples of the all compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, W.-L.; Liao, H.-R.; Chen, J.-J. Biflavans, Flavonoids, and a Dihydrochalcone from the Stem Wood of Muntingia calabura and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses. Molecules 2014, 19, 20521-20535. https://doi.org/10.3390/molecules191220521
Kuo W-L, Liao H-R, Chen J-J. Biflavans, Flavonoids, and a Dihydrochalcone from the Stem Wood of Muntingia calabura and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses. Molecules. 2014; 19(12):20521-20535. https://doi.org/10.3390/molecules191220521
Chicago/Turabian StyleKuo, Wen-Lung, Hsiang-Ruei Liao, and Jih-Jung Chen. 2014. "Biflavans, Flavonoids, and a Dihydrochalcone from the Stem Wood of Muntingia calabura and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses" Molecules 19, no. 12: 20521-20535. https://doi.org/10.3390/molecules191220521
APA StyleKuo, W.-L., Liao, H.-R., & Chen, J.-J. (2014). Biflavans, Flavonoids, and a Dihydrochalcone from the Stem Wood of Muntingia calabura and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses. Molecules, 19(12), 20521-20535. https://doi.org/10.3390/molecules191220521







