Abstract
Despite substantial advances in genotyping techniques and massively accumulated data over the past half century, a uniform measurement of neutral genetic diversity derived by different molecular markers across a wide taxonomical range has not yet been formulated. We collected genetic diversity data on seed plants derived by AFLP, allozyme, ISSR, RAPD, SSR and nucleotide sequences, converted expected heterozygosity (He) to nucleotide diversity (π), and reassessed the relationship between plant genetic diversity and life history traits or extinction risk. We successfully established a uniform π criterion and developed a comprehensive plant genetic diversity database. The mean population-level and species-level π values across seed plants were 0.00374 (966 taxa, 155 families, 47 orders) and 0.00569 (728 taxa, 130 families, 46 orders), respectively. Significant differences were recovered for breeding system (p < 0.001) at the population level and geographic range (p = 0.023) at the species level. Selfing taxa had significantly lower π values than outcrossing and mixed-mating taxa, whereas narrowly distributed taxa had significantly lower π values than widely distributed taxa. Despite significant differences between the two extreme threat categories (critically endangered and least concern), the genetic diversity reduction on the way to extinction was difficult to detect in early stages.
1. Introduction
According to the neutral theory of molecular evolution, current patterns of neutral genetic diversity that are maintained within species and populations reflect underlying molecular evolutionary processes involving mutation, genetic drift, and gene flow [1,2]. Because of the importance of genetic diversity in evolutionary and conservation genetics [3,4], one fundamental question is how to measure neutral genetic diversity using molecular markers. The capacity of molecular markers to measure genetic diversity has improved greatly over the past half century [5], including allozyme [6], DNA sequencing (since Kreitman) [7], microsatellites (simple sequence repeats, SSR) [8], random amplified polymorphic DNA (RAPD) [9], inter-simple sequence repeats (ISSR) [10] and amplified fragment length polymorphism (AFLP) [11]. Accumulated data and analyses using individual molecular markers have greatly enhanced our understanding of genetic diversity, however, a uniform measurement of neutral genetic diversity that is derived by different molecular markers and capable of being compared across a wide range of different species has not yet been formulated. In recent years, the booming of high-throughput genotyping approach using next-generation sequencing (NGS) [12] has been accompanied by the attenuated application of traditional molecular markers. Thus, it is urgently needed to summarize the previously accumulated genetic diversity data into a meaningful benchmark reference for future use with NGS techniques.
All measured heritable polymorphisms using any molecular marker are derivatives of indirect measurements of nucleotide differences of genomic sequences [5]. Nucleotide diversity (π), which is defined as the average number of nucleotide differences per site between two randomly chosen DNA sequences [13], is a fundamental criterion for measuring genetic diversity [14]. Although DNA sequencing is the direct measurement of π, the data were very limited because of the cost and time constraints in early stages, and instead allozyme, microsatellites, RAPD, ISSR and AFLP have been extensively used for estimating expected heterozygosity (He) under Hardy-Weinberg equilibrium [15], the most common parameter used to substitute π thus far. Although several methods have been reported to directly estimate π from AFLP and RAPD data [14,16,17], these methods were impractical for large-scale surveys because of the technical limitation of gel electrophoresis banding complexity. Thus, our first attempt in the present study is to determine whether a large number of reported He estimates derived by different molecular markers could be uniformly transformed to π. Few studies have attempted such work, although an evaluation of the correlation between allozyme and DNA sequencing has been performed [4,18].
To date, no solid statistical relationship has been developed between He and π, especially for different markers used in various genetic backgrounds across a wide spectrum of different taxa. The expected nucleotide diversity (π) under neutral equilibrium and infinite sites model is represented by 4Neμ0, where Ne is the effective population size and μ0 is the mutation rate per nucleotide [19]. Alternatively, the expected heterozygosity (He) under Hardy-Weinberg equilibrium and the infinite allele model is represented by 4Neμ/(4Neμ + 1), where the mutation rate μ refers to the entire protein or amplified fragment [20]. The expected values of both π and He depend on the effective population size and their corresponding mutation rates. Therefore the relationship between π and He is:
and the relationship between He estimates derived by two different markers is
If we assume that the mutation rate ratios μ0/μ and μ2/μ1 are constant across species and treat He/(1 − He) as a single parameter, both equations become simple linear formulae, i.e., the correlation and transformation among the estimates derived by different molecular markers could be realized under the assumption of constant mutation rate ratios. The mutation rate ratios could be obtained by performing regression analysis with linear functions through empirical data. In this study, we aimed to test the putative relationship and deduce the He-π transformation equations in seed plants, on the basis of a comprehensive compilation of all available data of He estimates derived by different molecular markers.
It is widely accepted that life history traits (taxonomic status, life form, geographic range, breeding system, seed dispersal, successional status, etc.) have significant impacts on plant genetic diversity levels based on evidence from several molecular markers, including allozyme [21,22], RAPD [23,24], SSR [24] and DNA sequences [25]. Main conclusions in those reviews and other related studies were quite consistent; however, exceptions have been found for two traits: geographic range at the population level and breeding system at the species level. Geographic range was significantly correlated with population-level He derived by allozyme [21] and SSR [24], but not in the RAPD data [23,24]; the impact of selfing on plant genetic diversity would be reflected at the population level instead of the species level [26,27]. In addition to these inconsistencies, according to the allozyme results, geographic range and breeding system accounted for the largest proportion of genetic variation at the species and population levels, respectively [21]. Thus, we aimed to reassess the association of plant genetic diversity with these two traits at both the species and population levels using a new subset of the compiled database.
There has been a great deal of controversy over the role of genetic factors in extinction risk in the past decades, especially after the “Lande scenario” [28], which has been widely interpreted to mean that most species would be driven to extinction by ecological and demographic factors before genetic factors have time to show impact [29]. Nevertheless, the direct empirical case studies were limited except those assessing Clarkia pulchella [30] and Melitaea cinxia [31], and most debates primarily relied on the theoretical deductions. Spielman et al. used an indirect method by comparing genetic heterozygosity between threatened species and their nonthreatened relatives across animals and plants listed in the International Union for Conservation of Nature (IUCN) Red List of Threatened species [32], but limited plant taxa and no detailed threat categories were included. Thus, reassessment of the association of plant genetic diversity among IUCN categorized plants across a wide taxonomic range was rigorously attempted in the present paper.
The purposes of the present study were three-fold: (1) to establish a uniform π criterion by transforming the He estimates using different molecular markers across a wide taxonomic spectrum of seed plants; (2) to reassess the relationship between plant genetic diversity and life history traits or extinction risk; (3) to provide baseline data for future evolutionary studies and conservation practice using NGS techniques.
2. Results
A total of 1901 records from 1434 papers including 1577 taxa at the species, subspecies or variety level were compiled in the primary literature survey (Table 1). After removing redundancy, the He estimates using the five different markers except SSR were quite similar, with mean values ranging 0.159–0.182 at the population level and 0.198–0.246 at the species level, whereas SSR-based He estimates were approximately three-fold larger (Mann-Whitney U test, p < 0.001), with a mean value of 0.55 at the population level and 0.643 at the species level (Figure 1; Table S1 and Table S2). The π values of 81 records derived by nucleotide sequences ranged from 0.00027 to 0.03318 with a mean value of 0.008 (Table S3).

Table 1.
The numbers of papers, taxa and records for the five molecular markers and nucleotide sequences included in this study.
| Markers | Papers | Taxons | Records |
|---|---|---|---|
| AFLP | 244 | 309 | 352 |
| Allozyme | 383 | 461 | 535 |
| ISSR | 158 | 173 | 183 |
| RAPD | 149 | 171 | 183 |
| SSR | 440 | 382 | 535 |
| Sequence | 60 | 81 | 113 |
| All | 1434 | 1577 | 1901 |
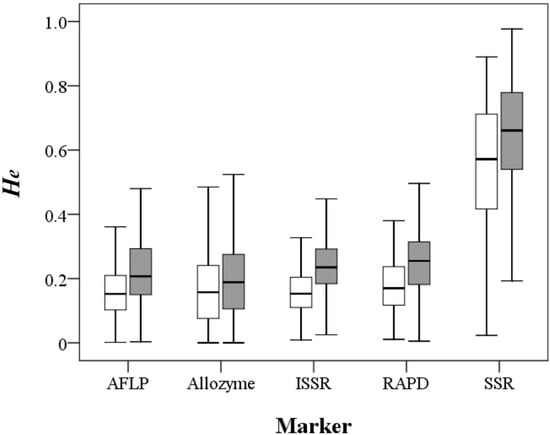
Figure 1.
Distribution of the expected heterozygosity values at the population (open box) and species (filled box) levels derived by the five molecular markers.
A summary of all pairwise correlation analyses between the π values derived by nucleotide sequences and the He/(1 − He) values derived by any of the five markers is shown in Table 2, and the taxa chosen for each marker pair are listed in Table S4. A majority of the correlation coefficients were significantly positive (p < 0.05), except for three pairs, Sequence/AFLP, Sequence/ISSR and ISSR/SSR, which was most likely due to limited data availability (6, 1 and 7). Five marker pairs (Sequence/Allozyme, Sequence/RAPD, Sequence/SSR, AFLP/SSR and RAPD/ISSR) were chosen for the regression analyses to convert He to π. The Allozyme-, RAPD-, and SSR-based He values were directly converted to π. However, the taxon numbers for the Sequence/AFLP and Sequence/ISSR pairs were too small; therefore, the AFLP- and ISSR-based He values were indirectly converted via SSR and RAPD, respectively. As shown in Figure 2 and Table S5, all five coefficients were significant (p < 0.001), and the R2 values ranged from 0.669 to 0.905. The linear coefficients for the five markers in the He-π conversion equation π = b He/(1 − He) were 0.021 (Allozyme), 0.013 (RAPD), 0.002 (SSR), 0.012 (AFLP, 6.333 × 0.002) and 0.014 (ISSR, 1.072 × 0.013).

Table 2.
Summary of the numbers of taxa, Spearman’s coefficients and one-tailed significance tests for correlation analyses among nucleotide sequences and the five molecular markers.
| Marker pair | N | rho | p |
|---|---|---|---|
| Sequence/AFLP | 6 | 0.600 | 0.104 |
| Sequence/Allozyme | 44 | 0.288 | 0.029 |
| Sequence/ISSR | 1 | / | / |
| Sequence/RAPD | 12 | 0.538 | 0.035 |
| Sequence/SSR | 49 | 0.240 | 0.048 |
| AFLP/Allozyme | 24 | 0.446 | 0.014 |
| AFLP/ISSR | 17 | 0.418 | 0.047 |
| AFLP/RAPD | 34 | 0.541 | <0.001 |
| AFLP/SSR | 47 | 0.302 | 0.020 |
| Allozyme/ISSR | 9 | 0.683 | 0.021 |
| Allozyme/RAPD | 39 | 0.631 | <0.001 |
| Allozyme/SSR | 67 | 0.235 | 0.028 |
| ISSR/RAPD | 27 | 0.825 | <0.001 |
| ISSR/SSR | 7 | 0.643 | 0.060 |
| RAPD/SSR | 29 | 0.335 | 0.038 |
All the Hs and Ht values derived by the five markers were converted to πs and πt with the deduced regression equations, whereas the π values measured by nucleotide sequences were directly used as πt. After removing redundancy, 1023 and 807 taxa were obtained in the πs and πt datasets, respectively. To eliminate the uncertainty of taxonomic status of cultivated taxa for the follow-up analyses, the cultivated taxa were removed and the taxon numbers were reduced to 966 and 728. The πs values ranged from 0.0000025 to 0.03285, with a mean value of 0.00374, involving 155 families and 47 orders, whereas the πt values ranged from 0.0000025 to 0.12900, with a mean value of 0.00569, involving 130 families and 46 orders (Table S6 and Table S7).
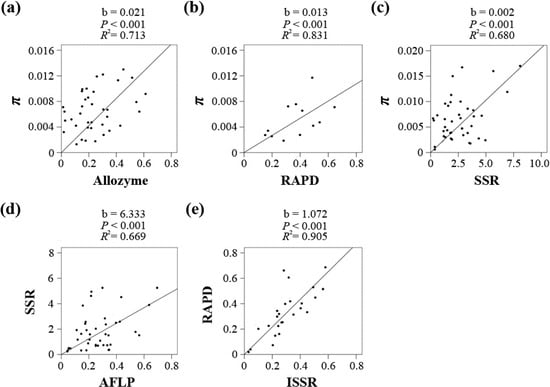
Figure 2.
Regression results for each of the five marker pairs using a linear model without intercept, including Sequence/Allozyme (a), Sequence/RAPD (b), Sequence/SSR (c), SSR/AFLP (d) and RAPD/ISSR (e).
The πs and πt values were then mapped onto 43 and 42 orders listed in APG III (2009), respectively (Figure 3). The top three most studied angiosperm families were Asteraceae, Fabaceae and Poaceae, whereas the orders were Asterales, Poales and Lamiales. For the gymnosperm species, the most studied family and order were Pinaceae and Pinales, respectively (Table S7). An approximately 10-fold variation covering two orders of magnitude (0.001 and 0.01) was detected for the mean πs and πt values across plant families and orders (Figure 3; Table S7).
The πs and πt values from a wide range of taxa were compared among the groups of breeding system, geographic range, and extinction risk (Table 3 and Table S6). One-way ANOVAs revealed significant differences for breeding system (p < 0.001) at the population level and geographic range (p = 0.023) at the species level, but not for extinction risk at either level. Multiple comparison analyses also revealed significant differences (p < 0.05) for several group pairs. Among the breeding system groups, selfing taxa had significantly lower values than other taxa, and asexual taxa had significantly lower values than outcrossing taxa at the population level. However, selfing taxa had significantly lower values than outcrossing and mixed-mating taxa at the species level. Narrowly distributed taxa had significantly lower values than widely distributed taxa at the species level. The CR category had significantly lower values than the LC category at both levels.
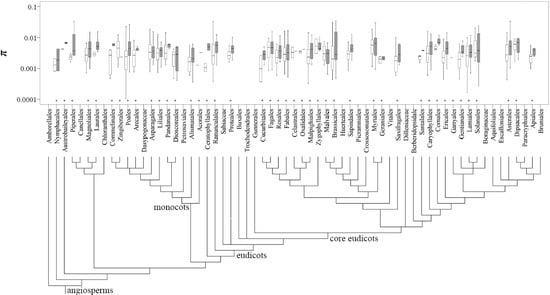
Figure 3.
Distribution of the π values at the population (open box) and species (filled box) levels across the angiosperm plants grouped by the orders listed in APG III (2009) (noted with asterisk).

Table 3.
Summary of the population-level (πs) and species-level (πt) nucleotide diversity of the sampled taxa grouped by different traits.
| Trait | πs | πt | ||||
|---|---|---|---|---|---|---|
| N | mean | SE | N | mean | SE | |
| Breeding system | * (p < 0.001) | NS (p = 0.133) | ||||
| asexual | 102 | 0.00336 b | 0.00310 | 71 | 0.00508 ab | 0.00390 |
| selfing | 70 | 0.00176 c | 0.00196 | 61 | 0.00427 b | 0.00495 |
| mixed-mating | 83 | 0.00360 ab | 0.00363 | 54 | 0.00633 a | 0.00594 |
| outcrossing | 490 | 0.00418 a | 0.00356 | 365 | 0.00597 a | 0.00641 |
| Geographic range | NS (p = 0.368) | * (p = 0.023) | ||||
| narrow | 461 | 0.00368 | 0.00324 | 336 | 0.00517 b | 0.00467 |
| wide | 433 | 0.00389 | 0.00356 | 307 | 0.00652 a | 0.00969 |
| Extinction risk | NS (p = 0.502) | NS (p = 0.493) | ||||
| CR | 18 | 0.00241 b | 0.00141 | 17 | 0.00326 b | 0.00231 |
| EN | 28 | 0.00332 ab | 0.00283 | 21 | 0.00590 ab | 0.00701 |
| VU | 41 | 0.00325 ab | 0.00314 | 31 | 0.00465 ab | 0.00318 |
| NT | 20 | 0.00367 ab | 0.00263 | 12 | 0.00576 ab | 0.00421 |
| LC | 80 | 0.00395 a | 0.00435 | 71 | 0.00685 a | 0.01088 |
| All | 966 | 0.00374 | 0.00343 | 728 | 0.00569 | 0.00730 |
Notes: NS (not significant) and * (p < 0.05) stand for the significance of one-way ANOVAs; SE stands for the standard errors; means followed by the same letter in a column are not significantly (p < 0.05) different.
3. Discussion
The most extensive data compiled and analyzed in this review provided us with valuable insights on the magnitude and variability of genetic diversity in plants (Table S1 and Table S3). Obviously, the results were quite different from what previous authors have concluded. The mean values of Allozyme-based Hs and Ht estimates summarized by Hamrick and Godt [21] were smaller (Hs, 0.113 vs. 0.173; Ht, 0.149 vs. 0.198). Such differences might be explained by the fact that we collected data accumulated until 2013 much later than those included in Hamrick and Godt [21]. More recently published papers tended to filter the monomorphic markers and use more polymorphic markers. However, larger mean Hs values (AFLP, 0.23 vs. 0.162; ISSR, 0.22 vs. 0.159; RAPD, 0.22 vs. 0.182; SSR, 0.61 vs. 0.550) summarized by Nybom et al. [24] were likely caused by smaller biased datasets compiled from the four markers (AFLP, 13 vs. 247; ISSR, 4 vs. 145; RAPD, 60 vs. 136; SSR, 104 vs. 260). Nevertheless, the comparison among different markers by Nybom et al. [24] showed a similar trend that AFLP-, ISSR-, and RAPD-based estimates were quite close whereas SSR-based estimates were three-fold larger.
Our results demonstrated that most of the 15 pairwise correlation coefficients were significantly positive (Table 2), confirming the utility of different molecular markers in population studies. Few assessment of the correlations among different molecular markers had been attempted before this study, except for the relationship between allozyme heterozygosity and nucleotide diversity [4,18]. Pyhäjärvi et al. detected a significant (p < 0.001) relationship in a dataset from 27 plant species [18], whereas the coefficient was marginally significant (p = 0.068) across 22 species studied by Leffler et al. [4]. In our study, although no significant correlation was observed for the three marker pairs (Sequence/AFLP, Sequence/ISSR and ISSR/SSR) because of limited data, significant correlations are expected to be recovered when more data become available. Theoretically, genetic variation estimated by molecular markers represents nucleotide differences in genomic sequences, however, the direct numeric relationship might be too difficult to formulate. Several methods were developed for estimating nucleotide diversity from AFLP and RAPD data [14,16,17] on the basis of electrophoresis band profiles, but these methods are impractical for large-scale data compilation. In this study, we confirmed the statistical validity of uniformly transforming the He estimates derived by the most commonly used molecular markers with the assumption that the mutation rate ratios of different markers are constant across species. The successful He-π conversion by using our deduced regression equations provided us with the first comprehensive plant nucleotide diversity database covering a wide range of plant families and orders.
The association of plant genetic diversity and life history traits (especially geographic range and breeding system) has been of great interest to evolutionary and conservation biologists. One-way ANOVAs in this study showed that genetic diversity revealed as π values was not significantly different among the taxa grouped by geographic range at the population level and by breeding system at the species level (Table 3). Our results suggested that narrowly distributed taxa (mean: 0.00368) were similar to widely distributed taxa (mean: 0.00389) at the population level, which was not in accordance with the conclusion reported for allozyme [21] and SSR [24], but consistence for RAPD [23,24]. One possible explanation was that geographic range is a trait of the whole species other than separate populations. In other words, it might happen that widely distributed taxa have a high level of genetic diversity over the whole species distribution range but a low level in some individual populations with high inter-population differentiation. It has been widely accepted that plant breeding system is a major trait underlying plant genome evolution and molecular diversity, and all of the aforementioned reviews using data from different markers have provided support for this idea. Unexpectedly, the effect of breeding system on genetic diversity was not significant (p = 0.133) at the species level in one-way ANOVAs in this study, although selfing taxa (mean: 0.00427) had significantly lower values than outcrossing (mean: 0.00597) and mixed-mating taxa (mean: 0.00633) in the multiple comparison. It was probably due to confounded partition of genetic diversity within and between populations of plant taxa with certain breeding systems. For example, selfer species usually have low intra-population genetic diversity, but high inter-population diversity, such as selfing Arabidopsis thaliana compared with its outcrossing relative A. lyrata [26,27]. Thus, such confounding attribute may explain that the impact of breeding system on plant genetic diversity was not significant at the species level. Nevertheless, significant differences of genetic diversity were recovered at the population level among the breeding system groups with mean π values ranging from 0.00176 to 0.00418 (Table 3).
The role of genetic factors in extinction risk has been controversial in the past decades [29], especially after Lande demonstrated that ‘demography may usually be of more immediate importance than population genetics in determining the minimum viable size of wild populations’ [28], although Lande has modified his views by readdressing the importance of mutational accumulation in extinction risk [33]. In fact, Lande also mentioned ‘the practical need in conservation for understanding the interaction of demographic and genetic factors in extinction’ [28]. Thus, there was no fundamentally irreconcilable point for the actual controversial debates on the role of genetic factors in increasing extinction risk of small plant populations. To the best of our knowledge, the practical issue is how to detect reductions in genetic diversity while eliminating other confounding factors. Spielman et al. compared genetic diversity levels between threatened species and their nonthreatened relatives and found that 77% of threatened species had lower diversity, providing convincing evidence for the importance of genetic factors in conservation [32]. Similar patterns of genetic diversity loss were also reported in mammals [34] and birds [35]. However, the drawbacks in Spielman et al. [32] were that a limited number of taxa of 21 angiosperms and 15 gymnosperms were sampled and no detailed threat categories were further investigated.
We further pursued this topic by sampling across a wide taxonomic range in seed plants and taking into account five detailed IUCN red list categories. Although one-way ANOVAs showed that genetic diversity levels among the extinction risk groups were not significantly different at either the population or species levels, which might be attributed to the wide range of the π values in the three categories (EN, VU, and NT) with moderate extinction risk, significant differences were detected between CR and LC at the two extremes of the categories in the multiple comparison, suggesting the influence of π on the threat categories ranked by IUCN. CR categorized taxa presented 39% (52%) lower mean πs (πt) value than LC categorized taxa, whereas the genetic diversity level was found to decline by 35% in Spielman et al. [32]. To examine the relationship, we assigned each category a numerical index from 1 to 5 (1, LC; 2, NT; 3, VU; 4, EN; 5, CR) and detected a marginally significant negative correlation (πs: Pearson’s r = −0.127, P = 0.082; πt: Pearson’s r = −0.13, p = 0.109) between π and IUCN red list categories, suggesting a weak tendency for genetic diversity to be lower in threat categories with higher extinction risk. Reduction of genetic diversity in plants on the way to extinction might be a gradual and slow process, in which the decline tends to appear far from distinguishable in the preliminary stages. The fuzzy patterns might be caused by confounding factors. By further examining the patterns of four traits (taxonomic status, life form, breeding system and geographic range) associated with the five IUCN red list categories in our dataset, we found a clear shift in elevated extinction risk from wide to narrow geographic range, but no similar pattern was detected for the three other traits (Figure 4). Thus, geographic range would be a good predictor for extinction risk among the plant life history traits. Nevertheless, genetic factors cannot be neglected in the conservation efforts, although the genetic signs of endangerment are difficult to detect in early stages.
It is worth noting that a number of limitations might arise in our data compilation and deduction. First, we assumed that the mutation rate ratios among different markers are constant across plant species. However, this assumption would be violated to some extent because mutation rates vary across species, genomic regions and through evolutionary time. Second, slight differences among several versions of basic equations for calculating He estimates [15,36,37,38] might bias the collected data from compiled papers, although this bias was unavoidable for such a large-scale data collection in this study. Finally, proportion of polymorphic loci may result in unexpected confounding deviations for genetic diversity estimates, which has been well documented in the previous reviews [21,23]. Some papers reported estimates of all loci including monomorphic ones, whereas some others only used polymorphic loci by filtering monomorphic ones. In the data collection process, we chose the latter for consistency.
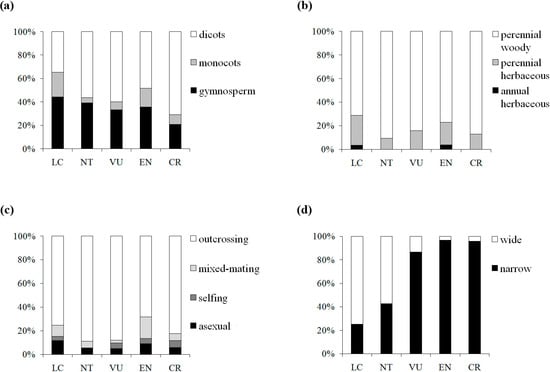
Figure 4.
Distribution of the taxon numbers of the five IUCN red list categories, grouped by taxonomic status (a), life form (b), breeding system (c) and geographic range (d).
4. Experimental Section
4.1. Data Collection
Genetic diversity data on seed plants were collected through a literature survey. Papers published before May 2013 that provided expected heterozygosity (He) [15,36,37] estimates derived by any of the five commonly used molecular markers (AFLP, Allozyme, ISSR, RAPD and SSR) or nucleotide diversity (π) [13] estimates derived by nuclear gene sequences were included in the data compilation. All available He estimates were separately recorded at the population (Hs) or species (Ht) levels, whereas all π values were recorded at the species level. The Hs estimates were averaged from at least two populations for each species, subspecies or variety, whereas the Ht estimates were calculated for all pooled samples. Only the estimates with polymorphic loci were chosen if both estimates of all loci (including monomorphic loci) and polymorphic loci were reported in the same paper, and only the estimates with more represented populations or loci were retained if there was more than one value assessed by the same marker for the same taxon.
4.2. Correlation and Regression Analyses, and Data Conversion
Pairwise correlation analyses were performed among the estimates derived by AFLP, Allozyme, ISSR, RAPD, SSR and nucleotide sequences for the same taxa available. The species-level estimates were preferentially included over the population-level estimates. All the He values were transformed to He/(1 − He) prior to the correlation analysis. For numeric conversion from He to π, regression analyses between these two values were performed for each taxon. The hypothetic equation was a simple linear function without intercept, π = b He/(1 − He). If the numbers of the same taxa for the markers were too few and the deduced coefficients were not significant, regression analyses via other markers were used for the He-π conversion, i.e. π = b1 H1/(1 − H1) = b2 H2/(1-H2). Polyploid taxa were excluded for the regression analyses. Subsequently, all the He values were converted to π at the population and species levels according to the regression equations. All the πs and πt values were gathered and prioritized in the sequential order, sequence > SSR > Allozyme > RAPD > AFLP > ISSR, so that only one value is retained for each taxon. Cultivated taxa (including crops, fruits and vegetables) were then removed for further analysis. Each species was also verified and classified in various taxonomic databases, and angiosperm species followed the APG III version (2009) [39].
4.3. Life History Traits and Extinction Risk
For each taxon, data were collected for the important life history traits breeding system (asexual, selfing, mixed-mating, outcrossing) and geographic range (narrow, wide), as well as for the threat category (EX-extinct, EW-extinct in the wild, CR-critically endangered, EN-endangered, VU-vulnerable, NT-near threatened, LC-least concern). For geographic range, “narrow” was equivalent to “endemic” and “narrow” used in Hamrick and Godt [21], whereas “wide” was equivalent to “regional” and “widespread”. The breeding system and geographic range information was obtained from the compiled papers, the USDA-NRCS database (http://plants.usda.gov), pertinent floras or other botanical literature. The threat category information was from the IUCN Red List of Threatened Species version 2013.1 [40]. Mean π values and standard errors were calculated for each group of the three traits at the population and species levels. To test the relationship between genetic diversity and life history traits or extinction risk, one-way analysis of variance (ANOVAs) and multiple comparison analyses were performed to determine the significance levels of the differences in the π values among the groups.
5. Conclusions
The present study established a uniform π criterion and developed a comprehensive genetic diversity database across a wide taxonomic spectrum of seed plants and all commonly used genetic markers, thus providing a meaningful benchmark reference for future studies with evolutionary biological and conservation concern. However, questions still remain wide open on the way to solve the old riddle about genetic diversity. The compiled large-scale comprehensive plant genetic diversity database in this study will serve as a solid reference resource for future work. Further discrimination between population-level and species-level with detailed trait hierarchies should be considered in future attempts.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/12/20113/s1.
Acknowledgments
We thank Jing Wang and Yifei Liu for their thoughtful comments on data compilation and analysis. This work was supported by the National Science and Technology Infrastructure Program of China (2009FY120200), the Knowledge Innovation Project of Chinese Academy of Sciences (KSCX2-EW-J-20) and the National Natural Science Foundation of China (31300178).
Author Contributions
The listed authors contributed as follows: HH and MK conceived and designed the project. BA collected, compiled and analyzed data. BA and HH drafted and revised the manuscript. All authors read and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lewontin, R.C. The Genetic Basis to Evolutionary Change; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Leffler, E.M.; Bullaughey, K.; Matute, D.R.; Meyer, W.K.; Ségurel, L.; Venkat, A.; Andolfatto, P.; Przeworski, M. Revisiting an old riddle: what determines genetic diversity levels within species? PLoS Biol. 2012, 10, e1001388. [Google Scholar] [CrossRef] [PubMed]
- Avise, J.C. Molecular Markers, Natural History, and Evolution, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Prakash, S.; Lewontin, R.C.; Hubby, J.L. A molecular approach to the study of genic heterozygosity in natural populations. IV. Patterns of genetic variation in central, marginal and isolated populations of Drosophila pseudoobscura. Genetics 1969, 61, 841–858. [Google Scholar] [PubMed]
- Kreitman, M. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature 1983, 304, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Litt, M.; Luty, J.A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am. J. Hum. Genet. 1989, 44, 397–401. [Google Scholar] [PubMed]
- Williams, J.G.K.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef] [PubMed]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequenc repeats (SSR)-anchored PCR amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, R.L. Estimating nucleotide diversity from random amplified polymorphic DNA and amplified fragment length polymorphism data. Mol. Phylogenet. Evol. 2001, 18, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.G.; Lanigan, C.M.S. Prospects for estimating nucleotide divergence with RAPDs. Mol. Biol. Evol. 1993, 10, 1096–1111. [Google Scholar] [PubMed]
- Innan, H.; Terauchi, R.; Kahl, G.; Tajima, F. A method for estimating nucleotide diversity from AFLP data. Genetics 1999, 151, 1157–1164. [Google Scholar] [PubMed]
- Pyhäjärvi, T.; Kujala, S.T.; Savolainen, O. Revisiting protein heterozygosity in plants--nucleotide diversity in allozyme coding genes of conifer Pinus sylvestris. Tree Genet. Genomes 2011, 7, 385–397. [Google Scholar] [CrossRef]
- Tajima, F. Evolutionary relationships of DNA sequences in finite populations. Genetics 1983, 105, 437–460. [Google Scholar] [PubMed]
- Kimura, M.; Crow, J.F. The number of alleles that can be maintained in a finite population. Genetics 1964, 49, 725–738. [Google Scholar] [PubMed]
- Hamrick, J.L.; Godt, M.J.W. Allozyme diversity in plant species. In Plant Population Genetics, Breeding and Genetic Resources; Brown, A.H.D., Clegg, M.T., Kahler, A.L., Weir, B.S., Eds.; Sinauer Associates: Sunderland, MA, USA, 1989; pp. 43–63. [Google Scholar]
- Hamrick, J.L.; Godt, M.J.W. Effects of life history traits on genetic diversity in plant species. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996, 351, 1291–1298. [Google Scholar] [CrossRef]
- Nybom, H.; Bartish, I.V. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect. Plant Ecol. 2000, 3, 93–114. [Google Scholar] [CrossRef]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Glémin, S.; Bazin, E.; Charlesworth, D. Impact of mating systems on patterns of sequence polymorphism in flowering plants. Proc. R. Soc. Lond. B. Biol. Sci. 2006, 273, 3011–3019. [Google Scholar] [CrossRef]
- Savolainen, O.; Langley, C.H.; Lazzaro, B.P.; Fréville, H. Contrasting patterns of nucleotide polymorphism at the alcohol dehydrogenase locus in the outcrossing Arabidopsis lyrata and the selfing Arabidopsis thaliana. Mol. Biol. Evol. 2000, 17, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.I.; Lauga, B.; Charlesworth, D. Subdivision and haplotype structure in natural populations of Arabidopsis lyrata. Mol. Ecol. 2003, 12, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Genetics and demography in biological conservation. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Newman, D.; Pilson, D. Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution 1997, 51, 354–362. [Google Scholar] [CrossRef]
- Saccheri, I.; Kuussaari, M.; Kankare, M.; Vikman, P.; Fortelius, W.; Hanski, I. Inbreeding and extinction in a butterfly metapopulation. Nature 1998, 392, 491–494. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Frankham, R. Most species are not driven to extinction before genetic factors impact them. Proc. Natl. Acad. Sci. USA 2004, 101, 15261–15264. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Mutation and conservation. Conserv. Biol. 1995, 9, 782–791. [Google Scholar] [CrossRef]
- Garner, A.; Rachlow, J.L.; Hicks, J.F. Patterns of genetic diversity and its loss in mammalian populations. Conserv. Biol. 2005, 19, 1215–1221. [Google Scholar] [CrossRef]
- Evans, S.R.; Sheldon, B.C. Interspecific patterns of genetic diversity in birds: Correlations with extinction risk. Conserv. Biol. 2008, 22, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [PubMed]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
- Lynch, M.; Milligan, B.G. Analysis of population genetic structure with RAPD markers. Mol. Ecol. 1994, 3, 91–99. [Google Scholar] [CrossRef] [PubMed]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2013.1. Available online: http://www.iucnredlist.org (accessed on 2 July 2013).
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).