Goniolactone C, a Styryl Lactone Derivative, Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Migration and Proliferation via PDGFR/ERK Signaling
Abstract
:1. Introduction
2. Results and Discussion
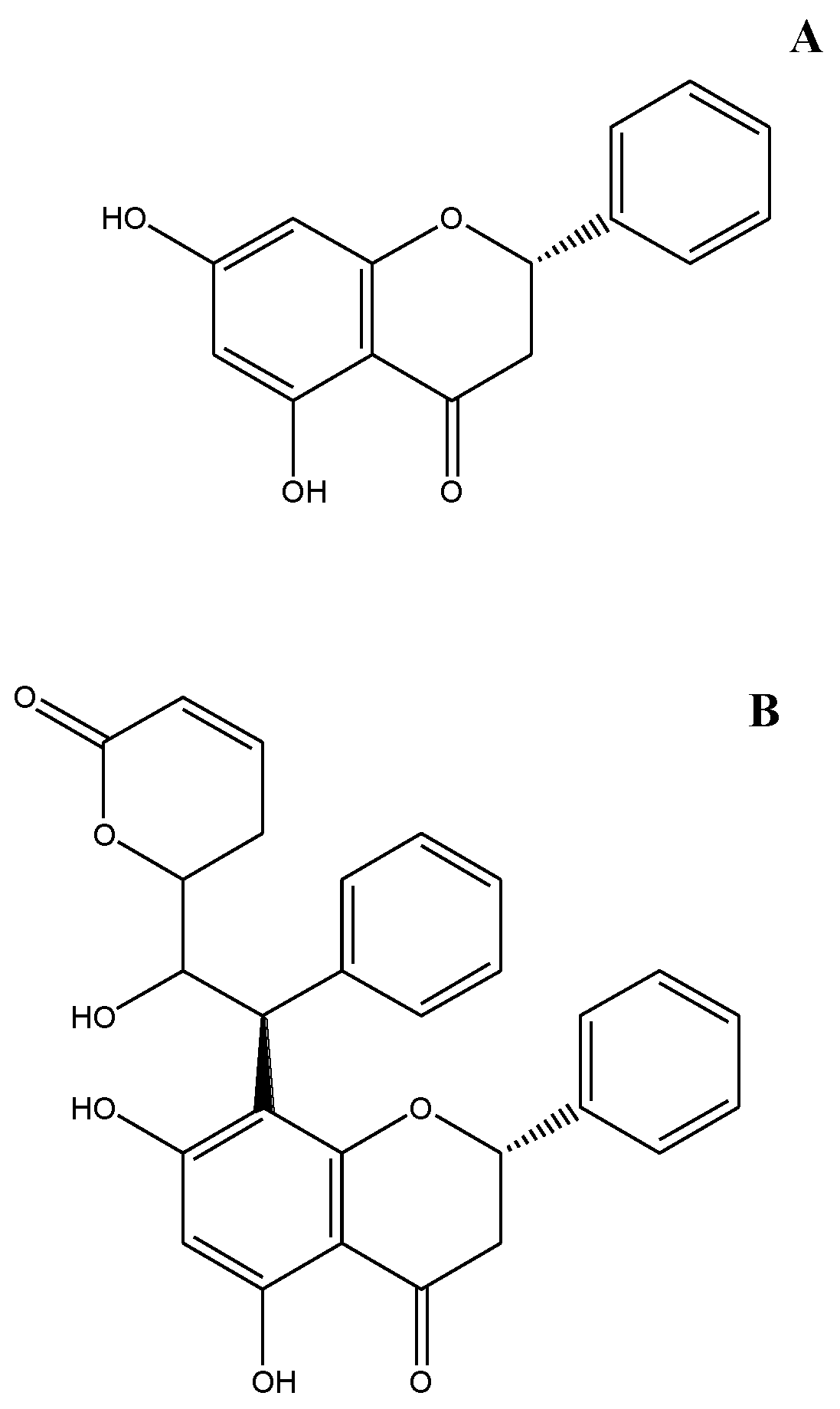
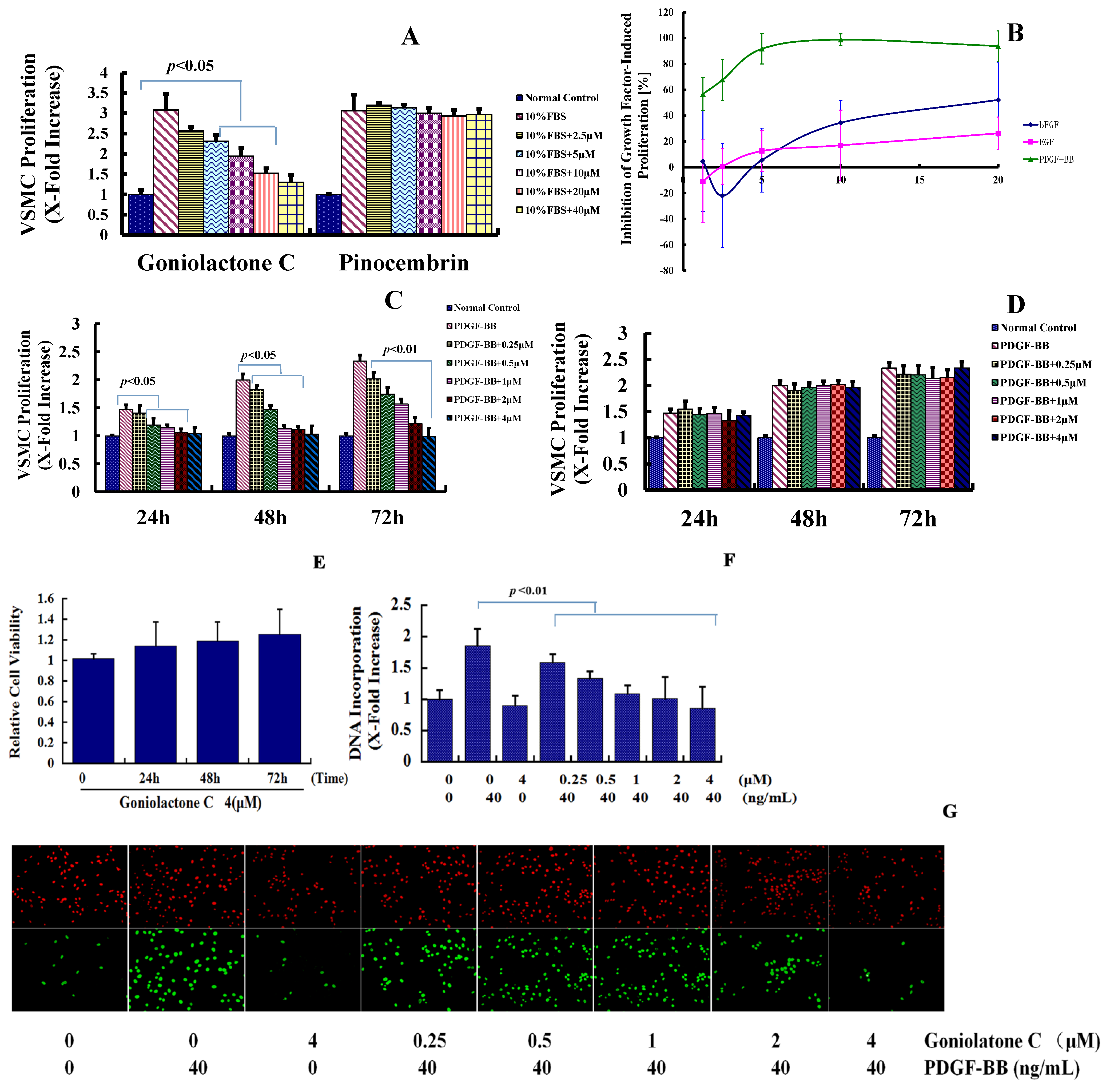
2.1. Goniolactone C Specifically Reduces PDGF-BB-Induced VSMC Proliferation and Migration
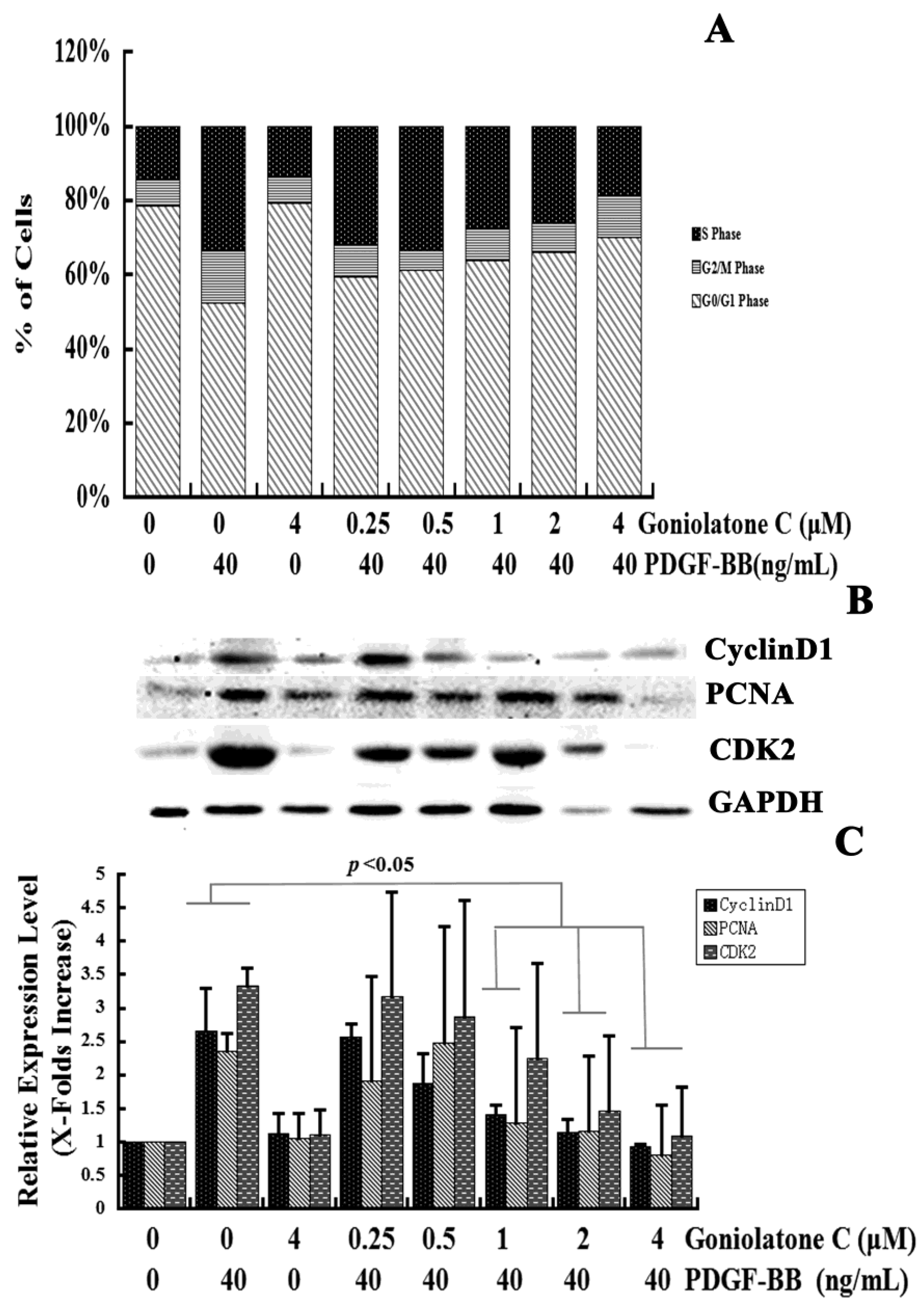
2.2. Goniolactone C-Mediated Inhibition of VSMC Proliferation Is Associated with Cell Cycle Arrest
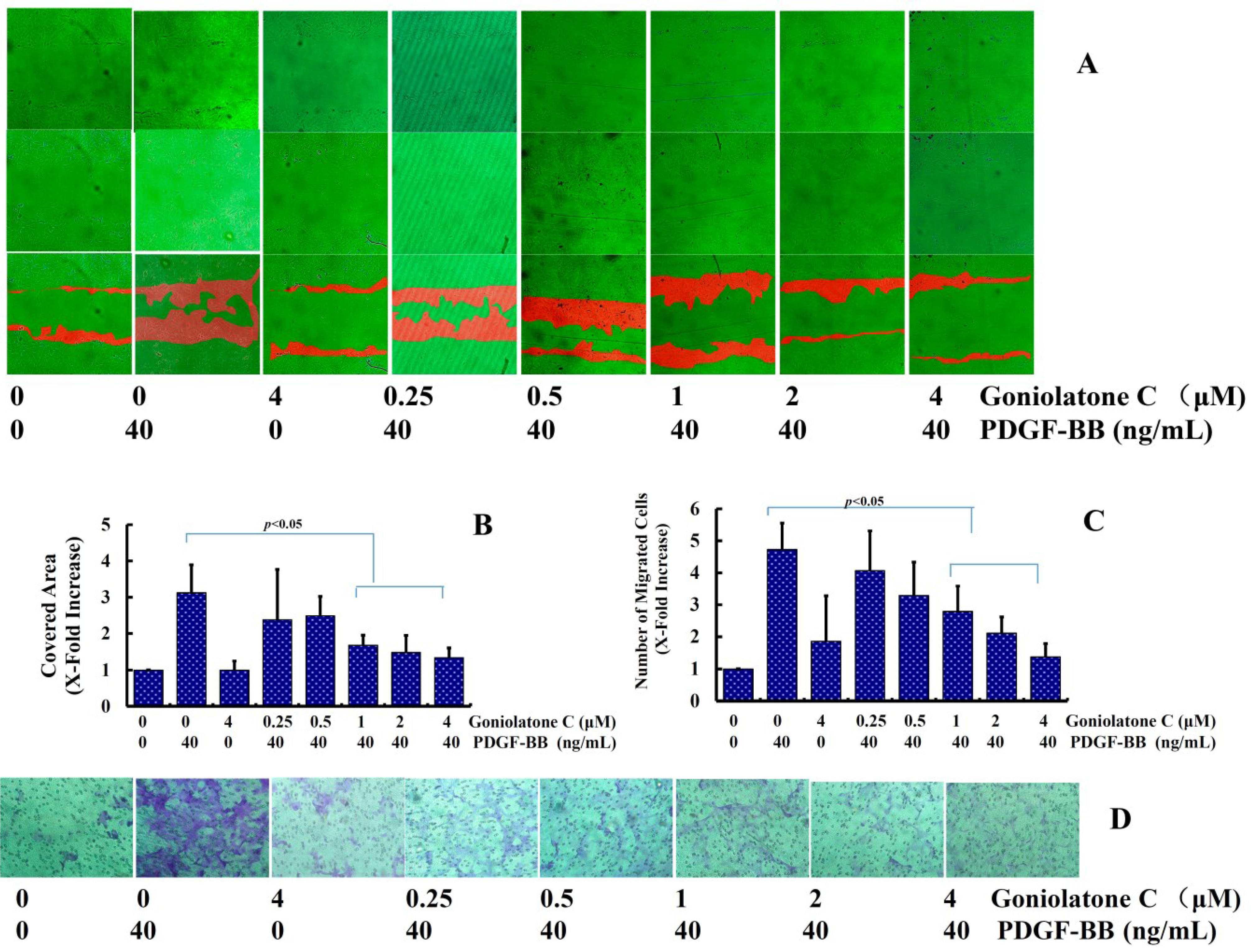
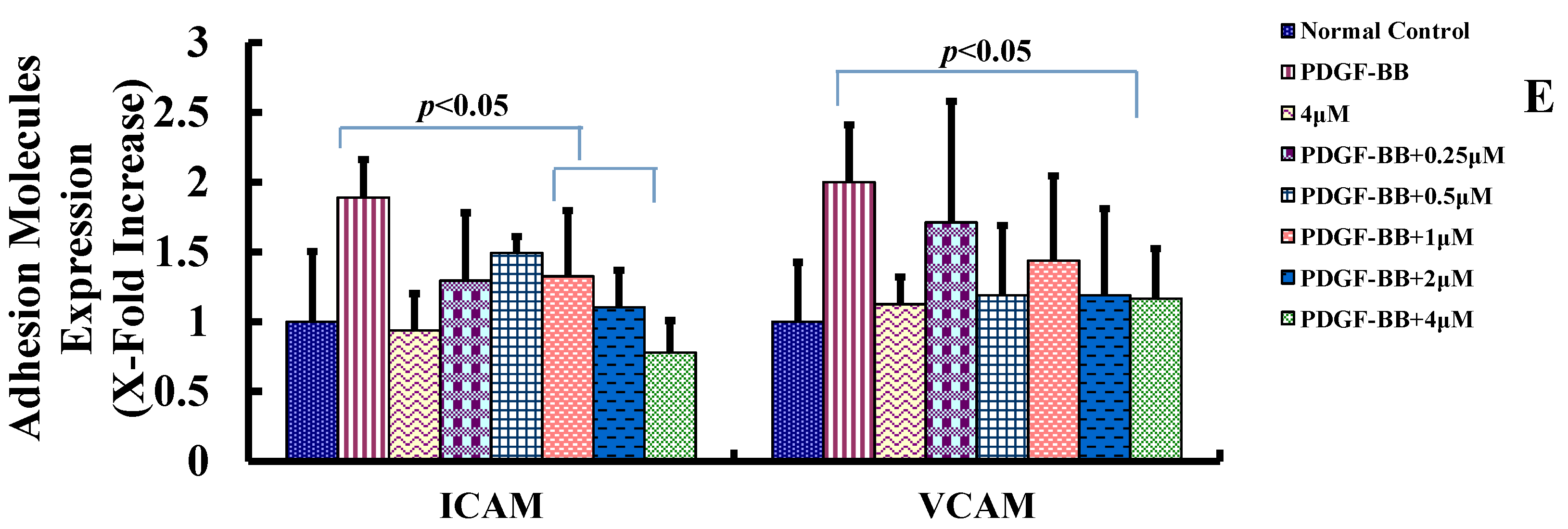
2.3. Goniolactone C Inhibits PDGF-BB-Induced VSMC Migration and Adhesion Molecule Expression
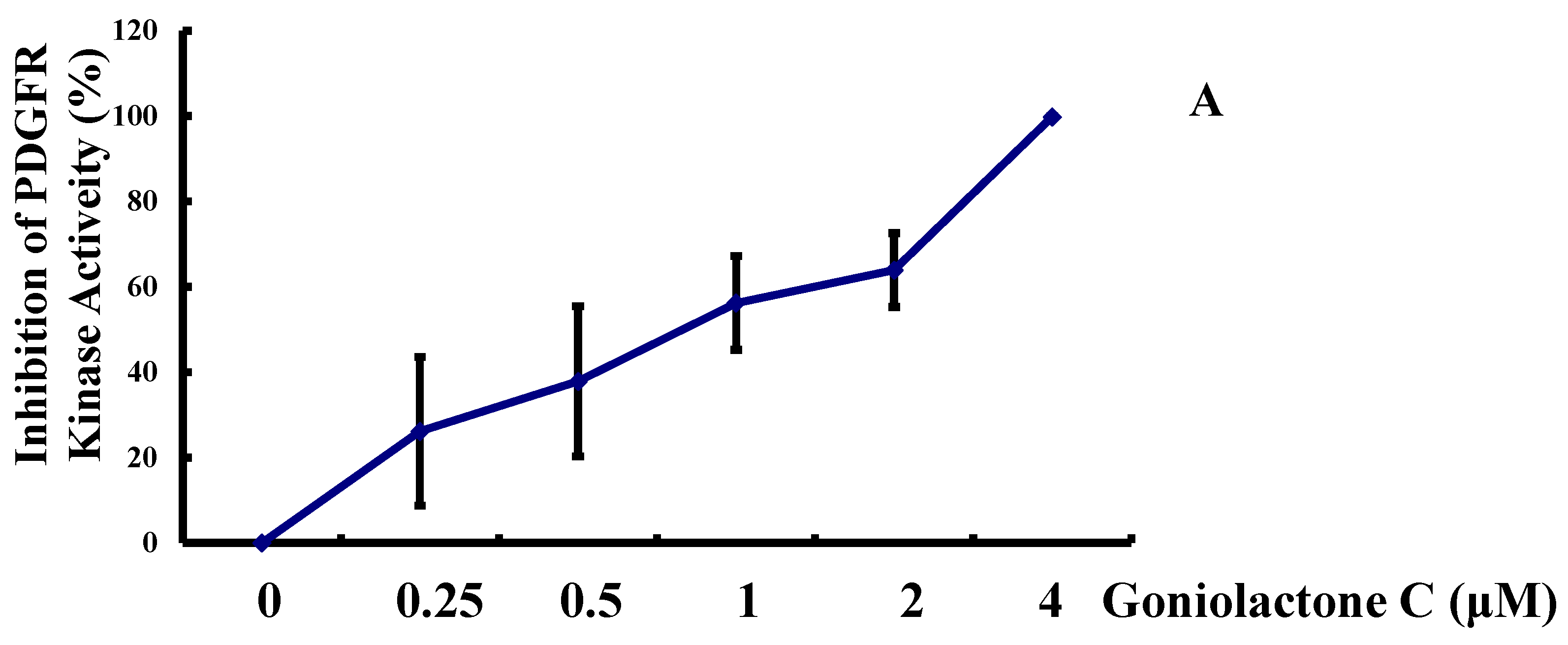
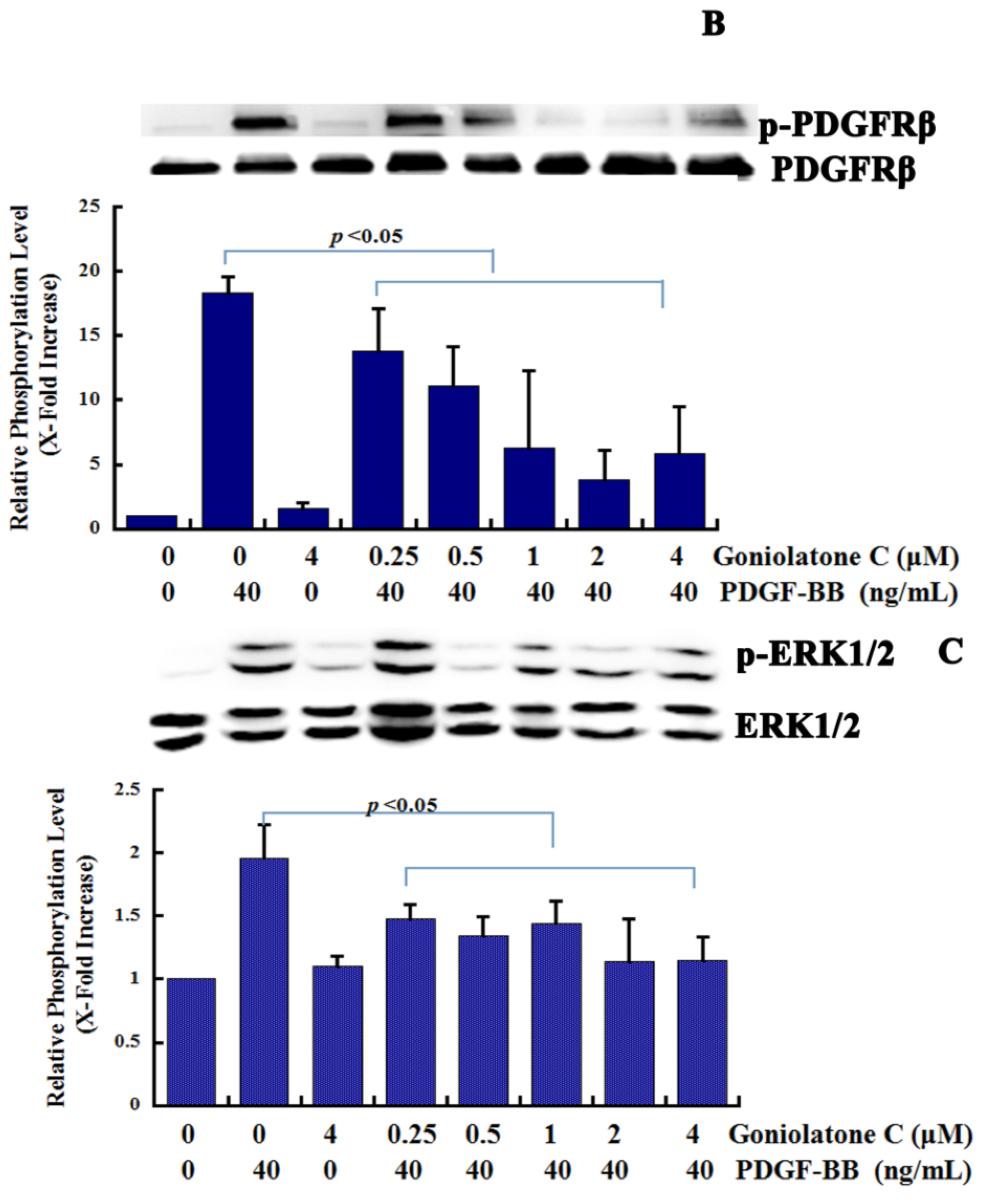
2.4. Goniolactone C Inhibits the PDGFR-β/ERK1/2 Cell Signaling Cascade
3. Experimental Section
3.1. General Information
3.2. Antibodies and Major Reagents
3.3. Proliration Assay
3.3.1. Serum-Induced VSMC Proliferation Assay
3.3.2. Growth Factor-Induced VSMC Proliferation Assay
3.3.3. BrdU Incorporation Assay
3.4. Kinase Activity Assays
3.5. Migration Assays
3.5.1. Wound-Healing Assay
3.5.2. Modified Boyden Chamber Assay
3.6. Western Blot Analyses
3.7. Cell Cycle Progression Analyses
3.8. Assessment of ICAM-1 and VCAM-1 Production
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orozco, L.D.; Liu, H.; Perkins, E.; Johnson, D.A.; Chen, B.B.; Fan, F.; Baker, R.C.; Roman, R.J. 20-Hydroxyeicosatetraenoic acid inhibition attenuates balloon injury-induced neointima formation and vascular remodeling in rat carotid arteries. J. Pharmacol. Exp. Ther. 2013, 346, 67–74. [Google Scholar]
- Sun, L.; Zhao, R.; Zhang, L.; Zhang, T.; Xin, W.; Lan, X.; Huang, C.; Du, G. Salvianolic acid A inhibits PDGF-BB induced vascular smooth muscle cell migration and proliferation while does not constrain endothelial cell proliferation and nitric oxide biosynthesis. Molecules 2012, 17, 3333–3347. [Google Scholar]
- Lannoy, M.; Slove, S.; Louedec, L.; Choqueux, C.; Journé, C.; Michel, J.B.; Jacob, M.P. Inhibition of ERK1/2 Phosphorylation: A New Strategy to Stimulate Elastogenesis in the Aorta. Hypertension 2014, 64, 423–430. [Google Scholar]
- Bruder, M.; Vendramini-Costa, D.B.; de Carvalho, J.E.; Pilli, R.A. Design, synthesis and in vitro evaluation against human cancer cells of 5-methyl-5-styryl-2,5-dihydrofuran-2-ones, a new series of goniothalamin analogues. Bioorg. Med. Chem. 2013, 21, 5107–5117. [Google Scholar]
- Wang, S.; Zhang, Y.J.; Chen, R.Y.; Yu, D.Q. Goniolactones A-F, six new styrylpyrone derivatives from the roots of Goniothalamus cheliensis. J. Nat. Prod. 2002, 65, 835–841. [Google Scholar]
- Popsavin, V.; Kovačević, I.; Benedeković, G.; Popsavin, M.; Kojić, V.; Bogdanović, G. Divergent synthesis of cytotoxic styryl lactones related to goniobutenolides A and B, and to crassalactone D. Org. Lett. 2012, 14, 5956–5959. [Google Scholar]
- Moharam, B.A.; Jantan, I.; Jalil, J.; Ahmad, F. Inhibitory effect of compounds from Goniothalamus tapis Miq and Goniothalamus uvaroides King on platelet-activating factor receptor binding. Phytother. Res. 2012, 26, 687–691. [Google Scholar]
- Fürst, R.; Zirrgiebel, U.; Totzke, F.; Zahler, S.; Vollmar, A.M.; Koch, E. The Crataegus extract WS 1,442 inhibits balloon catheter-induced intimal hyperplasia in the rat carotid artery by directly influencing PDGFR-beta. Atherosclerosis 2010, 211, 409–417. [Google Scholar]
- Jiang, L.P.; Lu, Y.; Nie, B.M.; Chen, H.Z. Antiproliferative effect of panaxynol on RASMCs via inhibition of ERK1/2 and CREB. Chem. Biol. Interact. 2008, 171, 348–354. [Google Scholar]
- Park, E.S.; Yoo, J.M.; Lim, Y.; Tudev, M.; Yoo, H.S.; Hong, J.T.; Yun, Y.P. Inhibitory effects of docetaxel on platelet-derived growth factor (PDGF)-BB-induced proliferation of vascular smooth muscle cells through blocking PDGF-receptor β phosphorylation. J. Pharmacol. Sci. 2011, 116, 204–213. [Google Scholar]
- Sachinidis, A.; Locher, R.; Vetter, W.; Tatje, D.; Hoppe, J. Different effects of platelet-derived growth factor isoforms on rat vascular smooth muscle cells. J. Biol. Chem. 1990, 265, 10238–10243. [Google Scholar]
- Yang, X.; Thomas, D.P.; Zhang, X.; Culver, B.W.; Alexander, B.M.; Murdoch, W.J.; Rao, M.N.; Tulis, D.A.; Ren, J.; Sreejayan, N. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 85–90. [Google Scholar]
- Wiart, C. Goniothalamus species: A source of drugs for the treatment of cancers and bacterial infections? Evid. Based Complement. Alternat. Med. 2007, 4, 299–311. [Google Scholar]
- Sun, L.; Zhang, T.; Yu, X.; Xin, W.; Lan, X.; Zhang, D.; Huang, C.; Du, G. Asymmetric dimethylarginine confers the communication between endothelial and smooth muscle cells and leads to VSMC migration through p38 and ERK1/2 signaling cascade. FEBS Lett. 2011, 585, 2727–2734. [Google Scholar]
- Sample Availability: Samples of the compounds Goniolactone C and pinocembrin are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Zhao, R.; Lan, X.; Chen, R.; Wang, S.; Du, G. Goniolactone C, a Styryl Lactone Derivative, Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Migration and Proliferation via PDGFR/ERK Signaling. Molecules 2014, 19, 19501-19515. https://doi.org/10.3390/molecules191219501
Sun L, Zhao R, Lan X, Chen R, Wang S, Du G. Goniolactone C, a Styryl Lactone Derivative, Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Migration and Proliferation via PDGFR/ERK Signaling. Molecules. 2014; 19(12):19501-19515. https://doi.org/10.3390/molecules191219501
Chicago/Turabian StyleSun, Lan, Rui Zhao, Xi Lan, Ruoyun Chen, Si Wang, and Guanhua Du. 2014. "Goniolactone C, a Styryl Lactone Derivative, Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Migration and Proliferation via PDGFR/ERK Signaling" Molecules 19, no. 12: 19501-19515. https://doi.org/10.3390/molecules191219501
APA StyleSun, L., Zhao, R., Lan, X., Chen, R., Wang, S., & Du, G. (2014). Goniolactone C, a Styryl Lactone Derivative, Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Migration and Proliferation via PDGFR/ERK Signaling. Molecules, 19(12), 19501-19515. https://doi.org/10.3390/molecules191219501




