Design, Synthesis, and Biological Evaluation of Artemisinin-Indoloquinoline Hybrids as Potent Antiproliferative Agents
Abstract
:1. Introduction
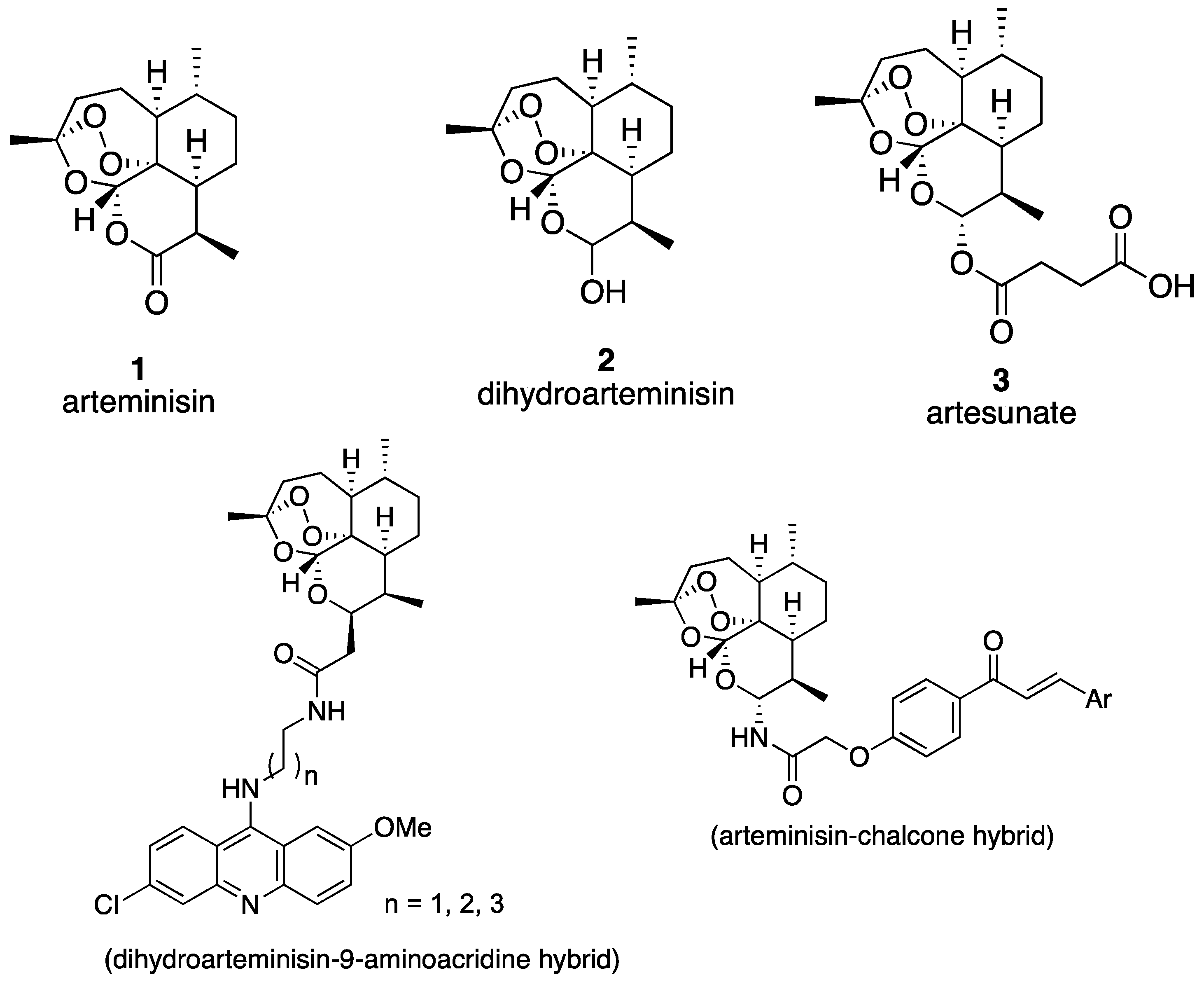
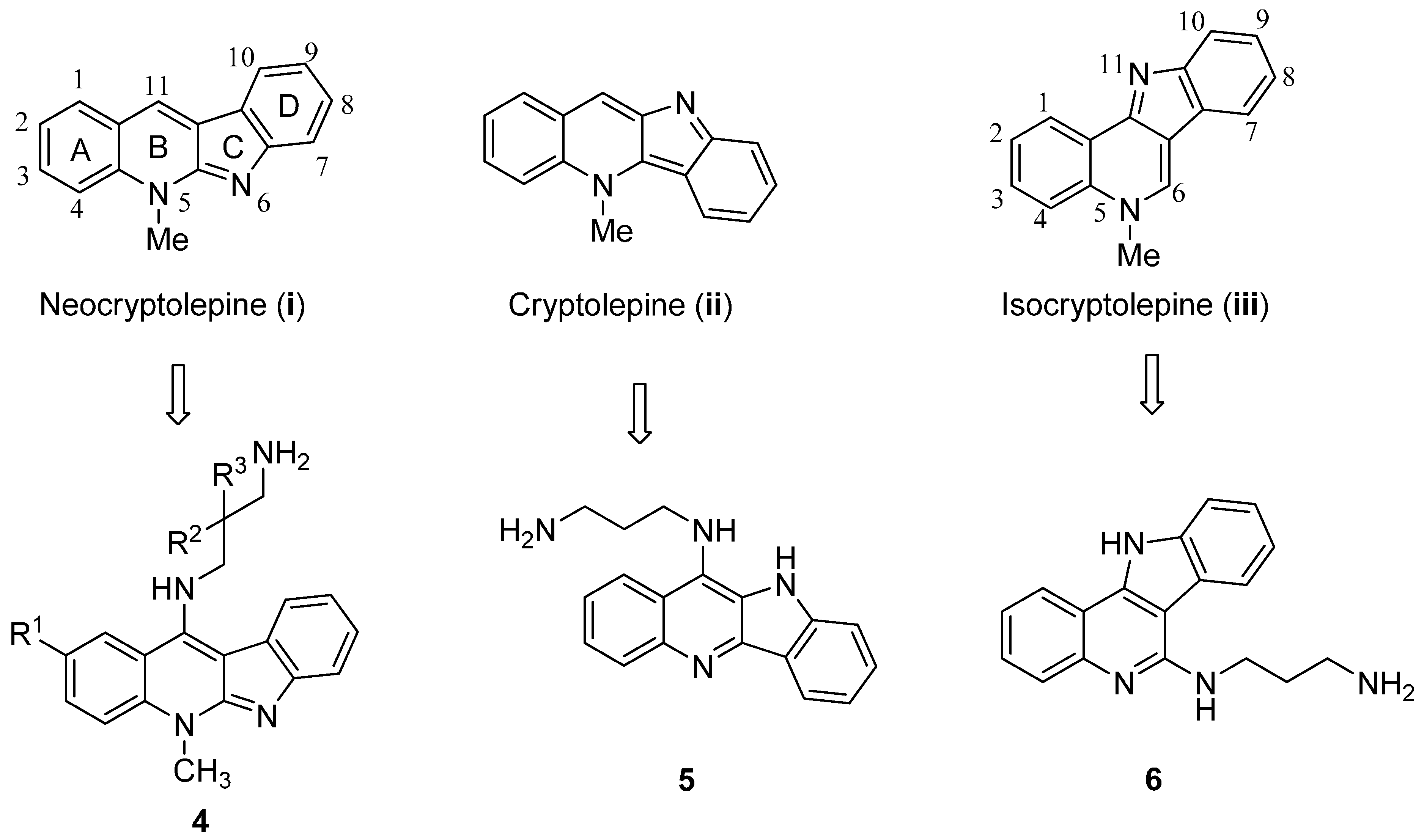
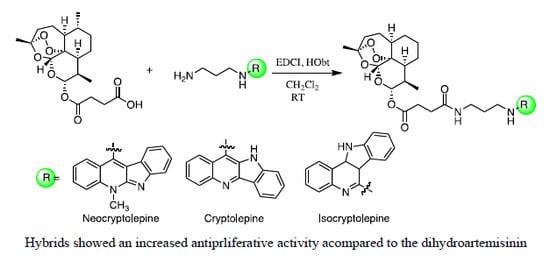
2. Results and Discussion
2.1. Chemistry
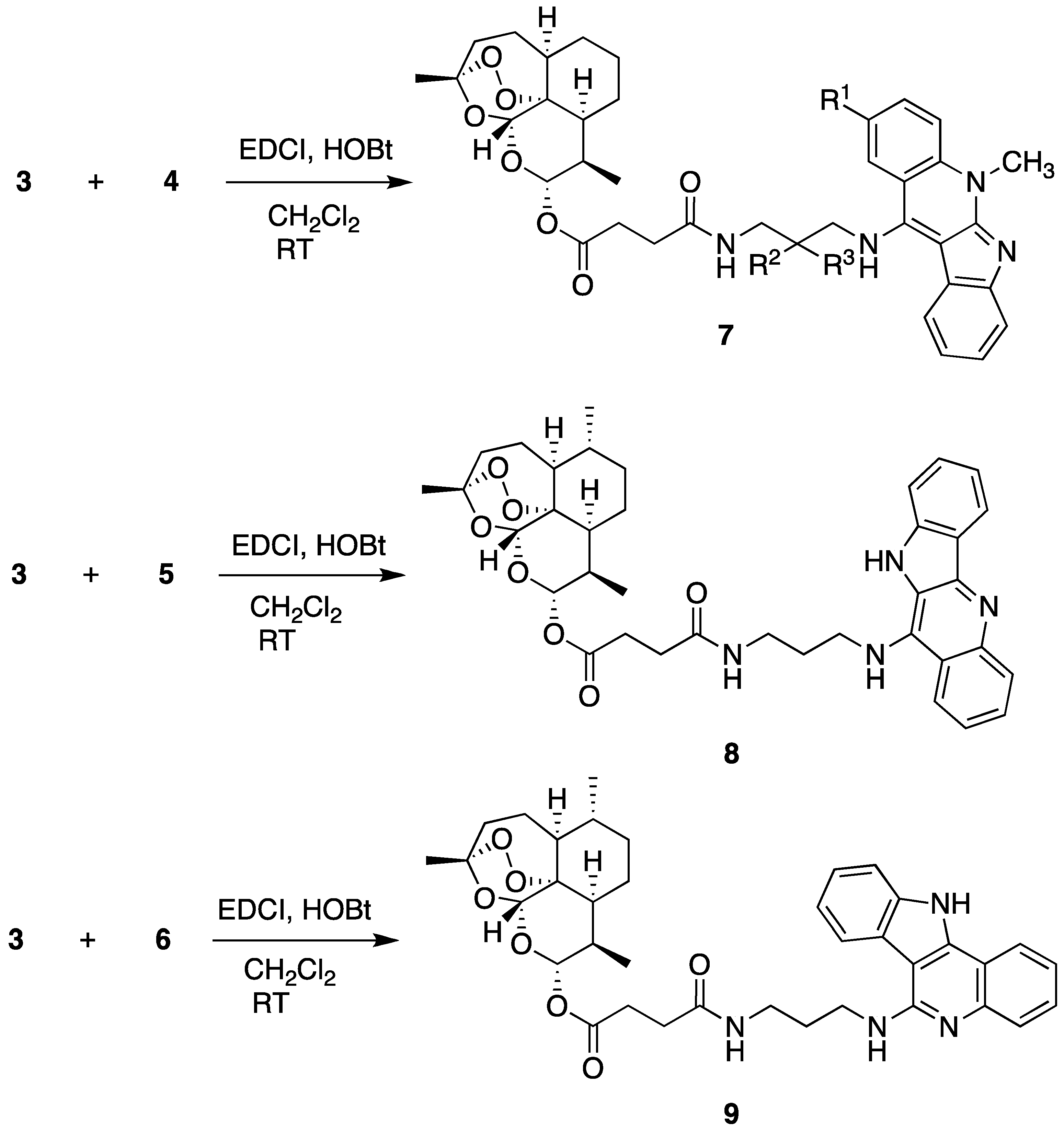
2.2. Biological Evaluation
| Compound | R1 | R2 | R3 | MV4-11 a IC50 (μM) |
|---|---|---|---|---|
| cisplatin | 2.820 ± 0.450 | |||
| doxorubicin HCl | 0.006 ± 0.002 | |||
| 7a | H | H | H | 0.286 ± 0.079 |
| 7b | Cl | CH3 | CH3 | 0.072 ± 0.022 |
| 7c | Br | CH3 | CH3 | 0.242 ± 0.031 |
| 7d | CO2Me | H | H | 0.148 ± 0.015 |
| 7e | CO2Me | CH3 | CH3 | 0.075 ± 0.001 |
| 7f | CO2Me | H | OH | 0.226 ± 0.019 |
| 8 | 0.286 ± 0.127 | |||
| 4a b | H | H | H | 0.066 ± 0.023 |
| 4d c | CO2Me | H | H | 0.086 ± 0.020 |
| 6 d | 0.124 ± 0.010 |
| Compound | BALB/3T3 a IC50 (μM) | A549 b IC50 (μM) | HCT116 c IC50 (μM) |
|---|---|---|---|
| cisplatin | 9.498 ± 0.500 | 8.965 ± 3.333 | 9.465 ± 1.300 |
| 7b | 6.423 ± 0.996 | 4.555 ± 2.086 | 0.893 ± 0.397 |
| 7d | 4.953 ± 0.220 | 3.663 ± 0.535 | 1.756 ± 0.329 |
| 7e | 5.945 ± 1.163 | 5.060 ± 0.911 | 2.206 ± 0.687 |
| 7f | 4.914 ± 0.430 | 4.444 ± 0.685 | 0.832 ± 0.216 |
| 8 | 2.725 ± 0.731 | 1.328 ± 0.586 | 0.557 ± 0.085 |
| 4d | 0.768 ± 0.155 | 0.649 ± 0.080 | 0.130 ± 0.014 |
| 6 d | 1.047 ± 0.127 | 0.172 ± 0.052 | 0.258 ± 0.107 |
| DHA (2) | - | >20 e | 1.34 ± 1.06 e |
3. Experimental Section
3.1. General Methods
3.1.1. General Procedure for the Synthesis of Artesunate-Indoloquinoline Hybrids 7
3.1.2. Physical Data for Compounds 7
3.1.3. Procedure for the Synthesis of Artesunate-Indoloquinoline Hybrid 8
3.1.4. Physical Data for Compound 8
3.2. Cell Line
3.3. Antiproliferative Assay in Vitro
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Poespoprodjo, J.R.; Fobia, W.; Kenangalem, E.; Lampah, D.A.; Sugiarto, P.; Tjitra, E.; Anstey, N.M.; Price, R.N. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. PLoS One 2014, 9, e84976. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.D.; Shen, C.C. The chemistry, pharmacology, and clinical applications of qinghaosu (Artemisinin) and its derivatives. Med. Res. Rev. 1987, 7, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.; Gallis, B.; Solazzi, J.W.; Kim, B.J.; Gulati, R.; Vakar-Lopez, F.; Goodlett, D.R.; Vessella, R.L.; Sasaki, T. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs 2010, 21, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr. Drug Targets 2006, 7, 407–421. [Google Scholar] [PubMed]
- Chen, T.; Li, M.; Zhang, R.W.; Wang, H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell. Mol. Med. 2009, 13, 1358–1370. [Google Scholar] [PubMed]
- Xie, L.J.; Zhai, X.; Liu, C.; Li, P.; Li, Y.X.; Guo, G.X.; Gong, P. Anti-tumor activity of new artemisinin–chalcone hybrids. Arch. Pharm. 2011, 344, 639–647. [Google Scholar] [CrossRef]
- Lee, S. Artemisinin, promising lead natural product for various drug developments. Mini Rev. Med. Chem. 2007, 7, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Soomro, S.; Langenberg, T.; Mahringer, A.; Konkimalla, V.B.; Horwedel, C.; Holenya, P.; Brand, A.; Cetin, C.; Fricker, G.; Dewerchin, M.; et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell. Mol. Med. 2011, 15, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ding, J.; Yang, C.; Gao, Y.; Li, X.; Chen, X.; Peng, Y.; Fang, J.; Xiao, S. Immunomodulatory and anti-inflammatory properties of artesunate in experimental colitis. Curr. Med. Chem. 2012, 19, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Yu, Y.; Ma, J.; Zhang, H.R.; Zhang, H.; Wang, X.Q.; Wang, J.C.; Zhang, X.; Zhang, Q. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, B.; Wang, S.; Pan, S.; Gao, Y.; Bai, X.; Xue, D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: Involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J. Cancer Res. Clin. Oncol. 2010, 136, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Marques, O.; da Silva, B.M.; Porto, G.; Lopes, C. Iron homeostasis in breast cancer. Cancer Lett. 2014, 28, 1–14. [Google Scholar] [CrossRef]
- Rahier, N.J. Camptothecin and Its Analogs. In Anticancer Agents from Natural Products, 2nd ed.; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC press: Boca Raton, FL, USA, 2012; pp. 5–25. [Google Scholar]
- Arcamone, F. Doxorubicin. In Anticancer Antibiotics; De Stevens, G., Ed.; Academic Press: New York, NY, USA, 1981; pp. 1–369. [Google Scholar]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Invest. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Buragohain, P.; Saikia, B.; Surinenia, N.; Barua, N.C.; Saxena, A.K.; Suri, N. Synthesis of a novel series of artemisinin dimers with potent anticancer activity involving Sonogashira cross-coupling reaction. Bioorg. Med. Chem. Lett. 2014, 24, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.M.; Wang, X.F.; Zhao, X.M.; Ren, T.R.; Wang, F.; Yu, B. Enhanced delivery of artemisinin and its analogues to cancer cells by their adducts with human serum transferrin. Int. J. Pharm. 2014, 467, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.G.; Fernandez-Dolon, M.; Sanchez-Vicente, L.; Maestre, A.D.; Gomez-San Miguel, A.B.; Alvarez, M.; Serrano, M.A.; Jansenc, H.; Efferth, T.; Marin, J.J.G.; et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg. Med. Chem. 2013, 21, 4432–4441. [Google Scholar] [PubMed]
- Xie, L.J.; Zhai, X.; Ren, L.X.; Meng, H.Y.; Liu, C.; Zhu, W.F.; Zhao, Y.F. Design, synthesis and antitumor activity of novel artemisinin derivatives using hybrid approach. Chem. Pharm. Bull. 2011, 59, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mercer, A.E.; Stocks, P.A.; la Pensée, L.J.I.; Cosstick, R.; Park, B.K.; Kennedy, M.E.; Piantanida, I.; Ward, S.A.; Davies, J.; et al. Antitumour and antimalarial activity of artemisinin-acridine hybrids. Bioorg. Med. Chem. Lett. 2009, 19, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, W.; Tan, J.; Song, D.; Li, M.; Liu, D.; Jing, Y.; Zhao, L. Synthesis of a series of novel dihydroartemisinin derivatives containing a substituted chalcone with greater cytotoxic effects in leukemia cells. Bioorg. Med. Chem. Lett. 2009, 19, 4385–4388. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; de Bruyne, T.; Pieters, L.; Claeys, M.; Vlietinck, A. New alkaloids from Cryptolepis. sanguinolenta. Tetrahedron Lett. 1996, 37, 1703–1706. [Google Scholar] [CrossRef]
- Paulo, A.; Gomes, E.T.; Steele, J.; Warhurst, D.C.; Houghton, P.J. Antiplasmodial activity of Cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Med. 2000, 66, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Bruyne, T.D.; Pieters, L.; Vlietinck, A.J.; Turger, C.A. In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 1997, 60, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. Isolation, biological activities, and synthesis of indoloquinoline alkaloids: Cryptolepine, isocryptolepine, and neocryptolepine. Curr. Org. Chem. 2011, 15, 1036–1057. [Google Scholar]
- Alexandra, P.; Elsa, T.G.; Jonathan, S.; Dave, C.W.; Peter, J.H. Antiplasmodial activity of cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Medica. 2000, 66, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, E.V.K.S.; Etukala, J.R.; Ablordeppey, S.Y. Indolo[3,2-b]quinolines: Synthesis, biological evaluation and structure activity-relationships. Mini-Rev. Med. Chem. 2008, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Lavrado, J.; Moreira, R.; Paulo, A. Indoloquinolines as scaffolds for drug discovery. Curr. Med. Chem. 2010, 17, 2348–2370. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, Md R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinolone. Eur. J. Med. Chem. 2014, in press. [Google Scholar]
- Cimanga, K.; de Bruyne, T.; Lasure, A.; van Poel, B.; Pieters, L.; Claeys, M.; Vanden, B.D.; Kambu, K.; Tona, L.; Vlietinck, A.J. In vitro biological activities of alkaloids from cryptolepis sanguinolenta. Planta Med. 1996, 62, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.T.; de Bruyne, T.; Pieters, L.; Totte, J.; Tona, L.; Kambu, K.; Vanden Berghe, D.; Vlietinck, A.J. Antibacterial and antifungal activities of neocryptolepine, biscryptolepine, and cryptoquindoline, alkaloids isolated from cryptolepis sanguinolenta. Phytomedicine 1998, 5, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Guittat, L.; Alberti, P.; Rosu, F.; van Miert, S.; Thetiot, E.; Pieters, L.; Gabelica, V.; de Pauw, E.; Ottaviani, A.; Riou, J.F.; et al. Interactions of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie 2003, 85, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, Y.Y.; Wang, J.W.; Zhu, Y.M. Cytotoxic Activities and DNA Binding Properties of 1-Methyl-7H-indeno[1,2-b]Quinolinium-7-(4-dimethylamino) Benzylidene Triflate. DNA Cell. Biol. 2012, 31, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Riechert-Krause, F.; Autenrieth, K.; Eick, A.; Weisz, K. Spectroscopic and calorimetric studies on the binding of an indoloquinoline drug to parallel and antiparallel DNA triplexes. Biochemistry 2013, 52, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Laine, W.; Baldeyrou, B.; de Pauw-Gillet, M.C.; Colson, P.; Houssier, C.; Cimanga, K.; van Miert, S.; Vlietinck, A.J.; Pieters, L. DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti. Cancer Drug Design 2000, 15, 191–201. [Google Scholar] [PubMed]
- Sidoryk, K.; Świtalska, M.; Wietrzyk, J.; Jaromin, A.; Pietka-Ottlik, M.; Cmoch, P.; Zagrodzka, J.; Szczepek, W.; Kaczmarek, Ł.; Peczyńska-Czoch, W. Synthesis and biological evaluation of new amino acid and dipeptide derivatives of neocryptolepine as anticancer agents. J. Med. Chem. 2012, 55, 5077–5087. [Google Scholar] [CrossRef] [PubMed]
- Seville, S.; Phillips, R.M.; Shnyder, S.D.; Wright, C.W. Synthesis of cryptolepine analogues as potential bioreducible anticancer agents. Bioorg. Med. Chem. 2007, 15, 6353–6360. [Google Scholar] [CrossRef] [PubMed]
- Dhanabal, T.; Sangeetha, R.; Mohan, P.S. Structure–activity relationship of antiparasitic and cytotoxic indoloquinoline alkaloids, and their tricyclic and bicyclic analogues. Bioorg. Med. Chem. 2009, 17, 7209–7217. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.M.; Yang, S.H.; Lin, K.L.; Chen, Y.L.; Chang, L.S.; Lin, S.R. Novel indoloquinoline derivative, IQDMA, suppresses STAT5 phosphorylation and induces apoptosis in HL-60 cells. Chem. Biol. Interact. 2008, 176, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Beauchard, A.; Jaunet, A.; Murillo, L.; Baldeyrou, B.; Lansiaux, A.; Chérouvrier, J.R.; Domon, L.; Picot, L.; Bailly, C.; Besson, T.; et al. Synthesis and antitumoral activity of novel thiazolobenzotriazole, thiazoloindolo[3,2-c]quinoline and quinolinoquinoline derivatives. Eur. J. Med. Chem. 2009, 44, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Dassonneville, L.; Lansiaux, A.; Wattelet, A.; Wattez, N.; Mahieu, C.; van Miert, S.; Pieters, L.; Bailly, C. Cytotoxicity and cell cycle effects of the plant alkaloids cryptolepine and neocryptolepine: Relation to drug-induced apoptosis. Eur. J. Pharm. 2000, 409, 9–18. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Edward, J.A.; Świtalska, M.; Stefańska, J.; Cmoch, P.; Zagrodzka, J.; Szczepek, W.; Peczyńska-Czoch, W.; Wietrzyk, J.; et al. Searching for new derivatives of neocryptolepine: Synthesis, antiproliferative, antimicrobial and antifungal activities. Eur. J. Med. Chem. 2014, 78, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, L.; Peczynska-Czoch, W.; Osiadacz, J.; Mordarski, M.; Sokalski, W.A.; Marcinkowska, E.; Glazman-Kusnierczyk, H.; Radzikowski, C. Synthesis, and cytotoxic activity of some novel indolo[2,3-b]quinoline derivatives: DNA topoisomerase II inhibitors. Bioorg. Med. Chem. 1999, 7, 2457–2464. [Google Scholar]
- Osiadacz, J.; Majka, J.; Czarnecki, K.; Kaczmarek, L.; Sokalski, W.A. Sequence-selectivity of 5,11-dimethyl-5H-indolo[2,3-b]quinoline binding to DNA. Footprinting and molecular modeling studies. Bioorg. Med. Chem. 2000, 8, 937–943. [Google Scholar]
- Godlewska, J.; Luniewski, W.; Zagrodzki, B.; Kaczmarek, L.; Bielawska-Pohl, A.; Dus, D.; Wietrzyk, J.; Opolski, A.; Siwko, M.; Jaromin, A.; et al. Biological evaluation of ω-(dialkylamino)alkyl derivatives of 6H-indolo[2,3-b]quinoline-novel cytotoxic DNA topoisomerase II inhibitors. Anticancer Res. 2005, 25, 2857–2868. [Google Scholar] [PubMed]
- Filak, L.K.; Goschl, S.; Heffeter, P.; Samper, K.G.; Egger, A.E.; Jakupec, M.A.; Keppler, B.K.; Berger, W.; Arion, V.B. Metal-arene complexes with indolo[3,2-c]-quinolines: Effects of ruthenium vs. osmium and modifications of the lactam unit on intermolecular interactions, anticancer activity, cell cycle, and cellular accumulation. Organometallics 2013, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Filak, L.K.; Goschl, S.; Hackl, S.; Jakupec, M.A.; Arion, V.B. Ruthenium- and osmium-arene complexes of 8-substituted indolo[3,2-c]quinolines: Synthesis, X-ray diffraction structures, spectroscopic properties, and antiproliferative activity. Inorg. Chim. Acta 2012, 393, 252–260. [Google Scholar] [CrossRef]
- Primik, M.F.; Goschl, S.; Jakupec, M.A.; Roller, A.; Keppler, B.K.; Arion, V.B. Structure-activity relationships of highly cytotoxic copper(II) complexes with modified indolo[3,2-c]quinoline ligands. Inorg. Chem. 2010, 49, 11084–11095. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Świtalska, M.; Mei, Z.W.; Lu, W.J.; Takahara, Y.; Feng, X.W.; El-Sayed, I.E.; Wietrzyk, J.; Inokuchi, T. Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure-activity relationships of neocryptolepines and 6-methyl congeners. Bioorg. Med. Chem. 2012, 20, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Świtalska, M.; Wang, L.; Yonezawa, M.; El-Sayed, I.E.; Wietrzyk, J.; Inokuchi, T. In vitro antiproliferative activity of 11-aminoalkylaminosubstituted 5H-indolo[2,3-b]quinolines; improving activity of neocryptolepines by installation of ester substituent. Med. Chem. Res. 2013, 22, 4492–4504. [Google Scholar] [CrossRef]
- Wang, N.; Świtalska, M.; Wu, M.Y.; Imai, K.; Ngoc, T.A.; Pang, C.Q.; Wang, L.; Wietrzyk, J.; Inokuchi, T. Synthesis and in vitro cytotoxic effect of 6-amino-substituted 11H- and 11Me-indolo[3,2-c]quinolones. Eur. J. Med. Chem. 2014, 78, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Lavrado, J.; Cabal, G.G.; Prudencio, M.; Mota, M.M.; Gut, J.; Rosenthal, P.J.; Diaz, C.; Guedes, R.C.; dos Santos, D.J.V.A.; Bichenkova, E.; et al. Incorporation of basic side chains into cryptolepine scaffold: Structure-antimalarial activity relationships and mechanistic studies. J. Med. Chem. 2011, 54, 734–750. [Google Scholar] [CrossRef] [PubMed]
- IC50 values of the compound 9 against the MV4–11 cell line: 0.128 ± 0.036 μM), BALB/3T3 cell line: 4.979 ± 0.471μM, A549 cell line: 1.806 ± 0.516 μM, HCT116 cell line: 0.855 ± 0.235 μM
- Lu, J.J.; Meng, L.H.; Cai, Y.J.; Chen, Q.; Tong, L.J.; Lin, L.P.; Ding, J. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol. Ther. 2008, 7, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 7a–7f are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Świtalska, M.; Wang, N.; Du, Z.-J.; Fukumoto, Y.; Diep, N.K.; Kiguchi, R.; Nokami, J.; Wietrzyk, J.; Inokuchi, T. Design, Synthesis, and Biological Evaluation of Artemisinin-Indoloquinoline Hybrids as Potent Antiproliferative Agents. Molecules 2014, 19, 19021-19035. https://doi.org/10.3390/molecules191119021
Wang L, Świtalska M, Wang N, Du Z-J, Fukumoto Y, Diep NK, Kiguchi R, Nokami J, Wietrzyk J, Inokuchi T. Design, Synthesis, and Biological Evaluation of Artemisinin-Indoloquinoline Hybrids as Potent Antiproliferative Agents. Molecules. 2014; 19(11):19021-19035. https://doi.org/10.3390/molecules191119021
Chicago/Turabian StyleWang, Li, Marta Świtalska, Ning Wang, Zhen-Jun Du, Yuta Fukumoto, Nguyen Kim Diep, Ryo Kiguchi, Junzo Nokami, Joanna Wietrzyk, and Tsutomu Inokuchi. 2014. "Design, Synthesis, and Biological Evaluation of Artemisinin-Indoloquinoline Hybrids as Potent Antiproliferative Agents" Molecules 19, no. 11: 19021-19035. https://doi.org/10.3390/molecules191119021
APA StyleWang, L., Świtalska, M., Wang, N., Du, Z.-J., Fukumoto, Y., Diep, N. K., Kiguchi, R., Nokami, J., Wietrzyk, J., & Inokuchi, T. (2014). Design, Synthesis, and Biological Evaluation of Artemisinin-Indoloquinoline Hybrids as Potent Antiproliferative Agents. Molecules, 19(11), 19021-19035. https://doi.org/10.3390/molecules191119021