Study on the Cytotoxic Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
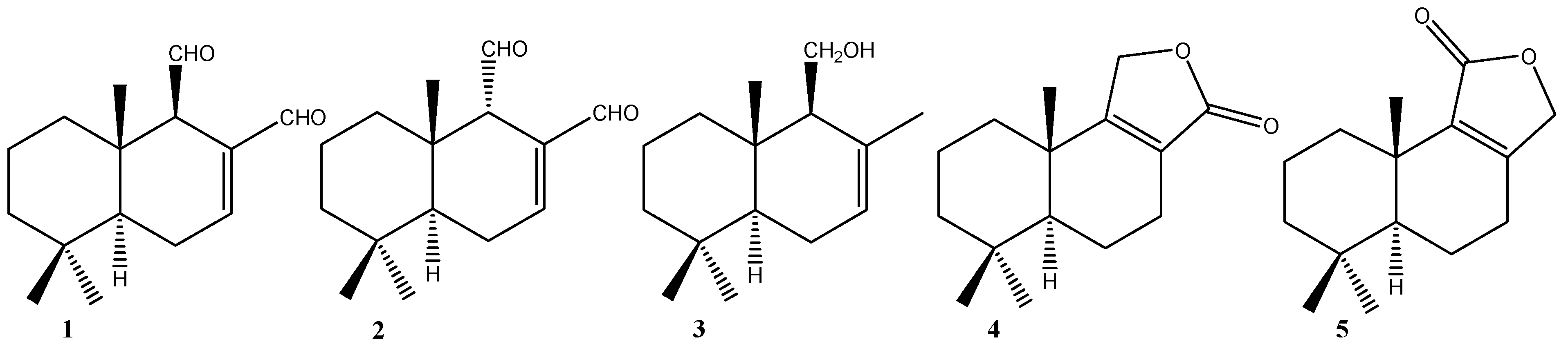
2.1.1. Compounds Obtained from 1
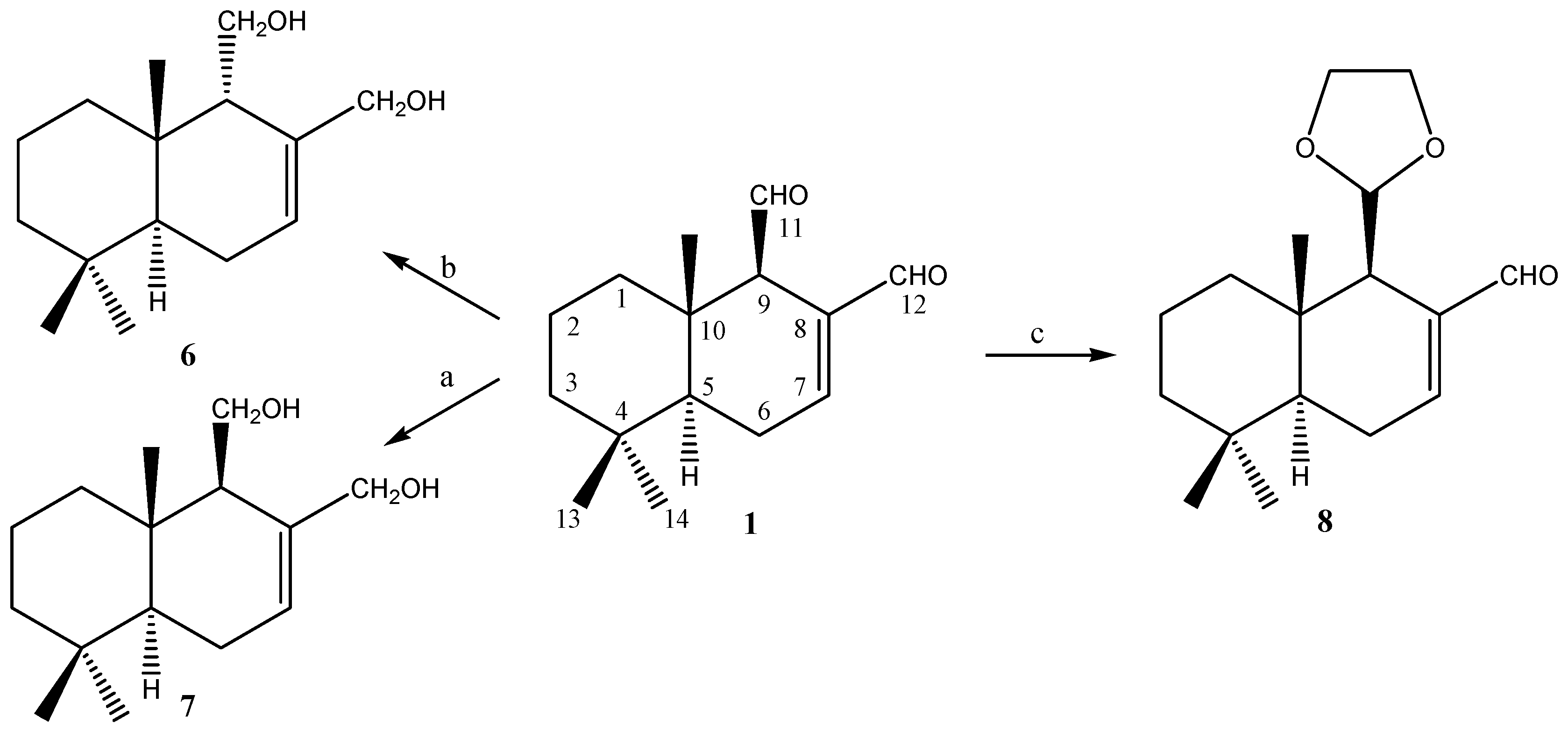
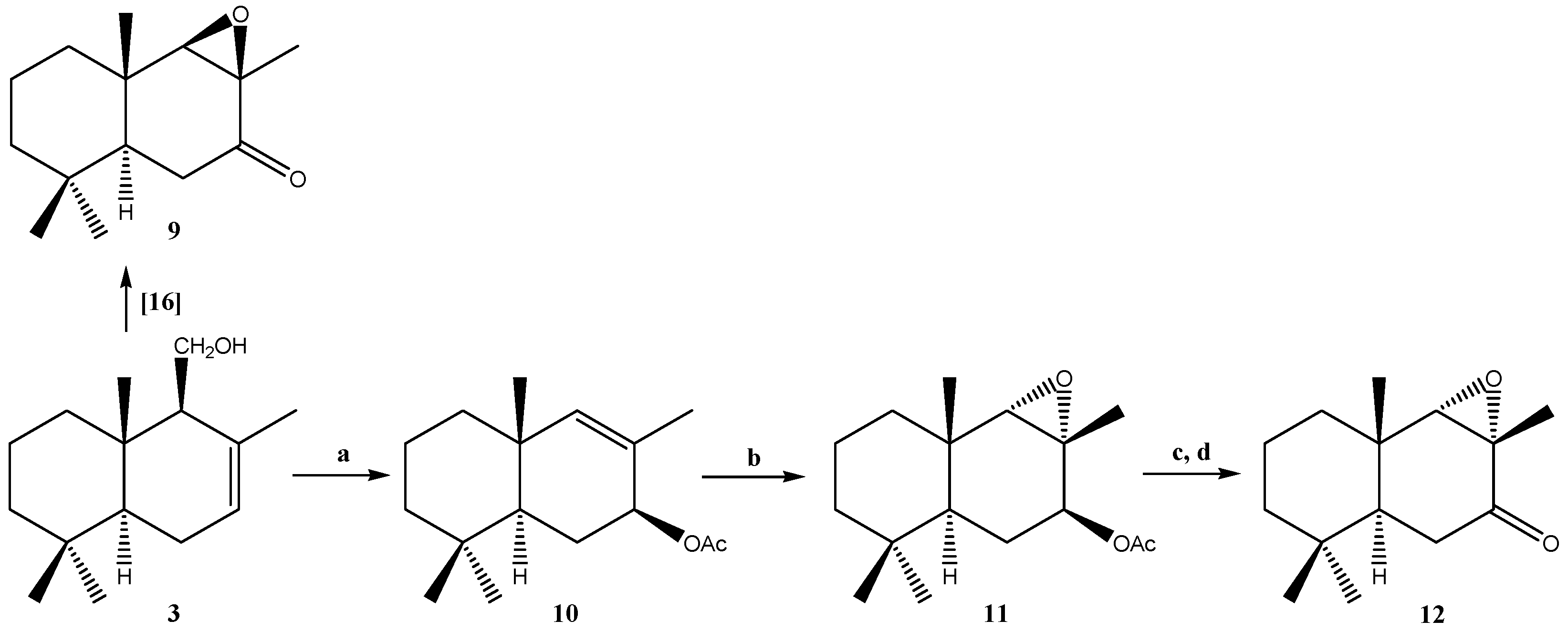
2.1.2. Compounds Obtained from 3
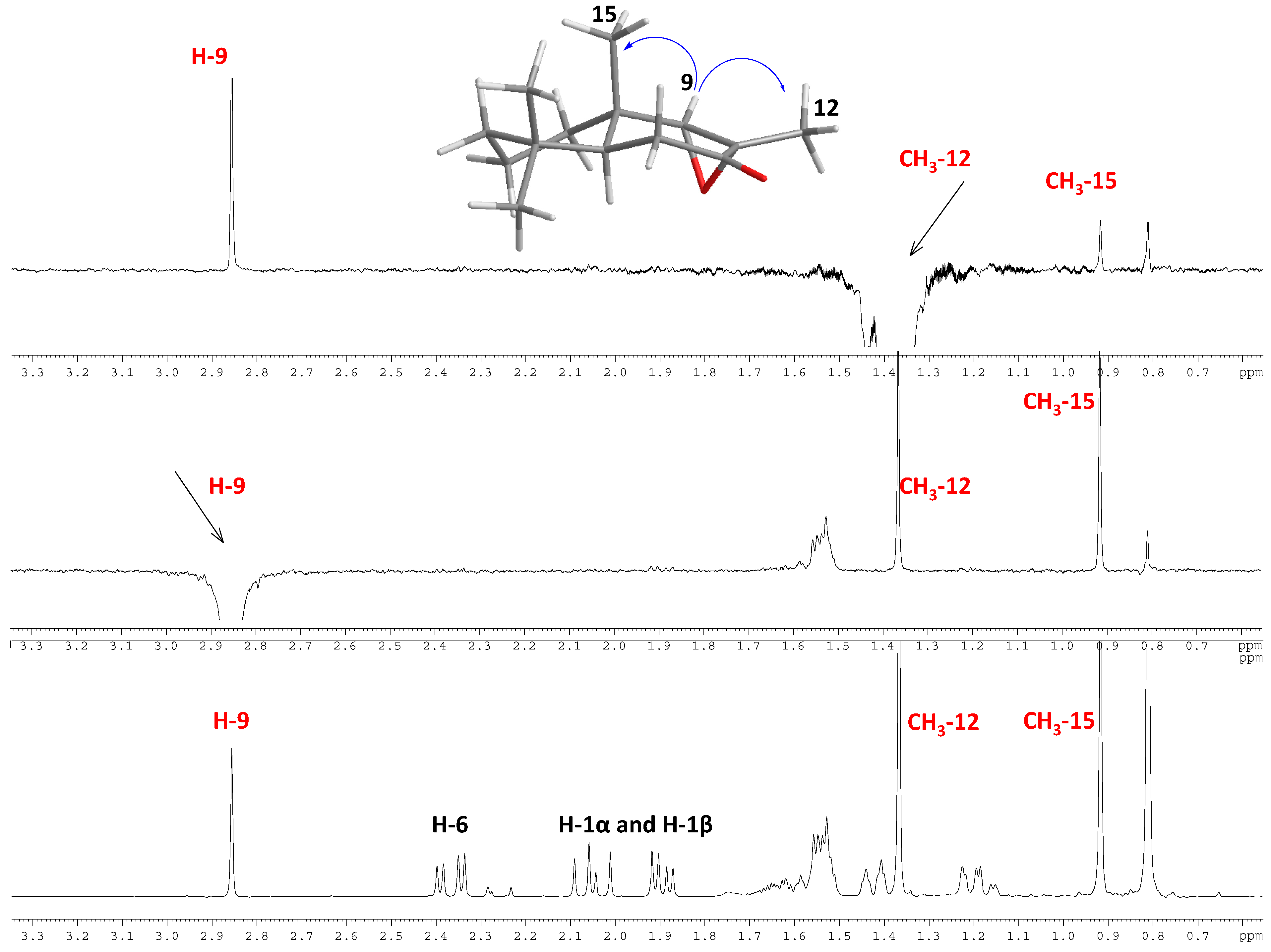
2.2. Biological Results
2.3. Viability Assay
| Compound | DU145 | PC-3 | MCF-7 | CoN | |
|---|---|---|---|---|---|
| 1 | IC50 | 71.4 ± 8.5 | 89.2 ± 6.8 | 93.7 ± 9.1 | >200 |
| 2 | IC50 | >200 | >200 | >200 | >200 |
| 3 | IC50 | >200 | >200 | >200 | >200 |
| 4 | IC50 | >200 | >200 | >200 | >200 |
| 5 | IC50 | 90.5 ± 8.2 | 87.6 ± 9.2 | >200 | >200 |
| 6 | IC50 | >200 | >200 | >200 | >200 |
| 7 | IC50 | >200 | >200 | >200 | >200 |
| 8 | IC50 | 70.6 ± 5.9 | 65.4 ± 5.5 | 97.1 ± 7.2 | >200 |
| 9 | IC50 | 93.5 ± 6.7 | 97.5 ± 10.4 | >200 | >200 |
| 10 | IC50 | >200 | >200 | >200 | >200 |
| 11 | IC50 | >200 | >200 | >200 | >200 |
| 12 | IC50 | >200 | 90.2 ± 8.8 | 88.4 ± 7.1 | >200 |

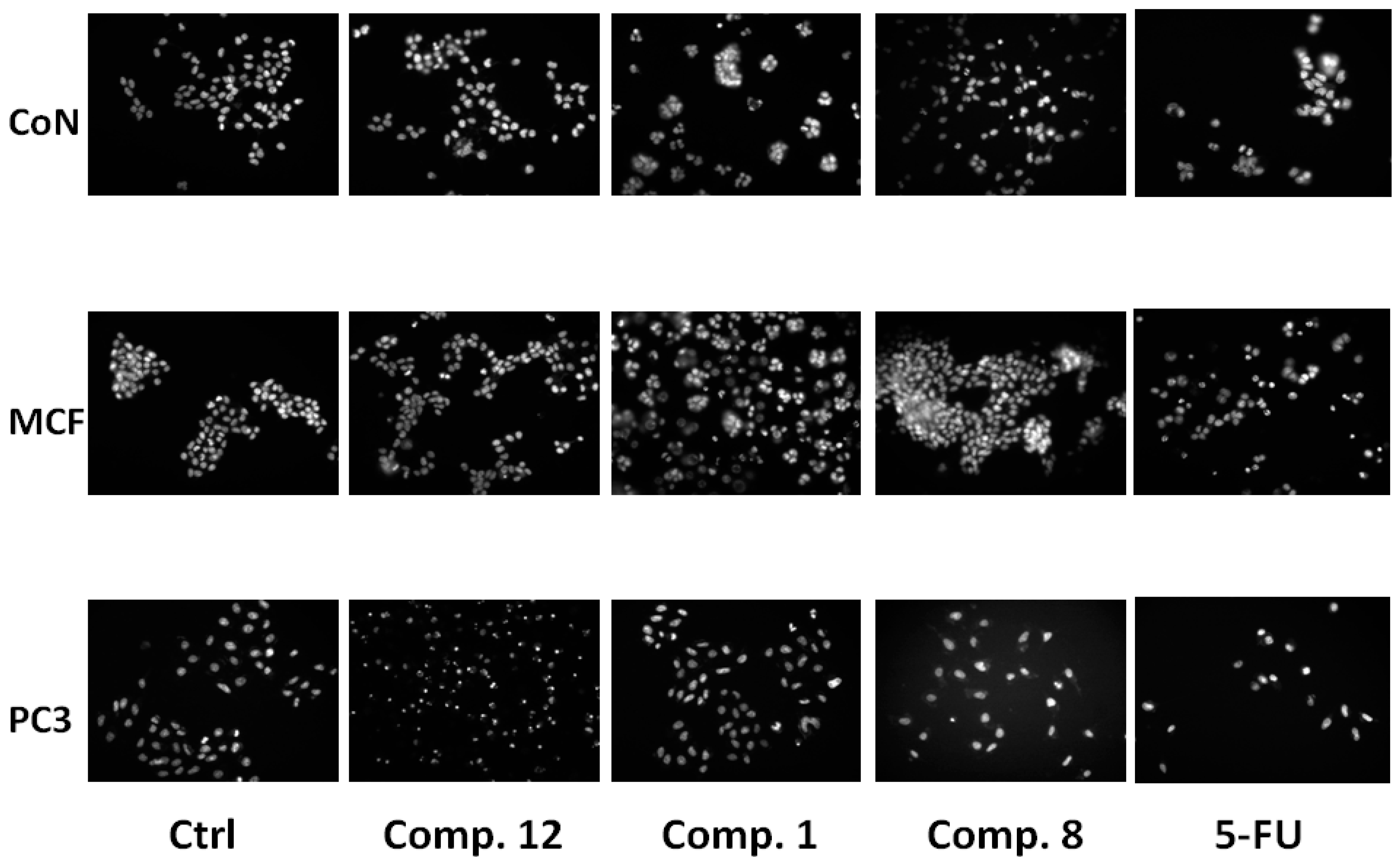
| CoN | MCF-7 | PC-3 | ||||
|---|---|---|---|---|---|---|
| 50 µM | 100 µM | 50 µM | 100 µM | 50 µM | 100 µM | |
| Comp. 12 | 102.6 ± 9.1 | 124.9 ± 9.7 | 111.4 ± 8.9 | 111.3 ± 9.8 | 139.1 ± 12.4 | 149.3 ± 21.3 |
| Comp. 8 | 96.0 ± 7.6 | 52.3 ± 8.4 * | 93.3 ± 7.1 | 72.3 ± 6.4 * | 136.2 ± 18.5 | 92.8 ± 10.4 |
| Comp. 1 | 49.1 ± 6.6 * | 11.8 ± 2.9 ** | 77.0 ± 6.5 * | 19.4 ± 1.5 ** | 111.6 ± 8.9 | 11.9 ± 2.4 ** |
| Control | 93.2 ± 8.7 | 95.8 ± 9.9 | 98.1 ± 10.5 | |||
| FCCP | 6.8 ± 1.3 ** | 7.4 ± 0.4 ** | 5.2 ± 0.4 ** | |||
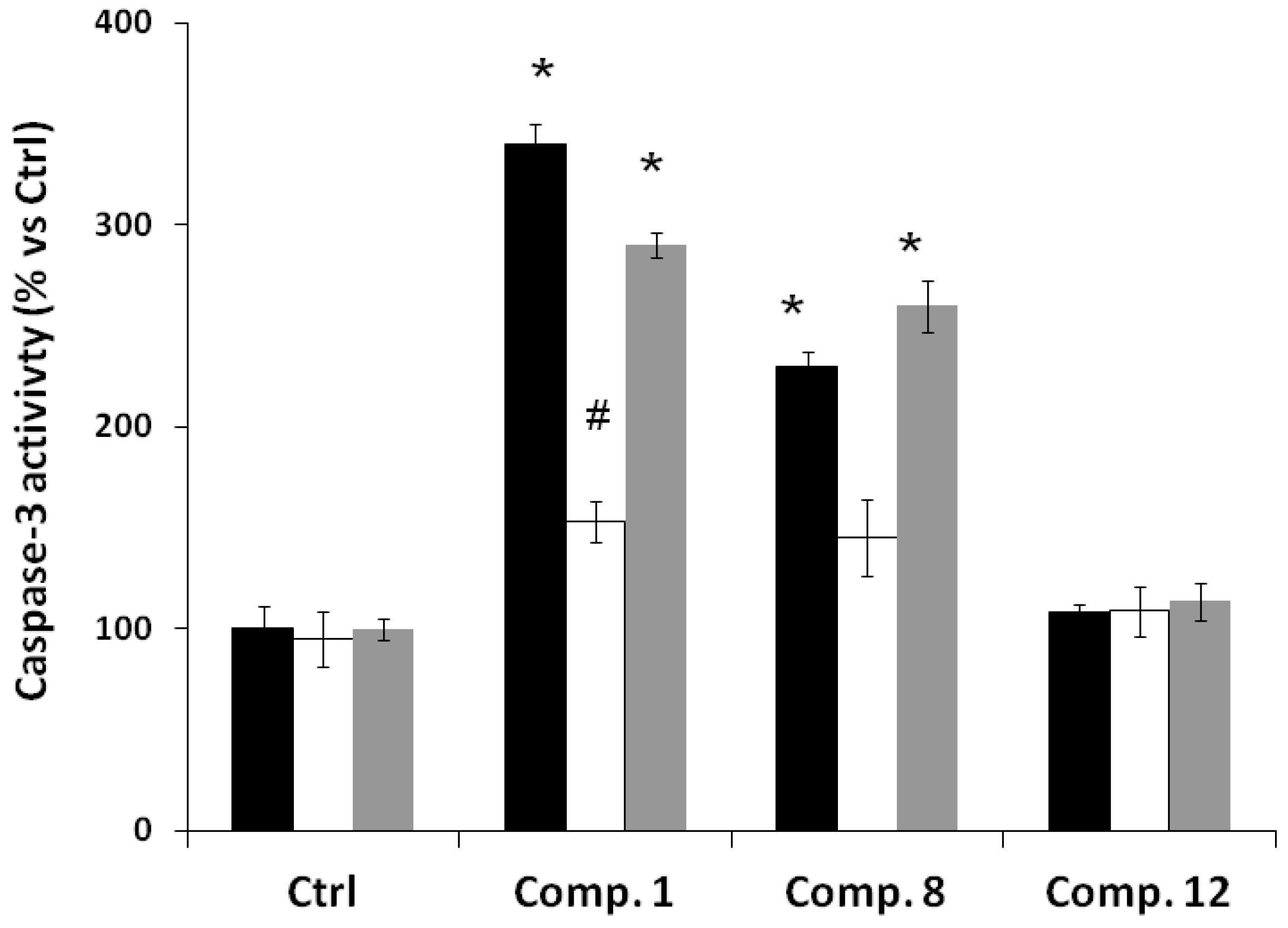
3. Experimental Section
3.1. Spectroscopic Analysis
3.2. Plant Material
3.3. Isolation of Natural Compounds 1–4
3.4. Preparation of Polygodial Derivatives 6–8 and Drimenol Derivatives 9–12
3.5. Cell Lines
3.6. Cell Viability
3.7. Morphological Assessment of Cell Apoptosis
3.8. Analysis of Mitochondrial Membrane Permeability
3.9. Caspase 3 Activity Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baechler, R.; Henríquez, R.; Aqueveque, X.; Martínez, M.; Soto, A. Prevalence of prostate cancer in the Seventh Region of Chile. Rev. Med. Chile 2001, 11, 1305–1310. [Google Scholar]
- Novoa, C.; Aliaga, A.; Badilla, S.; Reyes, D. Current reality of the screening for prostate cancer. Are we carrying out the recommendations? Rev. Chil. Urol. 2013, 78, 27–31. [Google Scholar]
- Echiburú-Chau, C.; Alfaro-Lira, S.; Brown, N.; Salas, C.; Cuellar, M.; Santander, J.; Ogalde, J.; Rothhammer, F. The selective cytotoxicity elicited by phytochemical extract from Senecio graveolens (Asteraceae) on breast cancer cells is enhanced by hypoxia. Int. J. Oncol. 2014, 44, 1357–1364. [Google Scholar]
- Estomba, D.; Ladio, A.; Lozada, M. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. J. Ethnopharmacol. 2006, 103, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.M.; de Groot, A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubo, I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: Involvement of pyrrole formation on cell surface in antifungal action. Bioorg. Med. Chem. 2005, 13, 6742–6747. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.; Fröde, T.; Mendes, G.; Malheiros, A.; Filho, V.C.; Yunes, R.A.; Calixto, J.B. Additional evidence for the anti-inflammatory and anti-allergic properties of the sesquiterpene polygodial. Life Sci. 2001, 70, 159–169. [Google Scholar] [CrossRef]
- Malheiros, A.; Filho, V.C.; Schmitt, C.; Yunes, R.; Escalante, A.; Svetaz, L.; Zacchino, S.; Delle Monache, F. Antifungal activity of drimane sesquiterpenes from Drimys brasiliensis using bioassay-guided fractionation. J. Pharm. Pharm. Sci. 2005, 8, 335–339. [Google Scholar] [PubMed]
- Sterner, O.; Carter, R.E.; Nilsson, L.M. Structure-activity relationships for unsaturated dialdehydes. 1. The mutagenic activity of 18 compounds in the Salmonella/microsome assay. Mutat. Res. 1987, 188, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Anke, H.; Sterner, O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta Med. 1991, 57, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Mc Callion, R.; Cole, A.; Walker, J.; Blunt, J.; Munro, M. Antibiotic substances from New Zealand plants. Planta Med. 1982, 44, 134–138. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Alves, T.; Ribeiro, F.; Kloos, H.; Zani, C. Polygodial, the fungitoxic component from the Brazilian medicinal plant Polygonum punctatum. Mem. Inst. Oswaldo Cruz 2001, 96, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Adachi, T.; Oi, S.; Kimura, A.; Katsumura, S.; Isoe, S.; Kubo, I. Structure-activity relationship of the Warburgia sesquiterpene dialdehydes. Agric. Biol. Chem. 1984, 48, 73–78. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lunde, C.; Kubo, I. In vitro antifungal susceptibilities of Candida albicans and other fungal pathogens to polygodial, a sesquiterpene dialdehyde. Planta Med. 1998, 65, 204–208. [Google Scholar] [CrossRef]
- Derita, M.; Zacchino, S. Validation of the ethnopharmacological use of Polygonum persicaria for its antifungal properties. Nat. Prod. Commun. 2011, 6, 931–933. [Google Scholar] [PubMed]
- Montenegro, I.; Pino, L.; Werner, E.; Madrid, A.; Espinoza, L.; Moreno, L.; Villena, J.; Cuellar, M. Comparative Study on the larvicidal activity of drimane sesquiterpenes and nordrimane compounds against Drosophila melanogaster til-til. Molecules 2013, 18, 4192–4208. [Google Scholar]
- Derita, M.; Montenegro, I.; Garibotto, F.; Enriz, R.; Cuellar, M.; Zacchino, S. Structural requirements for the antifungal activities of natural drimane sesquiterpenes and analogues, supported by conformational and electronic studies. Molecules 2013, 18, 2029–2051. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.D.; Newland, A.C. Apoptosis detection by DNA analysis. Methods Mol. Med. 1996, 6, 207–213. [Google Scholar] [PubMed]
- Fleischer, A.; Ghadiri, A.; Dessauge, A.F.; Duhamela, M.; Rebollo, M.P.; Alvarez-Franco, F.; Rebollo, A. Modulating apoptosis as a target for effective therapy. Mol. Immunol. 2006, 43, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Madrid, A.; Montenegro, I.; Werner, E.; Cuellar, M.; Espinoza, L. Diterpenylhydroquinones from Natural ent-Labdanes Induce Apoptosis through Decreased Mitochondrial Membrane Potential. Molecules 2013, 18, 5348–5359. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.; Kubo, I. Effect of Polygodial on the Mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2000, 44, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.I.; Lee, S.H. Antifungal mechanism of polygodial. J. Agric. Food Chem. 2001, 49, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Lodeyro, A.; Malheiros, A.; Zacchino, S.; Roveri, O. Inhibition of the mitochondrial ATP synthesis by polygodial, a naturally occurring dialdehyde unsaturated sesquiterpene. Biochem. Pharmacol. 2005, 70, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Emaus, R.K.; Grunwald, R.; Lemaster, J.J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: Spectral and metabolic properties. Biochim. Biophys. Acta 1986, 850, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Role of mitochondria as the gardens of cell death. Cancer Chemother. Pharmacol. 2006, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Kroemer, G. Mitochondria in cell death: Novel targets for neuroprotection and cardioprotection. Trends Mol. Med. 2003, 9, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Osório, L.; Cortés, M.; Armstrong, V.; Bailén, M.; González-Coloma, A. Antifeedant activity of some polygodial derivatives. Z. Naturforsch. C 2008, 63, 215–220. [Google Scholar]
- Derita, M.G.; Leivab, M.L.; Zacchino, S.A. Influence of plant part, season of collection and content of the main active constituent, on the antifungal properties of Polygonum acuminatum Kunth. J. Ethnopharmacol. 2009, 124, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, B.; Zapata, N.; Medina, P.; Viñuela, E. A complete 1H and 13C-NMR data assignment for four drimane sesquiterpenoids isolated from Drimys winteri. Magn. Reson. Chem. 2005, 43, 82–84. [Google Scholar] [CrossRef]
- Hueso-Rodríguez, J.; Rodríguez, B. A new and efficient route to optically active drimanes. Synthesis of (+)-winterin, (+)-confertifolin, (+)-isodrimenin, and (+)-bicyclofarnesol. Tetrahedron 1989, 45, 1567–1576. [Google Scholar] [CrossRef]
- Cortés, M.; Delgado, V.; Saitz, C.; Armstrong, V. Drimenol: A versatile synthon for compounds with trans-drimane skeleton. Nat. Prod. Commun. 2011, 6, 477–490. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Day, T.W.; Wu, C.H.; Safa, A.R. Etoposide induces protein kinase C Cδ- and caspase 3 dependent apoptosis in neuroblastoma cancer cells. Mol. Pharmacol. 2009, 76, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–12 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, I.; Tomasoni, G.; Bosio, C.; Quiñones, N.; Madrid, A.; Carrasco, H.; Olea, A.; Martinez, R.; Cuellar, M.; Villena, J. Study on the Cytotoxic Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Cancer Cell Lines. Molecules 2014, 19, 18993-19006. https://doi.org/10.3390/molecules191118993
Montenegro I, Tomasoni G, Bosio C, Quiñones N, Madrid A, Carrasco H, Olea A, Martinez R, Cuellar M, Villena J. Study on the Cytotoxic Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Cancer Cell Lines. Molecules. 2014; 19(11):18993-19006. https://doi.org/10.3390/molecules191118993
Chicago/Turabian StyleMontenegro, Ivan, Giacomo Tomasoni, Claudia Bosio, Natalia Quiñones, Alejandro Madrid, Hector Carrasco, Andres Olea, Rolando Martinez, Mauricio Cuellar, and Joan Villena. 2014. "Study on the Cytotoxic Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Cancer Cell Lines" Molecules 19, no. 11: 18993-19006. https://doi.org/10.3390/molecules191118993
APA StyleMontenegro, I., Tomasoni, G., Bosio, C., Quiñones, N., Madrid, A., Carrasco, H., Olea, A., Martinez, R., Cuellar, M., & Villena, J. (2014). Study on the Cytotoxic Activity of Drimane Sesquiterpenes and Nordrimane Compounds against Cancer Cell Lines. Molecules, 19(11), 18993-19006. https://doi.org/10.3390/molecules191118993




