Tissue-Specific Distribution of Ginsenosides in Different Aged Ginseng and Antioxidant Activity of Ginseng Leaf
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Ginsenosides and Quantitative Method Validation
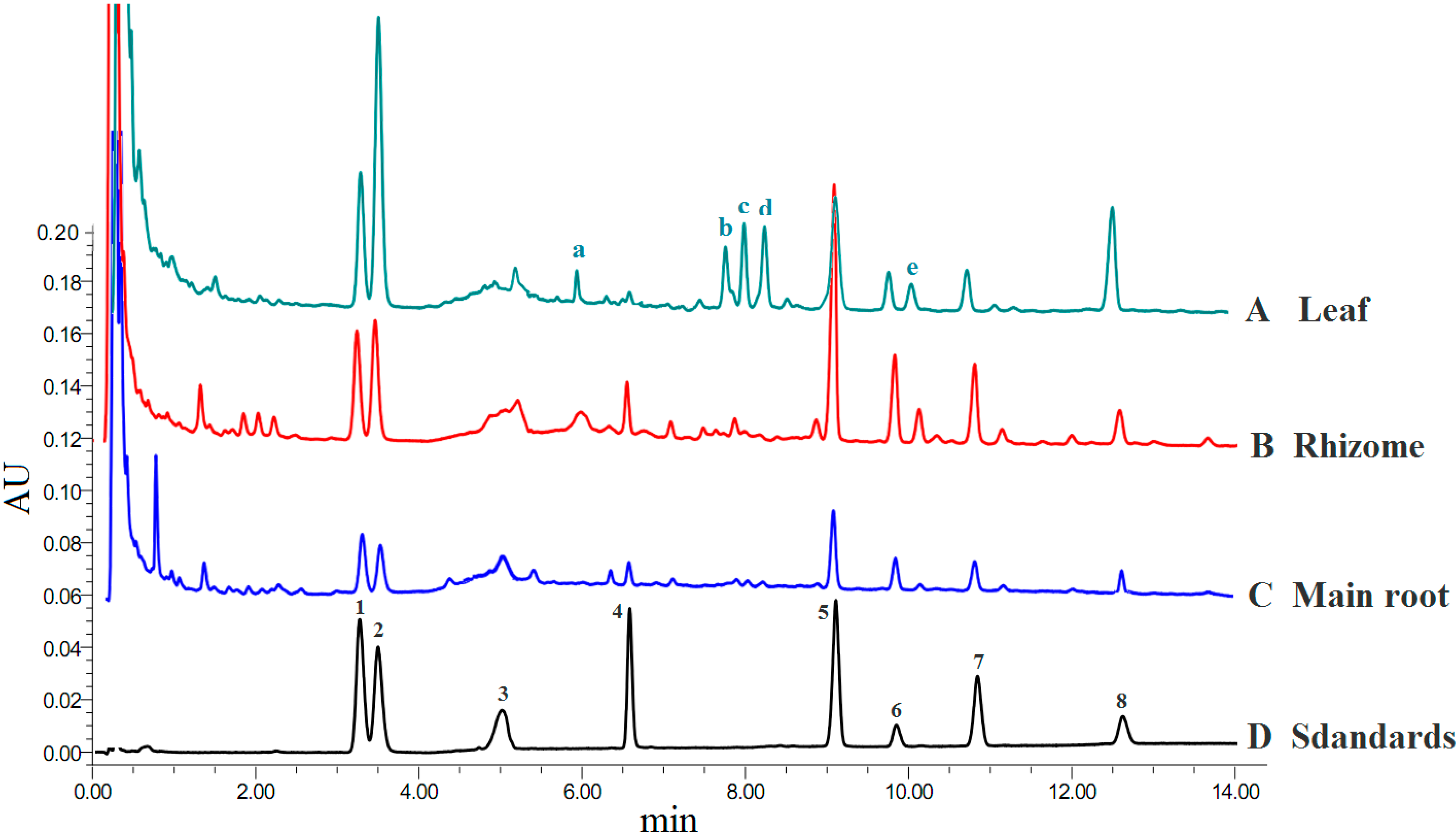
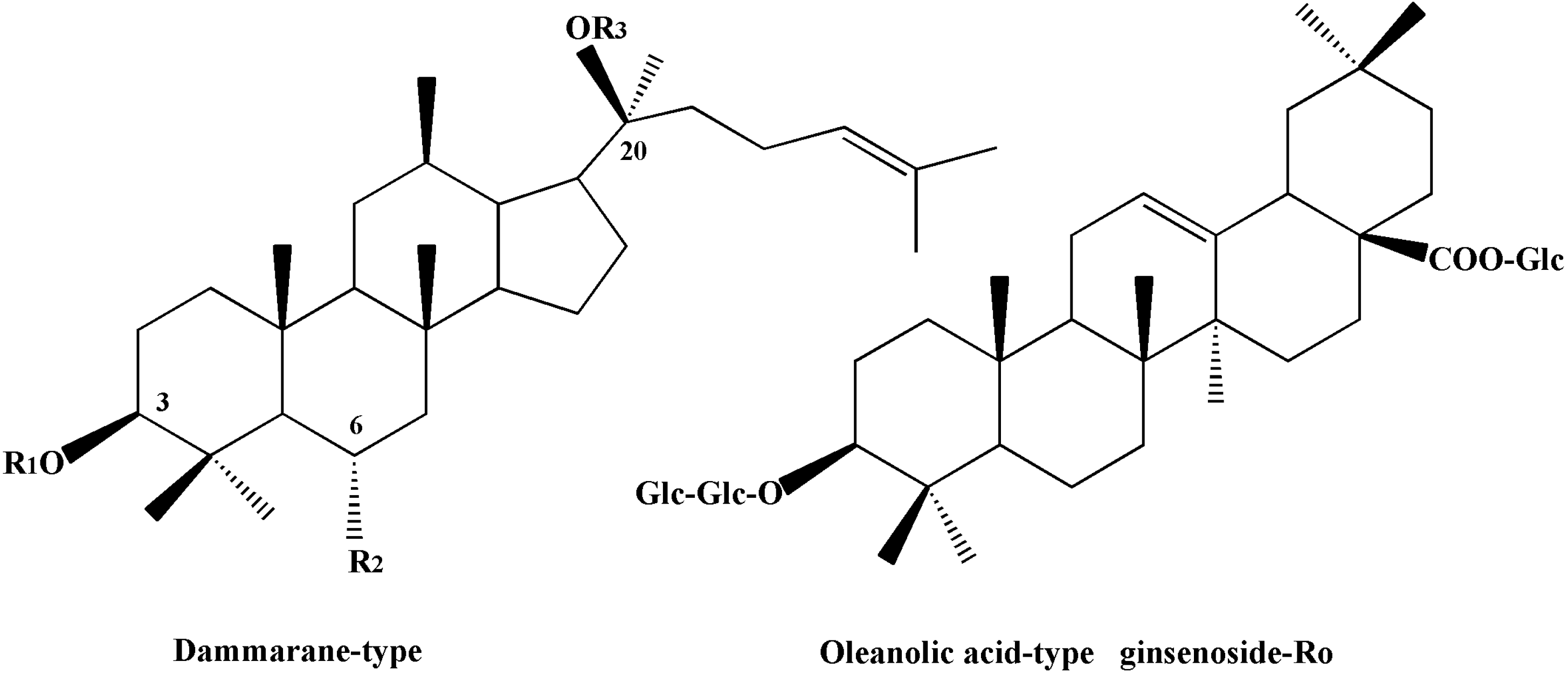
| Compounds | R1 | R2 | R3 |
|---|---|---|---|
| Ginsenoside-Rb1 | Glc(2-1) Glc | H | Glc(6-1) Glc |
| Ginsenoside-Rb2 | Glc(2-1) Glc | H | Glc(6-1) Arap |
| Ginsenoside-Rc | Glc(2-1) Glc | H | Glc(6-1) Araf |
| Ginsenoside-Rd | Glc(2-1) Glc | H | Glc |
| Ginsenoside-Re | H | O- Glc(2-1) Rha | Glc |
| Ginsenoside-Rf | H | O- Glc(2-1) Glc | H |
| Ginsenoside-Rg1 | H | O-Glc | Glc |
| Markers | Regression Equation | R2 | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Ginsenoside Rg1 | y = 785.09 x − 49305 | 0.9991 | 49.73–1823.33 | 2.10 | 5.21 |
| Ginsenoside Re | y = 1015.2 x − 86311 | 0.9990 | 39.73–910.42 | 2.19 | 4.86 |
| Ginsenoside Ro | y = 1256.8 x − 77168 | 0.9993 | 29.77–873.33 | 1.31 | 3.44 |
| Ginsenoside Rf | y = 1262.7 x − 80147 | 0.9994 | 25.86–948.33 | 1.72 | 4.09 |
| Ginsenoside Rb1 | y = 912.66 x − 85373 | 0.9993 | 47.73–1750.00 | 0.98 | 4.41 |
| Ginsenoside Rc | y = 577.06 x − 13760 | 0.9993 | 30.53–447.78 | 1.01 | 4.53 |
| Ginsenoside Rb2 | y = 992.69 x − 61958 | 0.9993 | 24.86–911.67 | 1.82 | 3.79 |
| Ginsenoside Rd | y = 991.01 x − 22351 | 0.9992 | 28.11–412.22 | 1.63 | 4.63 |
2.2. Ginsenosides Contents in Different Tissues of the Same Ginseng Plant
| Samples | Rg1 | Re | Ro | Rf | Rb1 | Rc | Rb2 | Rd | Total | PPT-/PPD-Type |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | ||||||||||
| 1L | 6.43 ± 0.12 | 10.45 ± 0.32 | 2.93 ± 0.32 | 2.63 ± 0.23 | 4.41 ± 0.08 | 2.26 ± 0.12 | 3.29 ± 0.05 | 4.26 ± 0.33 | 36.64 ± 0.56 | 1.37 |
| 2L | 5.98 ± 0.04 | 8.76 ± 0.14 | 2.79 ± 0.11 | 2.58 ± 0.21 | 3.88 ± 0.04 | 1.31 ± 0.25 | 2.73 ± 0.08 | 2.24 ± 0.34 | 30.27 ± 0.73 | 1.71 |
| 3L | 10.84 ± 0.10 | 16.34 ± 0.22 | 3.91 ± 0.43 | 2.68 ± 0.53 | 4.23 ± 0.42 | 1.53 ± 0.27 | 2.82 ± 0.13 | 2.43 ± 0.14 | 44.79 ± 1.29 | 2.71 |
| 4L | 14.70 ± 0.08 | 11.05 ± 0.31 | 4.14 ± 0.04 | 2.76 ± 0.08 | 4.06 ± 0.16 | 1.36 ± 0.16 | 2.77 ± 0.54 | 1.89 ± 0.10 | 42.74 ± 0.38 | 2.83 |
| 5L | 12.72 ± 0.26 | 20.18 ± 0.11 | 4.27 ± 0.56 | 2.68 ± 0.16 | 3.82 ± 0.02 | 1.79 ± 0.17 | 2.86 ± 0.20 | 2.87 ± 0.07 | 51.20 ± 1.10 | 3.14 |
| 6L | 11.03 ± 0.13 | 17.04 ± 0.07 | 4.18 ± 0.19 | 2.69 ± 0.32 | 3.90 ± 0.18 | 1.95 ± 0.33 | 3.17 ± 0.43 | 4.07 ± 0.22 | 48.04 ± 0.37 | 2.35 |
| 7L | 13.55 ± 0.07 | 15.27 ± 0.08 | 4.38 ± 0.42 | 2.74 ± 0.67 | 4.40 ± 0.38 | 2.19 ± 0.26 | 3.24 ± 0.11 | 3.75 ± 0.14 | 49.51 ± 1.15 | 2.32 |
| 8L | 9.59 ± 0.05 | 17.35 ± 0.43 | 5.00 ± 0.34 | 2.65 ± 0.32 | 4.15 ± 0.05 | 1.54 ± 0.05 | 2.82 ± 0.03 | 2.12 ± 0.55 | 45.22 ± 0.91 | 2.78 |
| 9L | 9.15 ± 0.08 | 17.11 ± 0.32 | 4.00 ± 0.09 | 2.74 ± 0.05 | 4.07 ± 0.25 | 1.51 ± 0.15 | 3.08 ± 0.25 | 2.10 ± 0.38 | 43.76 ± 0.39 | 2.70 |
| 10L | 13.24 ± 0.11 | 18.47 ± 0.29 | 4.63 ± 0.86 | 2.98 ± 0.19 | 4.11 ± 0.34 | 2.82 ± 0.10 | 3.52 ± 0.21 | 3.57 ± 0.29 | 53.34 ± 0.83 | 2.48 |
| 11L | 9.09 ± 0.21 | 17.01 ± 0.55 | 4.42 ± 0.57 | 2.75 ± 0.24 | 3.91 ± 0.41 | 1.62 ± 0.42 | 3.03 ± 0.10 | 2.77 ± 0.20 | 44.59 ± 0.30 | 2.55 |
| 12L | 10.81 ± 0.20 | 18.46 ± 0.37 | 4.20 ± 0.27 | 2.69 ± 0.15 | 5.16 ± 0.16 | 3.78 ± 0.37 | 4.46 ± 0,18 | 6.49 ± 0.17 | 56.06 ± 1.42 | 1.61 |
| 13L | 13.69 ± 0.34 | 20.87 ± 0.22 | 4.28 ± 0.09 | 2.69 ± 0.27 | 4.08 ± 0.06 | 3.10 ± 0.26 | 3.89 ± 0.06 | 6.44 ± 0.11 | 59.03 ± 0.63 | 2.13 |
| Rhizome | ||||||||||
| 1R | 2.57 ± 0.06 | 3.58 ± 0.30 | ND | ND | ND | 1.10 ± 0.06 | 2.55 ± 0.26 | 0.96 ± 0.02 | 10.76 ± 1.06 | 1.33 |
| 2R | 5.06 ± 0.10 | 5.55 ± 0.14 | 5.41 ± 0.12 | 2.58 ± 0.11 | 5.45 ± 0.10 | 3.16 ± 021 | 3.21 ± 0.52 | 1.46 ± 0.17 | 31.88 ± 0.89 | 0.99 |
| 3R | 3.40 ± 0.33 | 5.01 ± 0.19 | 2.63 ± 0.15 | 2.76 ± 0.10 | 5.16 ± 0.42 | 2.28 ± 0.17 | 3.33 ± 0.16 | 1.61 ± 0.21 | 26.19 ± 0.63 | 0.90 |
| 4R | 4.13 ± 0.26 | 4.68 ± 0.09 | 2.60 ± 0.48 | 2.97 ± 0.09 | 5.20 ± 0.69 | 2.25 ± 0.09 | 3.15 ± 0.25 | 1.46 ± 0.13 | 26.45 ± 0.73 | 0.98 |
| 5R | 3.66 ± 0.18 | 4.77 ± 0.31 | 2.69 ± 0.38 | 2.77 ± 0.39 | 5.24 ± 0.19 | 2.30 ± 0.37 | 3.35 ± 0.10 | 1.37 ± 0.19 | 26.15 ± 1.29 | 0.91 |
| 6R | 3.37 ± 0.14 | 4.23 ± 0.40 | 2.65 ± 0.35 | 2.69 ± 0.47 | 4.55 ± 0.05 | 1.59 ± 0.15 | 2.88 ± 0.18 | 1.13 ± 0.30 | 23.09 ± 0.30 | 1.01 |
| 7R | 3.54 ± 0.12 | 4.30 ± 0.59 | 2.59 ± 0.14 | 2.82 ± 0.35 | 5.10 ± 0.21 | 1.83 ± 0.28 | 3.00 ± 0.14 | 1.30 ± 0.28 | 24.48 ± 0.47 | 0.95 |
| 8R | 5.78 ± 0.30 | 8.29 ± 0.65 | 3.35 ± 0.14 | 3.29 ± 0.19 | 9.62 ± 0.09 | 5.71 ± 0.32 | 5.14 ± 0.08 | 2.75 ± 0.35 | 43.92 ± 1.28 | 0.75 |
| 9R | 5.96 ± 0.72 | 7.00 ± 0.21 | 3.25 ± 0.19 | 3.28 ± 0.18 | 9.96 ± 0.49 | 4.54 ± 0.07 | 4.72 ± 0.26 | 2.12 ± 0.14 | 40.83 ± 0.14 | 0.76 |
| 10R | 5.55 ± 0.36 | 6.96 ± 0.45 | 3.27 ± 0.05 | 3.49 ± 0.19 | 8.57 ± 0.22 | 5.05 ± 0.14 | 4.77 ± 0.14 | 2.14 ± 0.16 | 39.79 ± 0.51 | 0.78 |
| 11R | 5.85 ± 0.44 | 6.69 ± 0.33 | 3.30 ± 0.27 | 3.49 ± 0.19 | 9.49 ± 0.10 | 4.45 ± 0.26 | 4.53 ± 0.39 | 1.77 ± 0.23 | 39.56 ± 0.76 | 0.79 |
| 12R | 9.23 ± 0.07 | 9.42 ± 0.16 | 3.86 ± 0.23 | 4.24 ± 0.18 | 15.36 ± 0.20 | 7.53 ± 0.29 | 6.30 ± 0.52 | 2.78 ± 0.39 | 58.73 ± 1.37 | 0.72 |
| 13R | 11.95 ± 0.22 | 10.26 ± 0.10 | 4.19 ± 0.11 | 4.27 ± 0.12 | 16.43 ± 0.29 | 8.43 ± 0.47 | 7.04 ± 0.82 | 2.74 ± 0.29 | 65.30 ± 0.11 | 0.76 |
| Main root | ||||||||||
| 1 MR | 2.81 ± 0.17 | 4.16 ± 0.50 | ND | ND | 4.06 ± 0.26 | 1.34 ± 0.31 | 2.73 ± 0.26 | 1.17 ± 0.18 | 16.27 ± 0.57 | 0.75 |
| 2 MR | 2.84 ± 0.63 | 4.32 ± 0.24 | ND | 2.63 ± 0.53 | 4.26 ± 0.17 | 1.57 ± 0.30 | 2.8 ± 0.10 | 1.38 ± 0.09 | 19.79 ± 1.25 | 0.98 |
| 3 MR | 3.1 ± 0.30 | 4.39 ± 0.25 | ND | 2.68 ± 0.10 | 4.45 ± 0.18 | 1.91 ± 0.19 | 3.01 ± 0.16 | 1.23 ± 0.12 | 20.77 ± 0.41 | 0.96 |
| 4 MR | 3.77 ± 0.84 | 4.22 ± 0.19 | 2.51 ± 0.42 | 2.84 ± 0.37 | 4.51 ± 0.17 | 1.67 ± 0.13 | 2.76 ± 0.21 | 1.19 ± 0.28 | 23.48 ± 0.66 | 1.07 |
| 5 MR | 3.56 ± 0.12 | 4.43 ± 0.10 | 2.53 ± 0.37 | 2.78 ± 0.30 | 4.75 ± 0.20 | 2.03 ± 0.52 | 3.18 ± 0.28 | 0.90 ± 0.28 | 24.17 ± 0.53 | 0.99 |
| 6 MR | 3.5 ± 0.18 | 4.68 ± 0.37 | 2.53 ± 0.10 | 2.78 ± 0.72 | 4.89 ± 0.20 | 2.03 ± 0.22 | 3.06 ± 0.14 | 1.21 ± 0.21 | 24.68 ± 1.40 | 0.98 |
| 7 MR | 3.6 ± 0.24 | 4.44 ± 0.17 | 2.53 ± 0.19 | 2.79 ± 0.31 | 5.19 ± 0.28 | 1.82 ± 0.28 | 2.89 ± 0.10 | 1.08 ± 0.30 | 24.34 ± 0.39 | 0.99 |
| 8 MR | 3.48 ± 0.27 | 4.68 ± 0.06 | 2.51 ± 0.25 | 2.79 ± 0.19 | 5.23 ± 0.08 | 1.87 ± 0.14 | 2.9 ± 0.02 | 1.2 ± 0.17 | 24.68 ± 1.44 | 0.98 |
| 9 MR | 4.74 ± 0.38 | 5.39 ± 0.42 | 3.46 ± 0.19 | 2.97 ± 0.08 | 6.54 ± 0.04 | 2.55 ± 0.17 | 3.51 ± 0.11 | 1.1 ± 0.41 | 30.26 ± 0.18 | 0.96 |
| 10 MR | 4.48 ± 0.47 | 4.89 ± 0.11 | 3.49 ± 0.04 | 2.98 ± 0.59 | 5.59 ± 0.63 | 2.44 ± 0.21 | 3.34 ± 0.08 | 1.17 ± 0.60 | 28.39 ± 1.30 | 0.98 |
| 11 MR | 5.8 ± 0.26 | 5.21 ± 0.05 | 3.92 ± 0.51 | 3.13 ± 0.41 | 7.00 ± 0.37 | 2.72 ± 0.41 | 3.61 ± 0.13 | 1.16 ± 0.53 | 32.55 ± 0.77 | 0.98 |
| 12 MR | 5.98 ± 0.08 | 5.21 ± 0.15 | 3.79 ± 0.18 | 3.00 ± 0.36 | 4.8 ± 0.42 | 3.12 ± 0.15 | 2.79 ± 0.26 | 1.14 ± 0.59 | 29.83 ± 1.57 | 1.2 |
| 13 MR | 6.16 ± 0.25 | 5.77 ± 0.26 | 3.19 ± 0.10 | 3.26 ± 0.32 | 4.43 ± 0.27 | 3.23 ± 0.10 | 3.84 ± 0.51 | 1.26 ± 0.10 | 31.14 ± 0.62 | 1.19 |
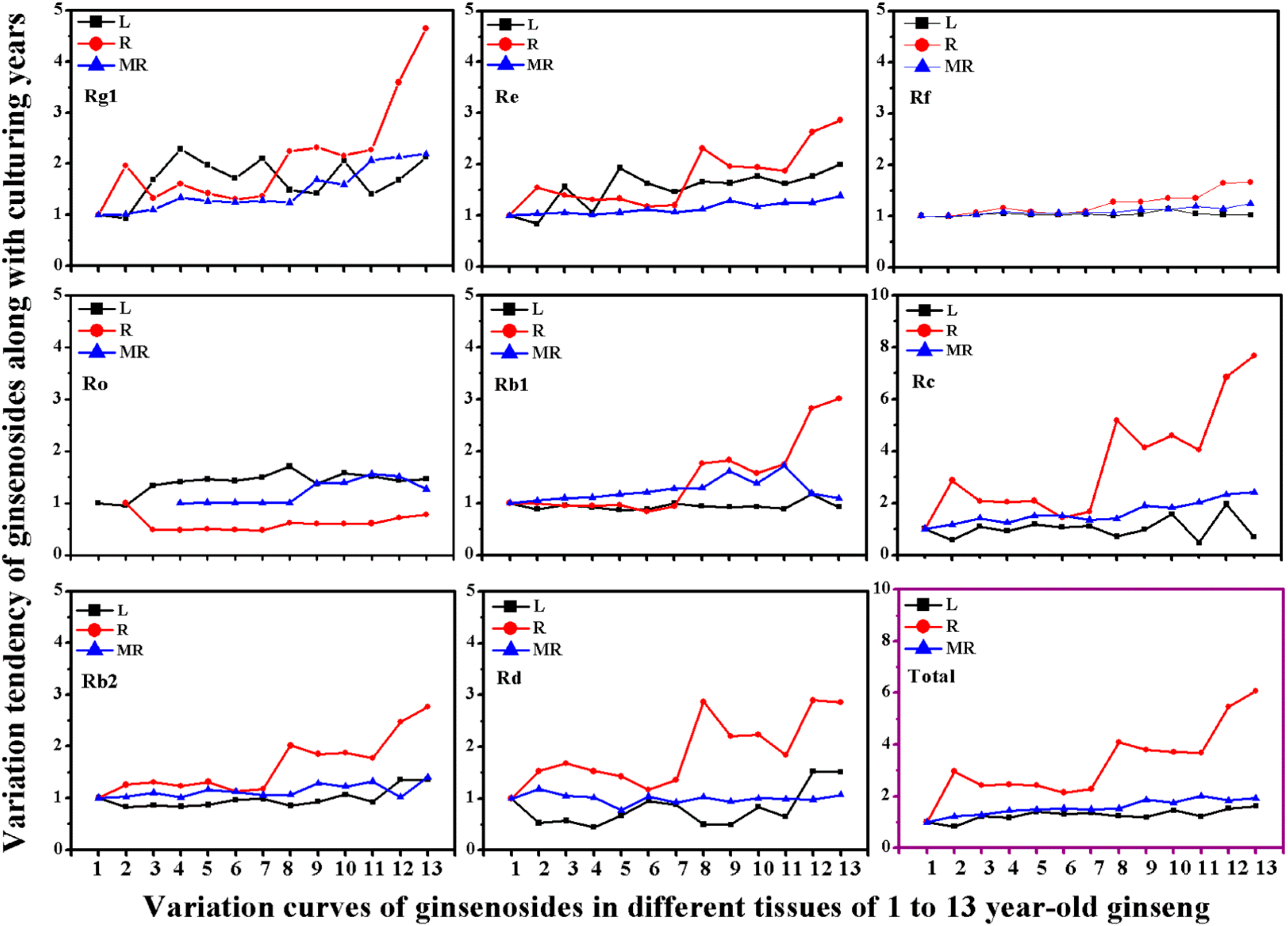
2.3. Variation of Ginsenoside Heterogeneity in Different Tissues
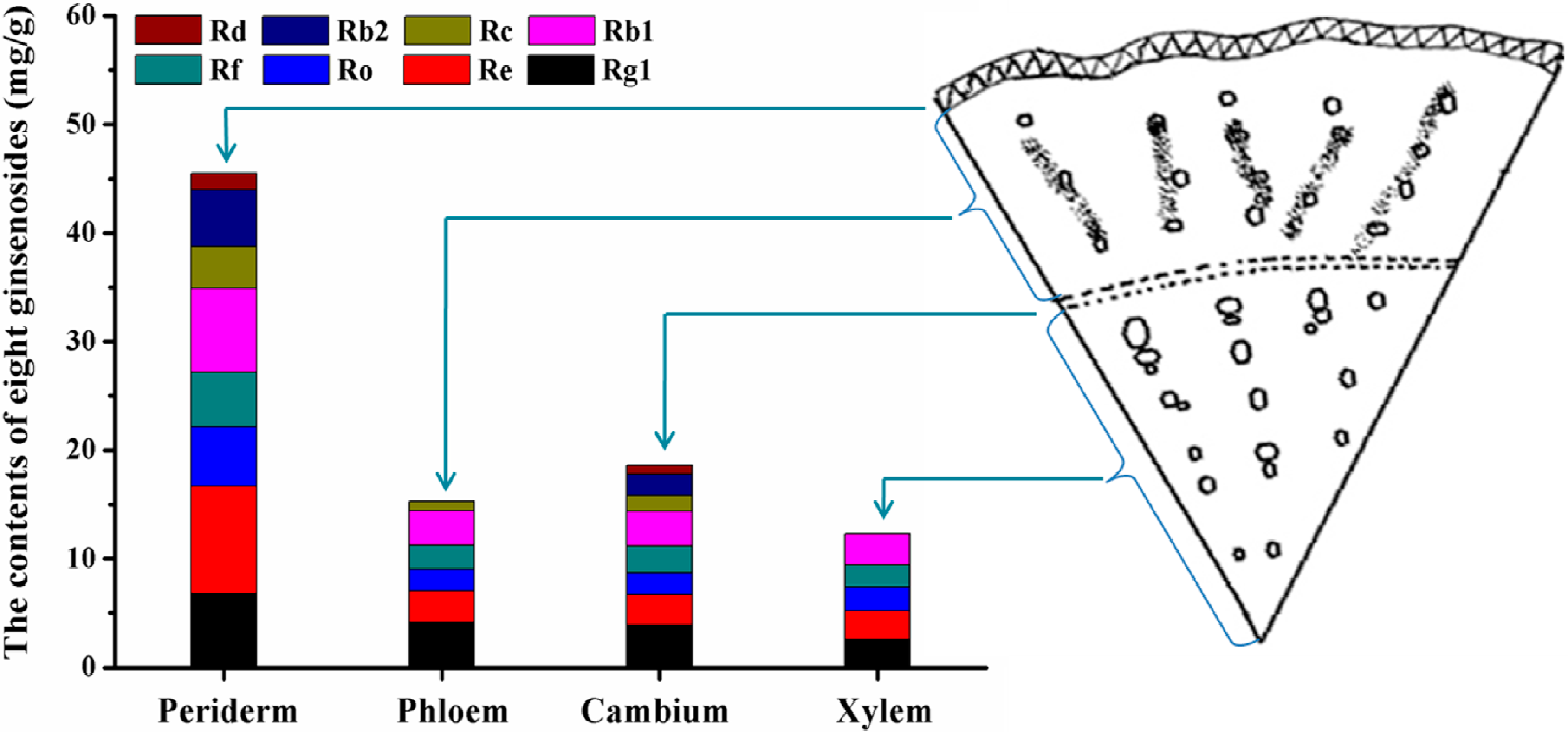
2.4. Ginsenoside Distribution Patterns in Four Parts of the Main Root
2.5. Antioxidant Evaluation of Ginseng Leaves
| Samples | DPPH (μg TE/mg) | ABTS (μg TE/mg) | HRSA (μg TE/mg) |
|---|---|---|---|
| 1 | 33.75 ± 1.57 | 52.45 ± 1.39 | 21. 87 ± 0.67 |
| 2 | 32.44 ± 2.32 | 51.02 ± 2.17 | 20.06 ± 0.34 |
| 3 | 38.73 ± 1.08 | 56.93 ± 0.90 | 23.52 ± 0.81 |
| 4 | 36.26 ± 0.79 | 57.45 ± 2.86 | 23.72 ± 1.45 |
| 5 | 46.10 ± 1.49 | 72.27 ± 0.52 | 29.73 ± 2.13 |
| 6 | 46.08 ± 0.21 | 71.08 ± 0.49 | 28.17 ± 0.87 |
| 7 | 46.36 ± 0.46 | 71.66 ± 2.25 | 28.66 ± 0.66 |
| 8 | 42.38 ± 1.15 | 66.38 ± 0.29 | 25.88 ± 0.62 |
| 9 | 41.12 ± 0.62 | 62.65 ± 1.02 | 25.08 ± 2.14 |
| 10 | 48.40 ± 3.61 | 74.26 ± 0.34 | 31.26 ± 0.68 |
| 11 | 41.82 ± 0.84 | 65.92 ± 1.54 | 25.49 ± 1.46 |
| 12 | 43.30 ± 0.05 | 71.30 ± 0.55 | 27.90 ± 0.82 |
| 13 | 41.06 ± 2.91 | 66.36 ± 1.01 | 25.96 ± 0.55 |
3. Experimental Section
3.1. Plant Materials
3.2. Reagents and Standards
3.3. Sample and Standard Solutions Preparation
3.4. Apparatus and UPLC Analytical Conditions
3.5. In Vitro Antioxidant Activity Assays
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef]
- Liu, Z.Q. Chemical insights into ginseng as a resource for natural antioxidants. Chem. Rev. 2012, 112, 3329–3355. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Hyam, S.R.; Jang, S.E.; Han, M.J.; Kim, D.H. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J. Agric. Food Chem. 2012, 60, 9595–9602. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Jensen, M.; Kidmose, U. Simultaneous determination of ginsenosides and polyacetylenes in American ginseng root (Panax quinquefolium L.) by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 8995–9003. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, C.J.; Zheng, B.; Gao, B.; Sun, A.M. Simultaneous determination of ten ginsenosides in American ginseng functional foods and ginseng raw plant materials by liquid chromatography tandem mass spectrometry. Food Anal. Methods 2013, 6, 112–122. [Google Scholar] [CrossRef]
- Sun, B.S.; Pan, F.Y.; Sung, C.K. Repetitious steaming-induced chemical transformations and global quality of black ginseng derived from Panax ginseng by HPLC-ESI-MS/MSn based chemical profiling approach. Biotechnol. Bioprocess Eng. 2011, 16, 956–965. [Google Scholar]
- Notice regarding the approval of ginseng (planted) for new resources of food. Available online: http://www.nhfpc.gov.cn/sps/s7891/201209/e94e15f2d9384b6795597ff2b101b2f1.shtml (accessed on 14 September 2012).
- Li, X.G.; Yan, Y.Z.; Jin, X.J.; Kim, Y.K.; Uddin, M.R.; Kim, Y.B.; Bae, H.H.; Kim, Y.C.; Lee, S.W.; Park, S.U. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. J. 2012, 9, 679–683. [Google Scholar]
- Shan, S.M.; Luo, J.G.; Huang, F.; Kong, L.Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J. Pharm. Biomed. Anal. 2014, 89, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Cho, J.G.; Bang, M.H.; Han, M.W.; Lee, M.H.; Yang, D.C.; Baek, N.I. Discrimination of Korean ginseng (Panax ginseng) roots using rapid resolution LC-QTOF/MS combined by multivariate statistical analysis. Food Sci. Biotechnol. 2011, 20, 1119–1124. [Google Scholar] [CrossRef]
- Kim, N.; Kim, K.; Choi, B.Y.; Lee, D.H.; Shin, Y.S.; Bang, K.H.; Cha, S.W.; Lee, J.W.; Choi, H.K.; Jang, D.S.; et al. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. J. Agric. Food Chem. 2011, 59, 10435–10441. [Google Scholar] [CrossRef]
- Jung, J.Y.; Jung, Y.; Kim, J.S.; Ryu, D.H.; Hwang, G.S. Assessment of peeling of astragalus roots using 1H-NMR- and UPLC-MS-based metabolite profiling. J. Agric. Food Chem. 2013, 61, 10398–10407. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lau, D.T.; Dong, T.T.; Zhang, J.; Choi, R.C.; Wu, H.Q.; Wang, L.Y.; Hong, R.H.; Li, S.H.; Song, X.; et al. Dual-index evaluation of character changes in Panax ginseng C.A. Mey stored in different conditions. J. Agric. Food Chem. 2013, 61, 6568–6573. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Luo, D.; Cheng, Y.J.; Ma, J.F.; Wang, Y.M.; Liang, Q.L.; Luo, G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MSn-based multicomponent quantification fingerprint. J. Agric. Food Chem. 2012, 60, 8213–8224. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Q.; Luo, J.G.; Kong, L.Y. Determination of 10 ginsenosides in Panax ginseng of different harvest times based on HPLC fingerprints and principal component analysis. Nat. Prod. Res. 2013, 27, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Myra, R.; Fang, B.; Christina, G.B. Neurotrophic and neuroprotective actions of ginsenosides Rb1 and Rg1. Planta Med. 2001, 67, 533–537. [Google Scholar]
- Saw, C.L.L.; Yang, A.Y.Q.; Cheng, D.C.; Boyanapalli, S.S.S.; Su, Z.Y.; Khor, T.O.; Gao, S.; Wang, J.R.; Jiang, Z.H.; Kong, A.N.T. Pharmacodynamics of ginsenosides: Antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem. Res. Toxicol. 2012, 25, 1574–1580. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Gyu, Y.S.; Woo, S.H.; Jin, W.H.; Young, S.K.; Kang, H.K.; Yukihiro, S.; Young, H.K. Ginsenosides from the leaves and flower buds of panax ginseng and their pharmacological effects. Curr. Bioact. Compd. 2011, 8, 159–166. [Google Scholar]
- Zhao, Y.L.; Wang, J.B.; Zhang, P.; Shan, L.M.; Li, R.S.; Xiao, X.H. Microcalorimetric study of the opposing effects of ginsenosides Rg1 and Rb1 on the growth of mice splenic lymphocytes. J. Therm. Anal. Calorim. 2011, 104, 357–363. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, L.H.; Jia, W.; Liu, X.M.; Dang, H.X.; Mai, W.L.; Wang, N.; Steinmetz, A.; Wang, Y.Q.; Xu, C.J. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother. Res. 2010, 24, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Luo, X.Y.; Liu, G.Z.; Chen, Y.P.; Wang, Z.C.; Sun, Y.X. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J. Agric. Food Chem. 2003, 51, 2555–2558. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Z.; Wu, J.P.; Xu, H.; Yuan, H.N. Research on the emetic effect of ginseng rhizome. Bull. Chin. Mater. Med. 1987, 6, 46–48. [Google Scholar]
- Zhang, D.W.; Zhang, S.C. Study on the emetic effect and medicinal value of ginseng rhizome. Ginseng Restaur. 1990, 2, 16–20. [Google Scholar]
- Luo, S.D.; Liu, A.Q.; Liu, Y.Y. Comparison of the active components and pharmacological effects between root and rhizome of ginseng. Pharm. Bull. 1983, 8, 22–24. [Google Scholar]
- Wu, Y.H.; Zheng, Y.; Zhang, L.Y.; Zhang, W.B.; Du, Y.P. Diversity evaluation in different parts of ginseng with HPLC coupled with principal component analysis. Pharm. Bull. 2011, 2, 161–164. [Google Scholar]
- China Pharmacopoeia Committee. China Pharmacopoeia; Chemical Industry Press: Beijing, China, 2010; Volume 1, p. 9. [Google Scholar]
- Lee, S.I.; Kwon, H.J.; Lee, Y.M.; Leed, J.H.; Hong, S.P. Simultaneous analysis method for polar and non-polar ginsenosides in red ginseng by reversed-phase HPLC-PAD. J. Pharm. Biomed. Anal. 2012, 60, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Li, P.Y.; Yeung, P. A simple HPLC assay for ginsenoside-Rh2 in plasma and its application for pharmacokinetic study in rats. Nat. Prod. Chem. Res. 2013, 1, 100103–100107. [Google Scholar]
- Wibo, B.S.; Ilco, A.L.A.B.; Alexander, L.L.D. Study on decomposition products of norbixin during bleaching with hydrogen peroxide and a peroxidase by means of UPLC-UV and mass spectrometry. Food Chem. 2012, 132, 1354–1359. [Google Scholar] [CrossRef]
- Pham, V.H.; David, W.H.; Wendy, B. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Chauveau-Duriot, B.; Doreau, M.; Nozière, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.T.; Li, J.; Zhang, H.Q.; Ding, L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007, 102, 1896–1901. [Google Scholar] [CrossRef]
- Qu, C.L.; Bai, Y.P.; Jin, X.Q.; Wang, Y.T.; Zhang, K.; You, J.Y.; Zhang, H.Q. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009, 115, 340–346. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Mihajilov-Krstev, T.M.; Nikolić, N.D.; Miladinović, L.C.; Cvetković, O.G. Investigation of the chemical composition—Antibacterial activity relationship of essential oils by chemometric methods. Anal. Bioanal. Chem. 2012, 403, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Zhang, H.; Zhou, P.P.; Han, S.N.; Han, Y.D.; Yuan, Y.J. Composition-activity relationship modeling to predict the antitumor activity for quality control of curcuminoids from Curcuma longa L. (turmeric). Anal. Methods 2013, 5, 641–647. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Li, X.G.; Zhang, C.X. Study on the chemical components of panax ginseng tissue. J. Jilin Agric. Univ. 1987, 9, 42–49. [Google Scholar]
- Kubo, M.; Tani, T.; Katsuki, T.; Ishizaki, K.; Arichi, S.; Histochemistry, I. Ginsenosides in Ginseng (Panax ginseng C.A. Meyer, root). J. Nat. Prod. 1980, 43, 278–284. [Google Scholar] [CrossRef]
- Sohala, R.S.; Orrb, W.C. The redox stress hypothesis of aging. Free Radic. Biol. Med. 2012, 52, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, R.; Lavranos, G.; Manolakou, P. ROS in the aging male: Model diseases with ROS-related pathophysiology. Reprod. Toxicol. 2009, 28, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Peng, D.C.; Xie, J.T. Ginseng leaf-stem: Bioactive constituents and pharmacological functions. Chin. Med. 2009, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Seog, H.M.; Choi, I.W.; Cho, H.Y. Antioxidant activities of cultivated and wild Korean ginseng leaves. Food Chem. 2005, 92, 535–540. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Schnee, S.; Andrey, Y.; Simoes-Pires, C.; Carrupt, P.A.; Wolfender, J.L.; Gindro, K. Study of leaf metabolome modifications induced by UV-C radiations in representative Vitis, Cissus and Cannabis Species by LC-MS based metabolomics and antioxidant assays. Molecules 2014, 19, 14004–14021. [Google Scholar] [CrossRef] [PubMed]
- China Pharmacopoeia Committee. China Pharmacopoeia; Chemical Industry Press: Beijing, China, 2010; Volume 1, p. 8. [Google Scholar]
- Lee, S.; Jung, E.S.; Do, S.G.; Jung, G.Y.; Song, G.; Song, J.M.; Lee, C.H. Correlation between species-specific metabolite profiles and bioactivities of blueberries (Vaccinium spp.). J. Agric. Food Chem. 2014, 62, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Antioxidant behavior in bulk oil: Limitations of polar paradox theory. J. Agric. Food Chem. 2012, 60, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Luan, L.Y.; Zhang, Z.W.; Huo, S.S.; Gao, X.; Fang, Y.L.; Xi, Z.M. Phenolic profiles and antioxidant properties of young wines made from Yan73 (Vitis vinifera L.) and cabernet sauvignon (Vitis vinifera L.) grapes treated by 24-Epibrassinolide. Molecules 2014, 19, 10189–10207. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fang, Y.L.; Gao, J.S.; Qiao, L.L.; Zhang, A.; Guo, M.Y. Phenolics composition and antioxidant activity of wine produced from spine grape (Vitis. davidiiFoex) and cherokee rose (Rosa laevigata Michx.) fruits from South China. J. Food Sci. 2012, 77, C8–C14. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-C.; Li, G.; Jiang, C.; Yang, B.; Yang, H.-J.; Xu, H.-Y.; Huang, L.-Q. Tissue-Specific Distribution of Ginsenosides in Different Aged Ginseng and Antioxidant Activity of Ginseng Leaf. Molecules 2014, 19, 17381-17399. https://doi.org/10.3390/molecules191117381
Zhang Y-C, Li G, Jiang C, Yang B, Yang H-J, Xu H-Y, Huang L-Q. Tissue-Specific Distribution of Ginsenosides in Different Aged Ginseng and Antioxidant Activity of Ginseng Leaf. Molecules. 2014; 19(11):17381-17399. https://doi.org/10.3390/molecules191117381
Chicago/Turabian StyleZhang, Ying-Chun, Geng Li, Chao Jiang, Bin Yang, Hong-Jun Yang, Hai-Yu Xu, and Lu-Qi Huang. 2014. "Tissue-Specific Distribution of Ginsenosides in Different Aged Ginseng and Antioxidant Activity of Ginseng Leaf" Molecules 19, no. 11: 17381-17399. https://doi.org/10.3390/molecules191117381
APA StyleZhang, Y.-C., Li, G., Jiang, C., Yang, B., Yang, H.-J., Xu, H.-Y., & Huang, L.-Q. (2014). Tissue-Specific Distribution of Ginsenosides in Different Aged Ginseng and Antioxidant Activity of Ginseng Leaf. Molecules, 19(11), 17381-17399. https://doi.org/10.3390/molecules191117381





