Effects of Ginsenoside Rg1 on the Expression of Toll-Like Receptor 3, 4 and Their Signalling Transduction Factors in the NG108-15 Murine Neuroglial Cell Line
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Ginsenoside Rg1 on TNF-α, IFN-β and iNOS Productions
| Group | Conc. (µg/mL) | TNF-α (pg/mL) | IFN-β (pg/mL) | iNOS (U/L) |
|---|---|---|---|---|
| Control | — | 27.43 ± 8.56 | 31.57 ± 7.31 | 1.09 ± 0.18 |
| Aβ25–35 | — | 73.12 ± 15.45 * | 92.25 ± 17.82 * | 3.67 ± 0.43 * |
| Rg1/Aβ25–35 | 2 | 67.48 ± 12.33 | 86.14 ± 15.33 | 3.21 ± 0.36 |
| 4 | 59.21 ± 13.14 Δ | 77.09 ± 10.89 | 2.94 ± 0.28 Δ | |
| 8 | 51.27 ± 13.06 ΔΔ | 72.32 ± 12.46 Δ | 2.56 ± 0.31 ΔΔ | |
| 16 | 46.21 ± 10.58 ΔΔ | 61.13 ± 9.33 ΔΔ | 2.14 ± 0.29 ΔΔ | |
| 32 | 42.16 ± 9.31 ΔΔ | 52.32 ± 7.56 ΔΔ | 1.97 ± 0.18 ΔΔ |
2.2. Effects of Ginsenoside Rg1 on the Levels of TLR3, TLR4, NF-κB and TRAF-6 mRNA
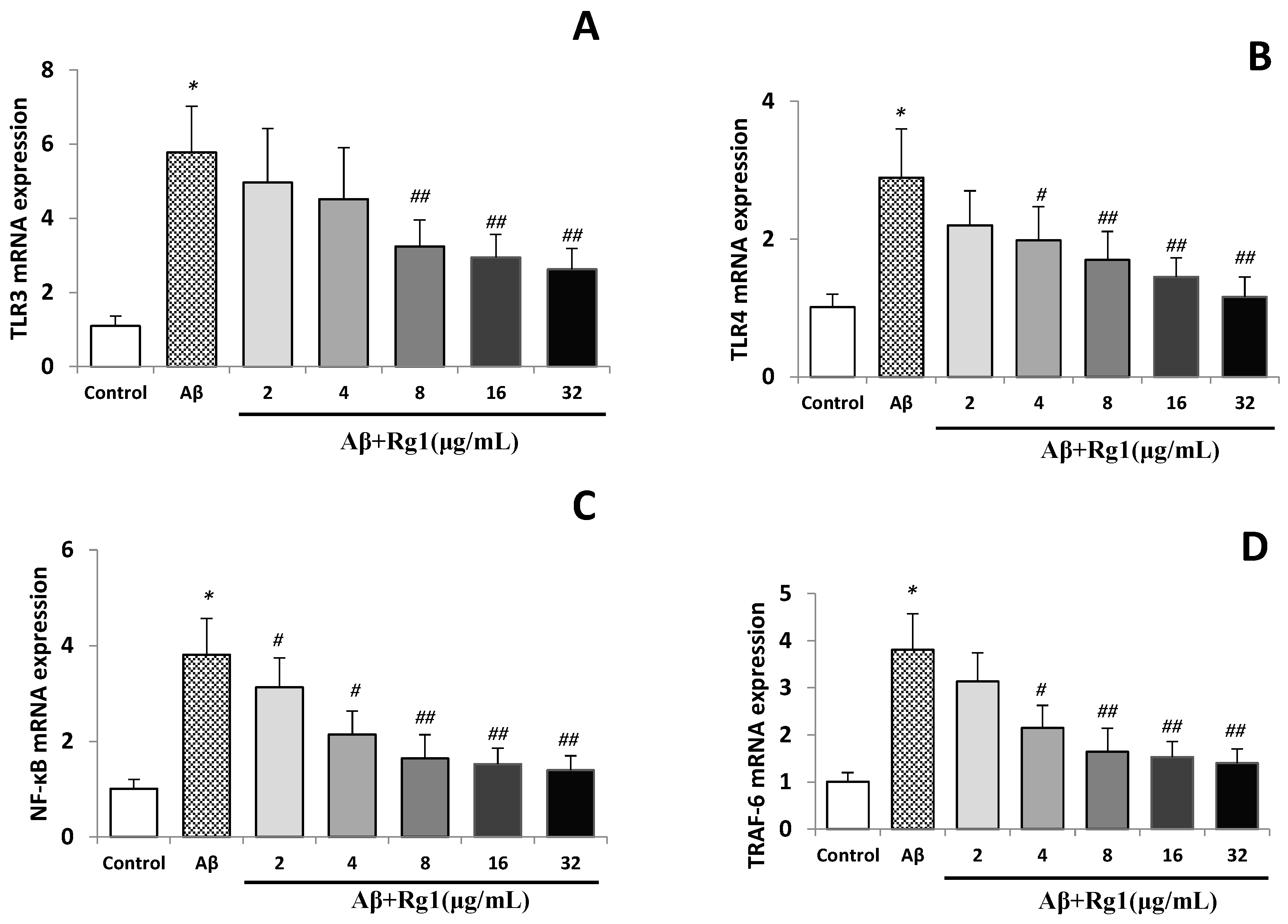
2.3. Effects of Ginsenoside Rg1 on the Protein Expressions of TLR3, TLR4, NF-κB and TRAF-6
2.4. Discussion
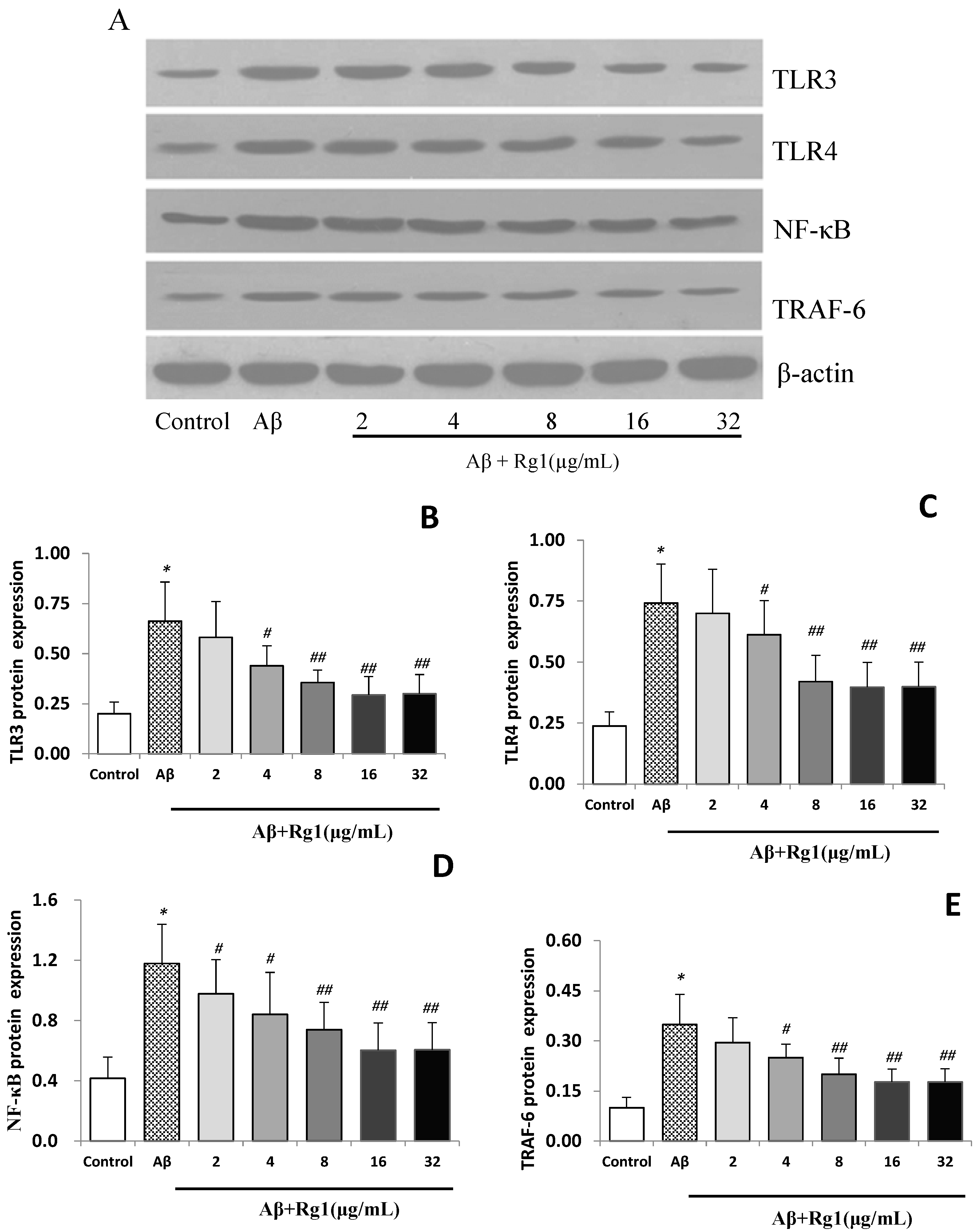
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Cell Culture and Treatments
3.3. Real-Time PCR Measurement
| Sense (F) | Anti-Sense (R) | |
|---|---|---|
| TLR3 | tGCCTTGGTCCCAAGCCTTCAACGA | TGGCCCGAAAACCTTCTTCTCAACGGA |
| TLR4 | CGCTTTCAGCTTTGCCTTCATTAC | TGCTACTTCCTTGTGCCCTGTGAG |
| NF-κB | GCTCGGCTGAATGAATCTACC | GTCTCCACGTATTTCCGCAACT |
| TRAF-6 | CAGGGATATGATGTGGAGT | TACCCTCAGGGAAAGAAT |
| β-actin | GTACCCCAGCATTGCTGACA | CTCCTGCTTGCTCATCCACATC |
3.4. Western Blotting Analysis
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daniilidou, M.; Koutroumani, M.; Tsolaki, M. Epigenetic mechanisms in Alzheimer’s disease. Curr. Med. Chem. 2011, 18, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; O’Brien, J. Clinical practice with anti-dementia drugs: A consensus statement from British Association for Psychopharmacology. J. Psychopharmacol. 2006, 20, 732–755. [Google Scholar] [CrossRef] [PubMed]
- Plant, L.D.; Webster, N.J.; Boyle, J.P.; Ramsden, M.; Freir, D.B.; Peers, C.; Pearson, H.A. Amyloid beta peptide as a physiological modulator of neuronal “A”-type K+ current. Neurobiol. Aging 2006, 27, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; McGeer, P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimer’s Dis. 2008, 13, 359–369. [Google Scholar]
- McGeer, P.L.; Rogers, J.; McGeer, E.G. Inflammation, anti-inflammatory agents and Alzheimer disease: The last 12 years. J. Alzheimer’s Dis. 2006, 9, 271–276. [Google Scholar]
- Akama, K.T.; van Eldik, L.J. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha TNFalpha-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J. Biol. Chem. 2000, 275, 7918–7924. [Google Scholar] [CrossRef] [PubMed]
- Weggen, S.L.; Eriksen, J.L.; Das, P.; Sagi, S.A.; Wang, R.; Pietrzik, C.U.; Findlay, K.A.; Smith, T.E.; Murphy, M.P.; Bulter, T.; et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 2001, 414, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef]
- Tahara, K.; Kim, H.D.; Jin, J.J.; Maxwell, J.A.; Li, L.; Fukuchi, K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain 2006, 129, 3006–3019. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Letiembre, M.; Liu, Y.; Heine, H.; Penke, B.; Hao, W.; Bode, B.; Manietta, N.; Walter, J.; Schulz-Schuffer, W.; et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell. Physiol. Biochem. 2007, 20, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [PubMed]
- Letiembre, M.; Hao, W.; Liu, Y.; Walter, S.; Mihaljevic, I.; Rivest, S.; Hartmann, T.; Fassbender, K. Innate immune receptor expression in normal brain aging. Neuroscience 2007, 146, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Copanaki, E.; Burbach, G.J.; Müller, U.C.; Deller, T. Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice. Neurosci. Lett. 2009, 453, 41–44. [Google Scholar] [CrossRef]
- Letiembre, M.; Liu, Y.; Walter, S.; Hao, W.; Pfander, T.; Wrede, A.; Schulz-Schaeffer, W.; Fassbender, K. Screening of innate immune receptors in neurodegenerative diseases: A similar pattern. Neurobiol. Aging 2009, 30, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.; Perry, N.S.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 2003, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wollen, K.A. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern. Med. Rev. 2010, 15, 223–244. [Google Scholar] [PubMed]
- Yu, L.C.; Chen, S.C.; Chang, W.C.; Huang, Y.C.; Lin, K.M.; Lai, P.H.; Sung, H.W. Stability of angiogenic agents, ginsenoside Rg1 and Re, isolated from Panax ginseng: In vitro and in vivo studies. Int. J. Pharm. 2007, 328, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T. Pathogensis and therapeutic strategies of senior dementia. Acta Pharmacol. Sin. 2000, 35, 635–640. [Google Scholar]
- Cheng, Y.; Shen, L.H.; Zhang, J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Shi, Y.L. The long-term effect of toosendanin on current through nifedipine-sensitive Ca2+ channels in NG108-15 cells. Toxicon 2005, 45, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Shi, Y.L. Characterization of an inward-rectifying potassium current in NG108-15 neuroblastoma × glioma cells. Pflug. Arch.-Eur. J. Physiol. 1997, 433, 617–625. [Google Scholar] [CrossRef]
- Cho, S.O.; Ban, J.Y.; Kim, J.Y.; Jeong, H.Y.; Lee, I.S.; Song, K.S.; Bae, K.; Seong, Y.H. Aralia cordata protects against amyloid beta protein 25–35-induced neurotoxicity in cultured neurons and has antidementia activities in mice. J. Pharmacol. Sci. 2009, 111, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Sáez, P.J.; Jiang, J.X.; Naus, C.C.; Sáez, J.C.; Giaume, C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Liang, Y.Q.; Tang, X.C.; He, X.C.; Bai, D.L. Stereoselectivities of enantiomers of huperzine A in protection against beta-amyloid25-35-induced injury in PC12 and NG108-15 cells and cholinesterase inhibition in mice. Neurosci. Lett. 2002, 317, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.; Rozemuller, J.M.; van Haastert, E.S.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Akira, S. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, C.; Nakayama, T. Toll-like receptors in the respiratory system: Their roles in inflammation. Curr. Allergy Asthma Rep. 2008, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.C.; Rasley, A.; Tranguch, S.L.; Marriott, I. Cultured astrocytes express toll-like receptors for bacterial products. Glia 2003, 43, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Arumugam, T.V.; Xu, X.; Cheng, A.; Mughal, M.R.; Jo, D.G.; Lathia, J.D.; Siler, D.A.; Chigurupati, S.; Ouyang, X.; et al. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. USA 2007, 104, 13798–13803. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Rossiter, J.P.; Lafon, M. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J. Neurovirol. 2006, 12, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.C.; Lorenz, E.; Schutte, B.C.; Zabner, J.; Kline, J.N.; Jones, M.; Frees, K.; Watt, J.L.; Schwartz, D.A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 2000, 25, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Huo, H.R.; Li, L.F.; Guo, S.Y.; Jiang, T.L. Sini tang prevents depression-like behavior in rats exposed to chronic unpredictable stress. Am. J. Chin. Med. 2009, 37, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Li, C.Y.; Ruan, Y.P.; Sun, M.; Qi, X.L.; Zhao, B.S.; Luo, F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur. J. Pharmacol. 2009, 612, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Zakrajsek, B.A.; Mills, A.G.; Gorn, V.; Singer, M.J.; Reed, M.W. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal. Biochem. 2000, 285, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.S.; Li, C.Y.; Yang, X.C.; Fang, J.; Yang, Y.X.; Guo, J.Y. Protective effect of gan mai da zao decoction in unpredictable chronic mild stress-induced behavioral and biochemical alterations. Pharm. Biol. 2010, 48, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound Ginsenoside Rg1 is not available from the authors (but is available from National Instutites for Food and Drug Control).
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.-S.; Liu, Y.; Gao, X.-Y.; Zhai, H.-Q.; Guo, J.-Y.; Wang, X.-Y. Effects of Ginsenoside Rg1 on the Expression of Toll-Like Receptor 3, 4 and Their Signalling Transduction Factors in the NG108-15 Murine Neuroglial Cell Line. Molecules 2014, 19, 16925-16936. https://doi.org/10.3390/molecules191016925
Zhao B-S, Liu Y, Gao X-Y, Zhai H-Q, Guo J-Y, Wang X-Y. Effects of Ginsenoside Rg1 on the Expression of Toll-Like Receptor 3, 4 and Their Signalling Transduction Factors in the NG108-15 Murine Neuroglial Cell Line. Molecules. 2014; 19(10):16925-16936. https://doi.org/10.3390/molecules191016925
Chicago/Turabian StyleZhao, Bao-Sheng, Yang Liu, Xiao-Yan Gao, Hua-Qiang Zhai, Jian-You Guo, and Xue-Yong Wang. 2014. "Effects of Ginsenoside Rg1 on the Expression of Toll-Like Receptor 3, 4 and Their Signalling Transduction Factors in the NG108-15 Murine Neuroglial Cell Line" Molecules 19, no. 10: 16925-16936. https://doi.org/10.3390/molecules191016925
APA StyleZhao, B.-S., Liu, Y., Gao, X.-Y., Zhai, H.-Q., Guo, J.-Y., & Wang, X.-Y. (2014). Effects of Ginsenoside Rg1 on the Expression of Toll-Like Receptor 3, 4 and Their Signalling Transduction Factors in the NG108-15 Murine Neuroglial Cell Line. Molecules, 19(10), 16925-16936. https://doi.org/10.3390/molecules191016925





