Abstract
We aimed to evaluate the in vitro effects of yerba maté, YGD (a herbal preparation containing yerba maté, guarana and damiana), and resveratrol on adipogenesis. The anti-adipogenic effects of yerba mate, YGD, resveratrol and YGD + resveratrol and yerba mate + resveratrol combinations were evaluated in 3T3-L1 cells by Oil Red staining, cellular triglyceride content, and PCR quantitative array. The results demonstrated that all of the tested compounds inhibited adipogenesis. Yerba maté extract significantly down-regulated the expression of genes that play an important role in regulating adipogenesis, such as Adig, Axin, Cebpa, Fgf10, Lep, Lpl, and Pparγ2. In addition, these genes, YGD also repressed Bmp2, Ccnd1, Fasn, and Srebf1. Resveratrol also modulated the expression of Adig, Bmp2, Ccnd1, C/EBPα, Fasn, Fgf10, Lep, Lpl, and Pparγ2. Moreover, resveratrol repressed Cebpb, Cdk4, Fgf2, and Klf15. The yerba maté extract and YGD up-regulated the expression of genes involved in inhibiting adipogenesis, such as Dlk-1, Klf2, and Ucp1. Resveratrol also induced the expression of Klf2 and Ucp1. In addition resveratrol modulated the Ddit3, Foxo1, Sirt1, and Sirt2. The combined effects of these compounds on gene expression showed similar results observed from individual treatments. Our data indicates that the synergy between the compounds favors the inhibition of adipogenesis.
1. Introduction
The high prevalence of obesity created a major public health concern because of the associated weight-related diseases that result in significant morbidity and mortality and reduced quality of life [1]. The increasing incidence of obesity suggests that this epidemic will continue to grow [2]. The global anti-obesity strategies focus on dietary and lifestyle modifications, i.e., restricting caloric intake and increasing physical activity, to slow down the development of obesity [3]. Research in the nutrition field has recently aroused considerable interest based on the potential of natural products to counteract obesity and the associated health complications [4,5].
Several studies have identified yerba maté (Ilex paraguariensis) as an excellent candidate in the struggle against obesity [6,7,8,9,10,11,12,13,14,15,16,17]. Data obtained from experiments conducted in diet-induced obesity models have shown that yerba maté suppresses body weight gain and visceral fat accumulation and decreases serum levels of cholesterol, triglycerides, LDL cholesterol, glucose, insulin, pancreatic lipase and leptin [6,10,14,15,16,17,18,19,20]. The molecular mechanisms by which yerba maté regulates obesity have also been studied. In vitro and in vivo studies have shown that yerba maté modulates signaling pathways, which regulate adipogenesis, antioxidant, anti-inflammatory and insulin signaling responses [6,7,8,9,10,11,12,14,15,16,17,21,22].
The anti-obesity role of yerba maté was also evaluated in humans. In a clinical study, it has been demonstrated that a herbal preparation containing yerba maté, Guarana and Damiana (“YGD”) significantly delayed gastric emptying, reduced the time to perceived gastric fullness, and induced significant weight loss over 45 days in overweight patients [23]. Subsequently, YGD has been demonstrated to produce a robust acute effect on caloric intake and eating duration, suggesting YGD strengthens within-meal satiation, an effect that may be mediated by changes in gastric emptying time [24].
Another important candidate to manage obesity is resveratrol (3,4,5-trihydroxystilbene), a naturally occurring polyphenol found in different plant species [25]. Resveratrol was first studied because of its cardioprotective effects. Throughout the years, epidemiological studies have shown that due to its high content of resveratrol, a moderate intake of wine, especially red wine, reduces the risk of cardiovascular disease [26]. Besides on initial cardioprotective effects, further on the anti-cancer, anti-inflammatory, antioxidant, and anti-obesity properties of resveratrol have been also studied and characterized [27,28,29,30].
Several in vitro and animal studies have demonstrated that resveratrol has an antiobesity potential by inhibiting adipocyte differentiation, decreasing proliferation, inducing apoptosis, decreasing lipogenesis, and promoting lipolysis and FA β-oxidation [30,31,32,33,34,35,36,37,38,39,40,41,42]. Additionally, clinical studies have shown antiobesity potential for resveratrol in obese volunteers [43,44,45,46]. Taken together, the data from clinical studies, in vitro and animal data indicate that the potential antiobesity effects and health promoting effects of resveratrol in the context of obesity should be better comprehended.
Considering the importance of adipogenesis in the obesity, the present study aimed to evaluate the in vitro effects of yerba maté, YGD, and resveratrol on expression level of key genes involved in the process of adipogenesis.
2. Results and Discussion
The MTT analysis revealed that YGD yerba maté and resveratrol were not cytotoxic to 3T3-L1 cells. Concerning the YGD + resveratrol and yerba maté + resveratrol mixtures the MTT revealed they were not cytotoxic.
The ability of YGD, yerba maté (50, 75, 100, 150, 200 or 300 μg/mL) or resveratrol (10, 20 or 30 μg/mL) to prevent lipid accumulation was examined by Oil Red O staining and evaluation of triglyceride content of 3T3-L1 adipocytes. Our data indicated that all of the tested compounds inhibited adipogenesis, but at different concentrations. YGD inhibited adipogenesis in both methods at a concentration of 100 µg/mL (Figure 1A and Figure 2A). Yerba maté significantly inhibited adipogenesis at a concentration of 150 µg/mL (Figure 1B and Figure 2B). Resveratrol had inhibitory activity at the lowest concentration tested (Figure 1C and Figure 2C). Next the effects of YGD + resveratrol was evaluated (100:10 or 150:10 μg/mL), or yerba maté + resveratrol (150:10 or 200:10 μg/mL) in the inhibition of adipogenesis. Our data also showed that all of the tested combination inhibit the adipogenesis (Figure 1D and Figure 2D). The Figure 3 illustrates the significative results obtained from Oil Red O staining.
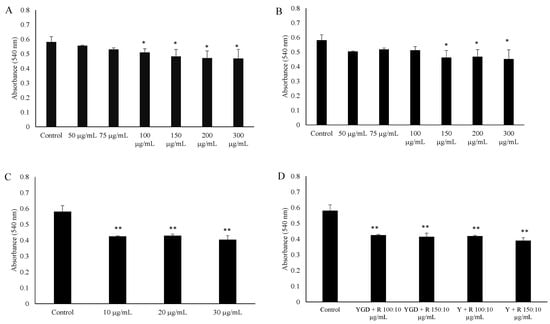
Figure 1.
Effect of (yerba maté, guarana and damiana) YGD (A); yerba maté (B); resveratrol (C) or mixed compounds (D) on adipogenesis in 3T3-L1 cells according to Oil Red O Assay. * p < 0.05 and ** p < 0.01 compared with control group.
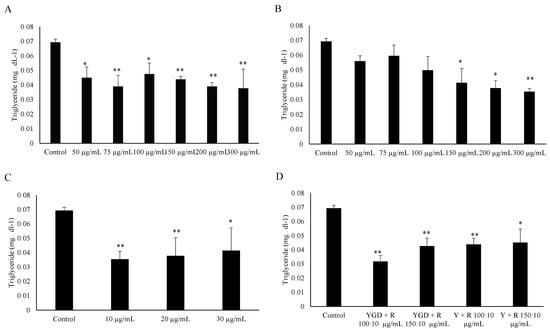
Figure 2.
Effect of YGD (A); yerba maté (B); resveratrol (C) or mixed compounds (D) on adipogenesis in 3T3-L1 cells according to evaluation of triglyceride content. * p < 0.05 and ** p < 0.01 compared with control group.
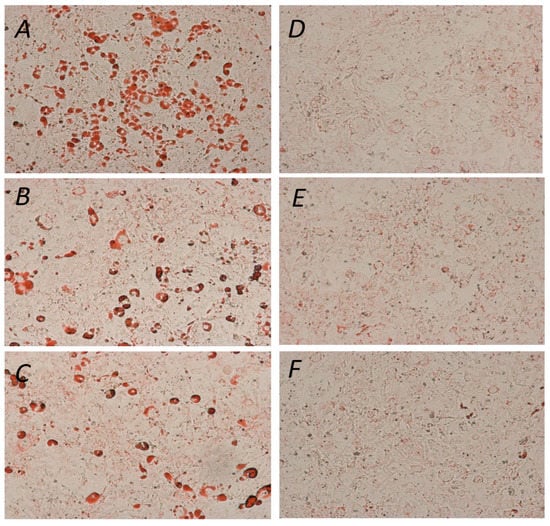
Figure 3.
Effect of yerba maté, YGD and resveratrol on 3T3-L1 differentiation. (A) Control; (B) YGD (100 μg/mL); (C) yerba maté (150 μg/mL); (D) resveratrol (10 μg/mL); (E) YGD + resveratrol (100:10 μg/mL); and (F) yerba maté + resveratrol (150:10 μg/mL). Adipogenic differentiation was assessed using Oil Red O staining for lipid droplets 100 ×.
The anti-adipogenic effects occurred at concentrations that did not affect cell viability. Regarding yerba maté, our data indicated that it inhibited adipogenesis, as previously described before [12,14]. The YGD also regulated this process negatively, but at a lower concentration. We could infer that this result might be explained by the presence of guarana and damiana in the YGD formulation. However, there is no evidence in the literature showing the anti-adipogenic effects of these compounds. Resveratrol also showed an effect on adipogenesis as reported previously [31,47]. The combined effects of these compounds on adipogenesis were also evaluated. The data presented shows that both combinations (YGD + resveratrol, and yerba maté + resveratrol) inhibited adipogenesis at the lowest concentration. It is noted that the level of inhibition of adipogenesis with the combinations is higher than that observed for resveratrol alone. The results are attributable to a synergistic effect obtained from the potent anti-adipogenic effect of resveratrol in combination with the other compounds.
To determine the effect of YGD (100 μg/mL), yerba maté (150 μg/mL), resveratrol (10 μg/mL), YGD + resveratrol (100:10 μg/mL), or yerba maté + resveratrol (150:10 μg/mL) on the expression of several genes that regulate adipogenesis, the Mouse Adipogenesis RT2 ProfilerTM PCR Array (SABiosciences) was utilized. This assay was performed at the lowest concentrations in which an inhibition of adipogenesis by both employed methods was observed. The triplicate samples from each group gave reproducible results.
The results from the PCR array revealed that the yerba maté extract modulated the expression of 12% (10/84) the genes. YGD modulated the expression of 17% (14/84) of the genes. Although YGD modulates the expression of more genes than yerba maté, we noted that both compounds act in the same pathways of adipogenesis (Table 1). Concerning the resveratrol effects on adipogenesis, our data showed that this compound modulated the expression of 24% (20/84) of the genes. Among these genes, 10 were exclusively modulated by resveratrol that were not modulated by other test compounds. Thus, these data allow us to infer that resveratrol could act in adipogenesis in a more comprehensive way and somewhat distinct from the yerba maté based compounds. The analysis of tested mixtures is also shown in Table 1. Our data indicated that the synergic effects of the combination resveratrol -yerba maté or -YGD might be responsible for the modulation of all studied genes.
The Yerba maté extract significantly down-regulated the expression of genes that play an important role in regulating adipogenesis, including Adig (Adipogenin), Axin (Axin 1), Cebpa (CCAAT/enhancer binding protein (C/EBP) alpha), Fgf10 (Fibroblast growth factor 10), Lep (Leptin), Lpl (Lipoprotein lipase), and Pparγ2 (Peroxisome proliferator-activated receptor, gamma 2). In addition to those genes, YGD also down-regulated Bmp2 (Bone morphogenetic protein 2), Ccnd1 (Cyclin D1), Fasn (Fatty acid synthase), and Srebf1 (Sterol regulatory element binding transcription factor 1). As observed for yerba maté and/or YGD, resveratrol also modulated the expression of Adig, Bmp2, Ccnd1, C/EBPα, Fasn, Fgf10, Lep, Lpl, and Pparγ2. Moreover, resveratrol repressed Cebpb (CCAAT/enhancer binding protein (C/EBP), beta), Cdk4 (Cyclin-dependent kinase 4), Fgf2 (Fibroblast growth factor 2), and Klf15 (Kruppel-like factor 15). The yerba maté extract and YGD up-regulated the expression of genes involved in inhibiting adipogenesis, such as Dlk-1 (Delta-like 1 homolog), Klf2 (Kruppel-like factor 2), and Ucp1 (Uncoupling protein 1). Resveratrol also induced the expression of Klf2 and Ucp1. Additionally, resveratrol modulated the Ddit3 (DNA-damage inducible transcript 3), Foxo1 (Forkhead box O1), Sirt1 (Sirtuin 1), and Sirt2 (Sirtuin 2) gene expression levels. The combined effects of these compounds on gene expression showed similar results on expression level of targeted genes.

Table 1.
Fold regulation results from the PCR array.
| Functional Gene Group | Gene | Yerba maté 150 μg/mL | YGD 100 μg/mL | Resveratrol 10 μg/mL | YGD 100 + R 10 μg/mL | Y 150 + R 10 μg/mL |
|---|---|---|---|---|---|---|
| Pro-adipogenic | Adig | −2.08 ± 0.31 * | −2.46 ± 0.93 * | −2.81 ± 0.32 * | −2.04 ± 0.47 * | −2.43 ± 0.59 * |
| Axin | −2.20 ± 0.83 * | −2.80 ± 0.86 * | −1.58 ± 0.11 | −2.99 ± 0.68 * | −2.60 ± 0.51 * | |
| Bmp2 | −1.95 ± 0.54 | −4.20 ± 1.58 * | −2.00 ± 0.11 * | −3.65 ± 0.94 * | −2.01 ± 0.93 * | |
| Cebpa | −4.13 ± 0.63 * | −7.20 ± 2.11 * | −2.29 ± 0.31 * | −5.58 ± 1.58 * | −4.41 ± 1.48 * | |
| Cebpb | −1.04 ± 0.14 | −1.24 ± 0.+23 | −2.28 ±0.88 * | −2.69 ± 0.69 * | −2.61 ± 0.62 * | |
| Ccnd1 | −1.92 ± 0.58 | −2.17 ± 0.83 * | −2.65 ± 0.65 * | −-2.00 ± 0.65 * | −2.06 ± 0.65 * | |
| Cdk4 | 1.12 ± 0.44 | 1.81 ± 0.28 | −2.39 ± 0.65 * | −2.88 ± 0.69 * | −3.74 ± 0.77 * | |
| Fasn | −1.97 ± 0.18 | −2.53 ± 0.64 * | −2.31 ±0.48 * | −2.52 ± 0.82 * | −2.22 ± 0.12 * | |
| Fgf2 | −1.41 ± 0.09 | −1.34 ± 0.49 | −2.36 ±0.81 * | −2.45 ± 0.51 * | −3.25 ± 0.31 * | |
| Fgf10 | −3.19 ± 0.69 * | −3.59 ± 1.64 * | −4.89 ± 1.94 * | −2.74 ± 0.34 * | −3.55 ± 0.84 * | |
| Klf15 | −1.25 ± 0.21 | −1.88 ± 0.22 | −3.83 ± 0.97 * | −4.55 ± 0.94 * | −4.77 ± 0.84 * | |
| Lep | −3.32 ± 0.45 * | −4.52 ± 1.21 * | −2.07 ± 0.41 * | −2.21 ± 0.61 * | −2.38 ± 0.96 * | |
| Lpl | −2.94 ± 0.69 * | −6.79 ± 1.65 * | −7.03 ± 0.91 * | −6.21 ± 2.01 * | −4.09 ± 1.19 * | |
| Pparg | −2.02 ± 0.22 * | −3.08 ±1.05 * | −2.41 ± 0.89 * | −2.69 ± 0.88 * | −2.24 ± 0.51 * | |
| Srebf1 | −1.87 ± 0.35 | −2.97 ± 1.18 * | -1.06 ± 0.58 | −2.32 ± 0.76 * | −2.88 ± 0.83* | |
| Anti-adipogenic | Ddit3 | 1.00 ± 0.14 | 1.47 ± 0.72 | 2.47 ± 0.57 * | 2.51 ± 0.40 * | 2.73 ± 0.52 * |
| Dlk1 | 4.82 ± 1.42 * | 1.01 ± 0.23 | 1.44 ± 0.81 | 2.98 ± 0.88 * | 3.61 ± 0.89 * | |
| Foxo1 | 1.87 ± 0.50 | 1.78 ± 0.29 | 2.30 ± 0.88 * | 3.54 ± 0.92 * | 3.65 ± 0.91 * | |
| Klf2 | 3.91 ±1.06 * | 4.82 ± 1.14 * | 5.57 ± 1.28 * | 3.65 ± 0.55 * | 4.63 ± 0.73 * | |
| Ucp1 | 3.47 ± 1.59 * | 4.15 ± 1.67 * | 6.29 ± 2.16 * | 6.02 ± 1.99 * | 6.80 ± 2.73 * | |
| Sirt1 | 1.23 ± 0.13 | 1.30 ± 0.50 | 2.94 ± 0.88 * | 3.20 ± 1.02 * | 3.08 ± 0.99 * | |
| Sirt2 | 1.79 ± 0.48 | 1.52 ± 0.22 | 2.08 ± 0.27 * | 2.88 ± 0.92 * | 2.76 ± 0.89 * |
* Significative down-regulation was considered when the fold regulation was ≤−2; significative up-regulation was considered when the fold regulation was ≥+2.
Adipogenesis is the developmental process by which a multipotent mesenchymal stem cell differentiates into a mature adipocyte. This process involves a highly regulated and coordinated cascade of transcription factors, including members of the PPAR, C/EBP, and SREBP families, which together lead to the establishment of the differentiated state [48]. In this context, it has been observed that yerba maté and resveratrol modulates adipogenesis by down regulating the levels of pro-adipogenic transcription factors, such as Pparγ2 and Cebpa, in vivo [6,14,23,33,35,37,38]. Similarly, our data also shows that yerba maté, YGD, resveratrol, and their combinations were able to down-regulate the expression of Pparγ2 and Cebpa. Additionally, Cebpb was repressed by resveratrol, as previously described [36], and by the mixed compounds.
SREBP1 is a transcription factor that controls fatty acid synthase, and functions an additional regulator of adipogenesis in parallel with C/EBPα and PPARγ pathways [49]. Here, we demonstrated that YGD and the mixed compounds down-regulated the downstream target gene Fasn. Fasn was also down regulated by resveratrol as previously described [50]. The data presented in this study also show that the tested compounds were able to repress Adig and Srebf1, adipogenic markers that mediate the initiation of adipogenesis.
Numerous effects of the KLF protein family have been reported in different tissues. In the 3T3-L1 cell line model, KLF2 inhibits adipogenesis by inhibiting PPARγ [51]. By assessing the expression of the Klf2, it was shown that all tested compounds increased the expression level of this gene, which may then contribute to the observed repression of Pparγ2 expression. Similar results were previously observed for yerba maté [14]. On the other hand, KLF15 plays an essential role in adipogenesis promoting the differentiation of 3T3-L1. It has been proposed that the cooperative effects of KLF15, PPARγ, and C/EBPα are responsible for the full range of adipocyte-specific gene expression during the differentiation process of adipocytes [52]. Our gene expression data showed that resveratrol and the mixed compounds down-regulated the expression of Klf15, further contributing to the inhibition of adipogenesis.
In addition, expression of C/EBPs and PPARγ is dependent on the activation of other genes that are essential for adipogenesis such as Dlk1.It has been reported that this gene contributes to inhibition of adipogenesis via inhibitory actions on C/EBP-β and C/EBP-δ in the pre-adipocyte cell line 3T3-L1. It is believed that down-regulation of Dlk1 is critical for adipogenesis to occur [53,54]. We observed that yerba maté, as previously described [14], and the combination of compounds tested in this study up-regulated Dlk1, which might be at least in part, responsible for the observed inhibition of adipogenesis. The results presented in this study show that all of the tested compounds down regulated the Pparγ and Cebpa mRNA expression, which leads to further down regulation of other genes such as Lep and Lpl. Similar data were previously observed for yerba maté [7] and resveratrol [30].
Bone morphogenetic proteins (Bmps), are members of the transforming growth factor-β (Tgf-β) family, which regulate diverse physiological processes including adipose tissue formation [55]. It has been demonstrated that Bmp activity prior to differentiation induction is responsible for adipocyte differentiation. Thus, it has been proposed that BMP-2 may act as a potent adipogenic agent if presented together with activators of PPARγ [56]. The data presented in this work shows that YGD, resveratrol and the mixed compounds were able to significantly down-regulate this pro-adipogenic gene.
FOXO1 is a transcription factor which negatively regulate the adipogenesis [57,58] . It has been shown that FOXO1 can bind to PPARγ promoter region and suppress its expression [58]. Complementarily, SIRT1 and SIRT2 have been shown to regulate the adipogenesis through modulation of FOXO1 acetylation/phosphorylation activity and may play a role in the inhibition of adipocyte differentiation [59,60]. It has been observed that resveratrol is an indirect activator of SIRT1 and SIRT2 leading to the suppression of adipogenesis [61,62]. Thus, the up-regulation of Sirt1, Sirt2 and Foxo1 by resveratrol and its combination with yerba mate or YGD observed in this study might contribute to the exhibited down-regulation of Pparg2.
It has been described that Ddit3 (also known as Chop-10) is a dominant-negative member of the C/EBP family. It is initially expressed by growth-arrested preadipocytes and sequesters/inactivates C/EBPβ during 3T3-L1 preadipocyte differentiation. Thus, its down-regulation is required for the complete adipocyte differentiation [63,64]. Therefore, the up-regulation observed after resveratrol and mixed compounds treatment might be one of the mechanisms by which these compounds exert their antiadipogenic effect. Another C/EBPβ regulator is Fgf10, which plays crucial roles in adipogenesis by contributing to the expression of C/EBPβ through an autocrine/paracrine mechanism [65]. Although our data shows that all of the tested products were able to significantly down-regulate Fgf10, a direct effect was observed only for resveratrol and mixed compounds. Additionally, Cdk4 activity has been proposed to be required for adipogenesis. Cdk4 participates in the clonal expansion phase of adipocyte differentiation through activation of PPARγ [66]. Our data showed that resveratrol and the mixed compounds were able to down-regulate Cdk4, which might contribute to the Pparg repression observed.
It has been proposed that WNT/β-catenin pathway exert a negative regulation on adipogenesis [67]. When the WNT/β-catenin pathway is inactive, β-catenin is proteasomally degraded by the destruction complex composed of adenomatous polyposis coli (APC), glycogen synthase kinase (GSK)3β and AXIN [68]. Activation of the WNT/β-catenin pathway induces the expression of its target genes including cyclin D1 (CCND1), which has been reported to down-regulate PPARγ, a major adipogenic transcription factor [69]. In our study, Axin, which is known to be a negative regulator of the WNT/β-catenin pathway, was down-regulated by yerba maté, YGD and the combination of compounds. Regarding Ccnd1, our data show an inhibition of its expression after YGD, resveratrol and mixed compound treatments. These results are in accordance with a previous study, which demonstrated an early effect of resveratrol on Ccnd1 down-regulation. The authors also observed a subsequent inhibition of cell cycle reentry and clonal expansion [70], which leads to an inhibition of adipogenesis.
Ucp1 is known to be a significant component of whole body energy expenditure, and its dysfunction contributes to the development of obesity. In this study, we observed that all of the tested compounds were able to up-regulate the expression of Ucp1. Similar results were previously described for yerba maté [6,7] and resveratrol [39,71].
3 Experimental Section
3.1. Materials
The soluble yerba maté powder and the patented herb extract formulation YGD (Zotrim, which contains 46% yerba mate, 39% guarana and 15% damiana extracts) were provided by Natures Remedies, Amersham, UK. Resveratrol and Oil Red O were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture reagents were purchased from Invitrogen (Invitrogen Life Technologies, Alameda, CA, USA).
3.2. In Vitro Evaluation of Adipogenesis
3.2.1. 3T3-L1 Cell Culture
The 3T3-L1 cell line was purchased from the Rio de Janeiro Cell Bank (Rio de Janeiro, Brazil) and it was cultured to confluence in DMEM supplemented with 10% fetal bovine serum and 10 mL/L penicillin/streptomycin at 37 °C and 5% CO2. Forty-eight hours after achieving confluence (day 0), the cells were incubated in differentiation medium (DMEM supplemented with 10% fetal bovine serum, 0.25 μM dexamethasone, 10 μg/mL insulin and 0.5 mM IBMX). After 96 h (day 4), the cells were then cultured in maturation medium (DMEM supplemented with 10% foetal bovine serum and 5 μg/mL insulin) for 10 days.
For the MTT and Oil Red O assays the cells were incubated with YGD, yerba maté (at the following concentrations: 50, 75, 100, 150, 200 or 300 μg/mL), resveratrol (at the following concentrations: 10, 20 or 30 μg/mL), YGD + resveratrol (100:10 or 150:10 μg/mL), or yerba maté + resveratrol (150:10 or 200:10 μg/mL) during the differentiation period (from day 0 to day 4).
3.2.2. Cytotoxicity Determination—MTT Assay
Cell toxicity was estimated by using the tetrazolium salt reduction test (MTT assay) on 3T3-L1 cells after exposure with the described compounds (at day 4). Briefly, the cells were cultured as described and incubated with the compounds or vehicle (0.1% DMSO) at 37 °C in 5% CO2. The supernatant was discarded and MTT was added (5 mg/mL in PBS). Cells were incubated for 3 h at 37 °C in 5% CO2, after which 100 µL 10% sodium dodecyl sulfate in 0.01 M HCl was added to each well. Samples were then incubated for 18 h at 37 °C and absorbance was measured at 540 nm in a microplate reader (Multiscan MS: Labsystems, Joensuu, Finland). Data are presented as mean ± SEM from three independent experiments (each in triplicate).
3.2.3. 3T3-L1 Differentiation Assay—Oil Red O
Differentiation was evaluated using an Oil Red O staining assay. Briefly, the cells were incubated with the compounds during the differentiation period (day 0). At the end of the maturation period, the cells were washed with PBS, fixed in 4% paraformaldehyde for 30 min and incubated for 1 h with 1% Oil Red O solution [72]. After multiple water washes, the Oil Red O was dissolved in 100% isopropyl alcohol, and the optical density was measured on a microplate spectrophotometer at 540 nm. The data are presented as mean ± SEM from three independent experiments (each in triplicate).
3.2.4. Quantification of the Triglyceride Content
Intracellular triglycerides content was assessed using commercial kit (LABORLAB, Guarulhos-SP, Brazil). Briefly, the cells were incubated with the compounds during the differentiation period (day 0) as previously described. At the end of the maturation period, the cells were deprived of fetal bovine serum 24 h before harvest. The absorbance at 550 nm is proportional to the concentration of triglycerides of each sample. All samples were determined in triplicate.
3.2.5. RNA Extraction and cDNA Synthesis
The 3T3-L1 cells were cultured as previously described. The material used for RNA extraction was harvested at the end of the differentiation period (day 4). To stabilize and protect the RNA, all samples were stored at −80 °C in RNAlater (Qiagen, Valencia, CA, USA). Total RNA was isolated using the RNeasy tissue kit (Qiagen Valencia, CA, USA). After the extraction, ~100 ng of RNA was used for cDNA synthesis using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol.
The Mouse Adipogenesis RT2 Profiler™ PCR Array (SABiosciences Corporation, Frederick, MD, USA) was utilized to determine the expression profile of 84 key adipogenesis genes.
The gene array profiled the expression of 84 genes, including regulatory genes, PPARγ targets, and genes related to adipogenesis. In addition, the array included controls for human genomic DNA contamination and the reverse transcription step, a positive control for the PCR, and 5 housekeeping genes (ribosomal protein large P1 (Rplp1), hypoxanthine phosphoribosyltransferase 1 (Hprt1), ribosomal protein L13A (Rpl13a), lactate dehydrogenase A (Ldha), and beta-actin (Actb)).
Quantitative PCR was performed on a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and the threshold cycle numbers were determined using RQ Study Software (Applied Biosystems, Foster, CA, USA). The reactions were performed in duplicate, and the threshold cycle numbers were averaged. The 50 µL reaction mixtures contained 25 µL Platinum SYBR Green Quantitative PCR SuperMix-UDG (Invitrogen™ Life Technologies, Alameda, CA, USA) and 100 ng of cDNA. The reactions were cycled through a preliminary UDG treatment for 2 min at 50 °C and 2 min denaturation at 95 °C, which was followed by 45 cycles of denaturation at 95 °C for 15 s, annealing for 15 s, and primer extension at 72 °C for 15 s. Subsequently, a melting point analysis of the double-stranded amplicons was performed that consisted of 40 cycles with a 1 °C decrement (15 s each) in each cycle, beginning at 95 °C. Data are presented as mean ± SD from three independent experiments (each in triplicate). The data were analyzed using web-based PCR data analysis software (SABiosciences) and normalised to the 5 housekeeping genes.
3.3. Statistical Analysis
The data obtained were analyzed using the SPSS 13.0 statistical Software (SPSS Incorporation Chicago, IL, USA). Comparisons between groups of data were performed using a one-way ANOVA followed by Dunnett’s Multiple Comparisons. An associated probability (p value) of less than 5% was considered to be significant (p < 0.05). For gene expression, significative down-regulation was considered when the fold regulation was ≤−2; significative up-regulation was considered when the fold regulation was ≥+2.
4. Conclusions
This study determined that yerba maté, YGD and resveratrol regulate the expression of genes that are involved in adipogenesis. Furthermore, these compounds are postulated to regulate adipogenesis through the WNT pathway, which would result in a significant repression of Pparγ2 and C/EBP-α. The results also showed that resveratrol regulates a greater number of targets than yerba maté and YGD alone. The combination of resveratrol -yerba mate or -YGD indicates that the synergy between the compounds might favors the inhibition of adipogenesis by modulation the expression of all studied genes.
Acknowledgments
This work was supported by Natures Remedies Ltd., Amersham, UK.
Author Contributions
Juliana C. Santos—Data collection and analysis; Érica M. F. Gotardo—Data collection and analysis; Mitsue T. Brianti—Data collection and analysis; Mahmood Piraee—Study design and conception; Alessandra Gambero—Analysis and interpretation of data and writing manuscript; Marcelo L. Ribeiro—Study design and conception, analysis and interpretation of data and writing manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar]
- Waxman, A. Why a global strategy on diet, physical activity and health? World Rev. Nutr. Diet. 2005, 95, 162–166. [Google Scholar]
- Santos, A.P.; Rogero, M.M.; Bastos, D.H. Edible plants, their secondary metabolites and antiobesogenic potential. Recent Pat. Food Nutr. Agric. 2010, 2, 195–212. [Google Scholar]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008, 19, 717–726. [Google Scholar]
- Pang, J.; Choi, Y.; Park, T. Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: Potential role of ampk in the visceral adipose tissue. Arch. Biochem. Biophys. 2008, 476, 178–185. [Google Scholar]
- Arcari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; Funck, A.; Pedrazzoli, J.; de Souza, M.F.; Saad, M.J.; Bastos, D.H.; Gambero, A.; et al. Antiobesity effects of yerba mate extract (Ilex paraguariensis) in high-fat diet-induced obese mice. Obesity 2009, 17, 2127–2133. [Google Scholar]
- Arcari, D.P.; Bartchewsky, W., Jr.; dos Santos, T.W.; Oliveira, K.A.; DeOliveira, C.C.; Gotardo, E.M.; Pedrazzoli, J., Jr.; Gambero, A.; Ferraz, L.F.; Carvalho Pde, O.; et al. Anti-inflammatory effects of yerba mate extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Mol. Cell Endocrinol. 2011, 335, 110–115. [Google Scholar]
- Hussein, G.M.; Matsuda, H.; Nakamura, S.; Hamao, M.; Akiyama, T.; Tamura, K.; Yoshikawa, M. Mate tea (Ilex paraguariensis) promotes satiety and body weight lowering in mice: Involvement of glucagon-like peptide-1. Biol. Pharm. Bull. 2011, 34, 1849–1855. [Google Scholar]
- Kang, Y.R.; Lee, H.Y.; Kim, J.H.; Moon, D.I.; Seo, M.Y.; Park, S.H.; Choi, K.H.; Kim, C.R.; Kim, S.H.; Oh, J.H.; et al. Anti-obesity and anti-diabetic effects of yerba mate (Ilex paraguariensis) in c57bl/6j mice fed a high-fat diet. Lab. Anim. Res. 2012, 28, 23–29. [Google Scholar]
- Pimentel, G.D.; Lira, F.S.; Rosa, J.C.; Caris, A.V.; Pinheiro, F.; Ribeiro, E.B.; Oller do Nascimento, C.M.; Oyama, L.M. Yerba mate extract (ilex paraguariensis) attenuates both central and peripheral inflammatory effects of diet-induced obesity in rats. J. Nutr. Biochem. 2013, 24, 809–818. [Google Scholar]
- Gosmann, G.; Barlette, A.G.; Dhamer, T.; Arcari, D.P.; Santos, J.C.; de Camargo, E.R.; Acedo, S.; Gambero, A.; Gnoatto, S.C.; Ribeiro, M.L. Phenolic compounds from mate (Ilex paraguariensis) inhibit adipogenesis in 3t3-l1 preadipocytes. Plant Foods Hum. Nutr. 2012, 67, 156–161. [Google Scholar]
- Arcari, D.P.; Santos, J.C.; Gambero, A.; Ferraz, L.F.; Ribeiro, M.L. Modulatory effects of yerba mate (Ilex paraguariensis) on the pi3k-akt signaling pathway. Mol. Nutr. Food Res. 2013, 57, 1882–1885. [Google Scholar]
- Arcari, D.P.; Santos, J.C.; Gambero, A.; Ribeiro, M.L. The in vitro and in vivo effects of yerba mate (Ilex paraguariensis) extract on adipogenesis. Food Chem. 2013, 141, 809–815. [Google Scholar]
- Carmo, L.S.; Rogero, M.M.; Cortez, M.; Yamada, M.; Jacob, P.S.; Bastos, D.H.; Borelli, P.; Ambrosio Fock, R. The effects of yerba mate (Ilex paraguariensis) consumption on il-1, il-6, tnf-α and il-10 production by bone marrow cells in wistar rats fed a high-fat die. Int. J. Vitam. Nutr. Res. 2013, 83, 26–35. [Google Scholar]
- Lima Nda, S.; Franco, J.G.; Peixoto-Silva, N.; Maia, L.A.; Kaezer, A.; Felzenszwalb, I.; de Oliveira, E.; de Moura, E.G.; Lisboa, P.C. Ilex paraguariensis (yerba mate) improves endocrine and metabolic disorders in obese rats primed by early weaning. Eur. J. Nutr. 2014, 53, 73–82. [Google Scholar]
- Borges, M.C.; Vinolo, M.A.; Nakajima, K.; de Castro, I.A.; Bastos, D.H.; Borelli, P.; Fock, R.A.; Tirapegui, J.; Curi, R.; Rogero, M.M. The effect of mate tea (Ilex paraguariensis) on metabolic and inflammatory parameters in high-fat diet-fed wistar rats. Int. J. Food Sci. Nutr. 2013, 64, 561–569. [Google Scholar]
- Martins, F.; Suzan, A.J.; Cerutti, S.M.; Arcari, D.P.; Ribeiro, M.L.; Bastos, D.H.; Carvalho Pde, O. Consumption of mate tea (Ilex paraguariensis) decreases the oxidation of unsaturated fatty acids in mouse liver. Br. J. Nutr. 2009, 101, 527–532. [Google Scholar]
- Paganini Stein, F.L.; Schmidt, B.; Furlong, E.B.; Souza-Soares, L.A.; Soares, M.C.; Vaz, M.R.; Muccillo Baisch, A.L. Vascular responses to extractable fractions of Ilex paraguariensis in rats fed standard and high-cholesterol diets. Biol. Res. Nurs. 2005, 7, 146–156. [Google Scholar]
- Pomilio, A.B.; Trajtemberg, S.; Vitale, A.A. High-performance capillary electrophoresis analysis of mate infusions prepared from stems and leaves of Ilex paraguariensis using automated micellar electrokinetic capillary chromatography. Phytochem. Anal. 2002, 13, 235–241. [Google Scholar]
- Martinet, A.; Hostettmann, K.; Schutz, Y. Thermogenic effects of commercially available plant preparations aimed at treating human obesity. Phytomedicine 1999, 6, 231–238. [Google Scholar]
- Resende, P.E.; Verza, S.G.; Kaiser, S.; Gomes, L.F.; Kucharski, L.C.; Ortega, G.G. The activity of mate saponins (Ilex paraguariensis) in intra-abdominal and epididymal fat, and glucose oxidation in male wistar rats. J. Ethnopharmacol. 2012, 144, 735–740. [Google Scholar]
- Andersen, T.; Fogh, J. Weight loss and delayed gastric emptying following a south american herbal preparation in overweight patients. J. Hum. Nutr. Diet. 2001, 14, 243–250. [Google Scholar]
- Harrold, J.A.; Hughes, G.M.; O’Shiel, K.; Quinn, E.; Boyland, E.J.; Williams, N.J.; Halford, J.C. Acute effects of a herb extract formulation and inulin fiber on appetite, energy intake and food choice. Appetite 2013, 62, 84–90. [Google Scholar]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar]
- Lippi, G.; Franchini, M.; Favaloro, E.J.; Targher, G. Moderate red wine consumption and cardiovascular disease risk: Beyond the “french paradox”. Semin. Thromb. Hemost. 2010, 36, 59–70. [Google Scholar]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar]
- Zhang, X.H.; Huang, B.; Choi, S.K.; Seo, J.S. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3t3-l1 adipocytes differentiation. Nutr. Res. Pract. 2012, 6, 286–293. [Google Scholar]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Resveratrol inhibits tnf-α-induced changes of adipokines in 3t3-l1 adipocytes. Biochem. Biophys. Res. Commun. 2007, 364, 972–977. [Google Scholar]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3t3-l1 adipocytes via activation of ampk. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar]
- Chen, S.; Xiao, X.; Feng, X.; Li, W.; Zhou, N.; Zheng, L.; Sun, Y.; Zhang, Z.; Zhu, W. Resveratrol induces sirt1-dependent apoptosis in 3t3-l1 preadipocytes by activating ampk and suppressing akt activity and survivin expression. J. Nutr. Biochem. 2012, 23, 1100–1112. [Google Scholar]
- Costa Cdos, S.; Rohden, F.; Hammes, T.O.; Margis, R.; Bortolotto, J.W.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. Resveratrol upregulated sirt1, foxo1, and adiponectin and downregulated pparγ1-3 mrna expression in human visceral adipocytes. Obes. Surg. 2011, 21, 356–361. [Google Scholar]
- Kang, L.; Heng, W.; Yuan, A.; Baolin, L.; Fang, H. Resveratrol modulates adipokine expression and improves insulin sensitivity in adipocytes: Relative to inhibition of inflammatory responses. Biochimie 2010, 92, 789–796. [Google Scholar]
- Kang, N.E.; Ha, A.W.; Kim, J.Y.; Kim, W.K. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3t3-l1 preadipocytes. Nutr. Res. Pract. 2012, 6, 499–504. [Google Scholar]
- Kwon, J.Y.; Seo, S.G.; Yue, S.; Cheng, J.X.; Lee, K.W.; Kim, K.H. An inhibitory effect of resveratrol in the mitotic clonal expansion and insulin signaling pathway in the early phase of adipogenesis. Nutr. Res. 2012, 32, 607–616. [Google Scholar]
- Lasa, A.; Schweiger, M.; Kotzbeck, P.; Churruca, I.; Simon, E.; Zechner, R.; Portillo, M.P. Resveratrol regulates lipolysis via adipose triglyceride lipase. J. Nutr. Biochem. 2012, 23, 379–384. [Google Scholar]
- Rayalam, S.; Yang, J.Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3t3-l1 adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar]
- Wang, A.; Liu, M.; Liu, X.; Dong, L.Q.; Glickman, R.D.; Slaga, T.J.; Zhou, Z.; Liu, F. Up-regulation of adiponectin by resveratrol: The essential roles of the akt/foxo1 and amp-activated protein kinase signaling pathways and dsba-l. J. Biol. Chem. 2011, 286, 60–66. [Google Scholar]
- Zhang, H.Y.; Du, Z.X.; Meng, X. Resveratrol prevents tnfα-induced suppression of adiponectin expression via pparγ activation in 3t3-l1 adipocytes. Clin. Exp. Med. 2013, 13, 193–199. [Google Scholar]
- Zhu, J.; Yong, W.; Wu, X.; Yu, Y.; Lv, J.; Liu, C.; Mao, X.; Zhu, Y.; Xu, K.; Han, X.; et al. Anti-inflammatory effect of resveratrol on tnf-α-induced mcp-1 expression in adipocytes. Biochem. Biophys. Res. Commun. 2008, 369, 471–477. [Google Scholar]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized ldl and apob in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar]
- Rayalam, S.; Della-Fera, M.A.; Yang, J.Y.; Park, H.J.; Ambati, S.; Baile, C.A. Resveratrol potentiates genistein’s antiadipogenic and proapoptotic effects in 3t3-l1 adipocytes. J. Nutr. 2007, 137, 2668–2673. [Google Scholar]
- Brown, M.S.; Goldstein, J.L. The srebp pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar]
- Kim, J.B.; Sarraf, P.; Wright, M.; Yao, K.M.; Mueller, E.; Solanes, G.; Lowell, B.B.; Spiegelman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through add1/srebp1. J. Clin. Investig. 1998, 101, 1–9. [Google Scholar]
- Lasa, A.; Churruca, I.; Eseberri, I.; Andres-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3t3-l1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1559–1568. [Google Scholar]
- Banerjee, S.S.; Feinberg, M.W.; Watanabe, M.; Gray, S.; Haspel, R.L.; Denkinger, D.J.; Kawahara, R.; Hauner, H.; Jain, M.K. The kruppel-like factor klf2 inhibits peroxisome proliferator-activated receptor-γ expression and adipogenesis. J. Biol. Chem. 2003, 278, 2581–2584. [Google Scholar]
- Mori, T.; Sakaue, H.; Iguchi, H.; Gomi, H.; Okada, Y.; Takashima, Y.; Nakamura, K.; Nakamura, T.; Yamauchi, T.; Kubota, N.; et al. Role of kruppel-like factor 15 (klf15) in transcriptional regulation of adipogenesis. J. Biol. Chem. 2005, 280, 12867–12875. [Google Scholar]
- Wang, Y.; Kim, K.A.; Kim, J.H.; Sul, H.S. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 2006, 136, 2953–2956. [Google Scholar]
- Kopan, R.; Ilagan, M.X. The canonical notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar]
- Chang, H.; Brown, C.W.; Matzuk, M.M. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr. Rev. 2002, 23, 787–823. [Google Scholar]
- Sottile, V.; Seuwen, K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with brl 49653 (rosiglitazone). FEBS Lett. 2000, 475, 201–204. [Google Scholar]
- Dowell, P.; Otto, T.C.; Adi, S.; Lane, M.D. Convergence of peroxisome proliferator-activated receptor γ and foxo1 signaling pathways. J. Biol. Chem. 2003, 278, 45485–45491. [Google Scholar]
- Armoni, M.; Harel, C.; Karni, S.; Chen, H.; Bar-Yoseph, F.; Ver, M.R.; Quon, M.J.; Karnieli, E. Foxo1 represses peroxisome proliferator-activated receptor-γ1 and -γ2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J. Biol. Chem. 2006, 281, 19881–19891. [Google Scholar]
- Jing, E.; Gesta, S.; Kahn, C.R. Sirt2 regulates adipocyte differentiation through foxo1 acetylation/deacetylation. Cell Metab. 2007, 6, 105–114. [Google Scholar]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado de Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing ppar-gamma. Nature 2004, 429, 771–776. [Google Scholar]
- Wang, F.; Tong, Q. Sirt2 suppresses adipocyte differentiation by deacetylating foxo1 and enhancing foxo1's repressive interaction with ppargamma. Mol. Biol. Cell 2009, 20, 801–808. [Google Scholar]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. Srt1720, srt2183, srt1460, and resveratrol are not direct activators of sirtl. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar]
- Tang, Q.Q.; Lane, M.D. Role of c/ebp homologous protein (chop-10) in the programmed activation of ccaat/enhancer-binding protein-beta during adipogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 12446–12450. [Google Scholar]
- Huang, H.; Lane, M.D.; Tang, Q.Q. Effect of serum on the down-regulation of chop-10 during differentiation of 3t3-l1 preadipocytes. Biochem. Biophys. Res. Commun. 2005, 338, 1185–1188. [Google Scholar]
- Sakaue, H.; Konishi, M.; Ogawa, W.; Asaki, T.; Mori, T.; Yamasaki, M.; Takata, M.; Ueno, H.; Kato, S.; Kasuga, M.; et al. Requirement of fibroblast growth factor 10 in development of white adipose tissue. Genes Dev. 2002, 16, 908–912. [Google Scholar]
- Abella, A.; Dubus, P.; Malumbres, M.; Rane, S.G.; Kiyokawa, H.; Sicard, A.; Vignon, F.; Langin, D.; Barbacid, M.; Fajas, L. Cdk4 promotes adipogenesis through ppargamma activation. Cell Metab. 2005, 2, 239–249. [Google Scholar]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar]
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science 2000, 287, 1606–1609. [Google Scholar]
- Fu, M.; Rao, M.; Bouras, T.; Wang, C.; Wu, K.; Zhang, X.; Li, Z.; Yao, T.P.; Pestell, R.G. Cyclin d1 inhibits peroxisome proliferator-activated receptor γ-mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 2005, 280, 16934–16941. [Google Scholar]
- Mitterberger, M.C.; Zwerschke, W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3t3-l1 preadipocytes. J. Gerontol. Ser. A 2013, 68, 1356–1376. [Google Scholar]
- Andrade, J.M.; Frade, A.C.; Guimaraes, J.B.; Freitas, K.M.; Lopes, M.T.; Guimaraes, A.L.; de Paula, A.M.; Coimbra, C.C.; Santos, S.H. Resveratrol increases brown adipose tissue thermogenesis markers by increasing sirt1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar]
- Moon, H.S.; Chung, C.S.; Lee, H.G.; Kim, T.G.; Choi, Y.J.; Cho, C.S. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3t3-l1 cells. Obesity 2007, 15, 2571–2582. [Google Scholar]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).